Abstract
Ultrasound (US) has gained widespread use in diagnostic cardiovascular applications. At amplitudes and frequencies typical of diagnostic use, its biomechanical effects on tissue are largely negligible. However, these parameters can be altered to harness US’s thermal and non-thermal effects for therapeutic indications. High-intensity focused ultrasound (HIFU) and extracorporeal shock wave therapy (ECWT) are two therapeutic US modalities which have been investigated for treating cardiac arrhythmias and ischemic heart disease, respectively. Here, we review the biomechanical effects of HIFU and ECWT, their potential therapeutic mechanisms, and pre-clinical and clinical studies demonstrating their efficacy and safety limitations. Furthermore, we discuss other potential clinical applications of therapeutic US and areas in which future research is needed.
Keywords: Ultrasound, Atrial fibrillation, Ablation, Angiogenesis
1 Introduction
In the 1950s, cardiologist Inge Edler and his physicist colleague, Carl Hellmuth Hertz, collaborated to apply ultrasound (US) to visualize the heart and intracardiac structures. This pioneering work led to the field of echocardiography [1], and further sophistication of echocardiography revolutionized the field of cardiology [2]. Subsequently, the use of diagnostic US spread into gastroenterology, obstetrics, and gynecology [3]. The use of US for therapeutic indications preceded these early diagnostic applications. Lynn et al. demonstrated the use of focused US for tissue ablation as early as 1942 [4], and the Fry brothers used US as an ablative approach for the treatment of Parkinson disease [5]. Further advancement of therapeutic US awaited the development of improved imaging technologies, and only recently has it gained acceptance as a promising clinical modality. Therapeutic US technologies are currently approved by the United States Food and Drug Administration (FDA) and used for the treatment of solid tumors, uterine fibroids, glaucoma, kidney stones, deep venous thrombosis, and musculoskeletal injuries, and as an adjunct to transdermal drug delivery and liposuction [6]. More recently, the use of therapeutic US for cardiac applications has advanced, particularly as an approach to catheter-based ablation of arrhythmias and to treatment of ischemic heart disease (IHD). This article summarizes the mechanisms, modalities, clinical efficacy, and safety profile of therapeutic US in the treatment of cardiovascular diseases.
2 Biomechanical effects of ultrasound
Both therapeutic and diagnostic US modalities rely on piezoelectric crystals which, when electrically stimulated, release high frequency sound waves. As these sound waves propagate through tissue, their energy is partially absorbed and partially reflected by fluid, cells, and connective tissue. Based on the clinical application and tissue characteristics, US parameters (frequency, amplitude, pulse duration) can be optimized to maximize reflection and minimize absorption (for diagnostic imaging), or to maximize absorption (for therapeutic applications).
The effects of US on tissue can generally be divided into thermal and non-thermal mechanisms (Table 1). Absorption of the US waves by tissue leads to increased local temperatures. While diagnostic US leads to low or negligible increases in tissue temperature [7], increasing the pulse length, or power can lead to rapid heating, coagulation, liquefactive necrosis, tissue vaporization, or some combination of the above. This effect of therapeutic US is typically harnessed for applications that require tissue necrosis, such as ablation of soft tissue tumors, and, in cardiovascular applications, for treatment of cardiac arrhythmias.
Table 1.
Biomechanical effects of ultrasound
|
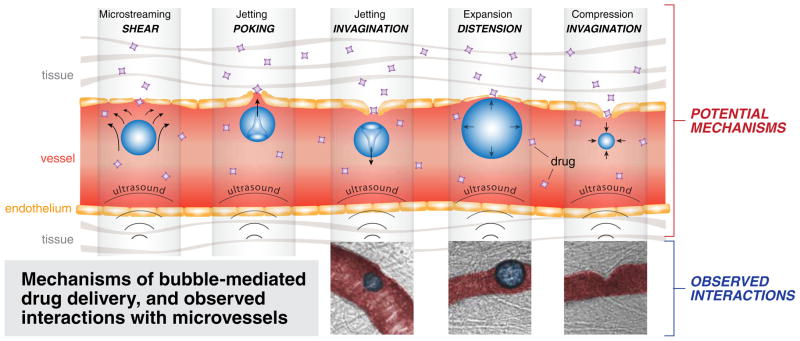
Alternatively, relatively high acoustic amplitude potentiates the non-thermal effects. These effects generally rely on the process of acoustic cavitation—the creation of small gas bubbles in the blood via transmission of US energy. Acoustic cavitation mediates US’s vascular effects through three potential mechanisms. One of these mechanisms is microstreaming (Fig. 1a), the transmission of shear stress from fluid motion generated by oscillating bubbles to cell membranes and endothelial surfaces (US waves may also directly induce shear stress onto cell membranes) [8]. Another mechanism is jetting (Fig. 1b and c) from asymmetric collapse of a bubble. Jetting of bubbles from vessel lumen into tissue (called poking), or from tissue into the lumen (leading to invagination of the local vessel wall), can potentially increase vascular permeability [9]. Permeability may also be enhanced by expanding and compressing bubbles, which, in turn, causes distension and invagination of the vessel wall, respectively (Fig. 1d and e) [10].
Fig. 1.
Schematic and ex vivo microscopy images of non-thermal vascular effects of therapeutic ultrasound. Top row demonstrates microstreaming (a), jetting (b, c), bubble expansion/compression (d, e). Bottom row images demonstrate interaction of lipid-coated perfluoropropane ultrasound contrast bubbles with capillary walls (injected with Green India Ink for contrast) in ex vivo rat mesentery, during HIFU treatment, under 40× high-speed microscopy [9, 10]
The non-thermal effects of therapeutic US, namely increased vascular permeability [11] and shear stress [12], may be the principal effects by which it has been shown to promote angiogenesis and improved myocardial contractility in IHD.
These mechanisms could also lead to soft tissue or vascular damage, particularly in gas-containing organs such as lung and bowel, in which gas bubbles readily cavitate [13]. However, with careful titration of US parameters, non-thermal effects can be therapeutically harnessed to increase vascular permeability without causing vascular damage [14]. In in vitro experiments, applying US therapy up to 0.5 mJ/mm2 to the aortic wall did not demonstrate any histologic evidence of vascular damage [15].
Finally, studies have suggested direct cellular effects of therapeutic US that may not necessarily act via acoustic cavitation (Table 1). Application of US to human umbilical vein endothelial cells (HUVECs) led to upregulation of vascular endothelial growth factor (VEGF) and its receptor, Flt-1 [16]. In vitro studies of therapeutic US on C6 rat glioma cells [17] and on HUVECs [18] showed the therapy to have anti-inflammatory properties, and to up-regulate nitric oxide synthesis. Another in vitro study applying therapeutic US to myocardial autopsy and biopsy specimens of patients with ischemic cardiomyopathy and normal controls showed increased differentiation of stem cells into endothelial cells, cardiac myocytes, and smooth muscle cells after application of US [19]. An in vivo study in rats showed therapeutic US to increase populations of cardiac stem cells [20].
3 Ultrasound modalities
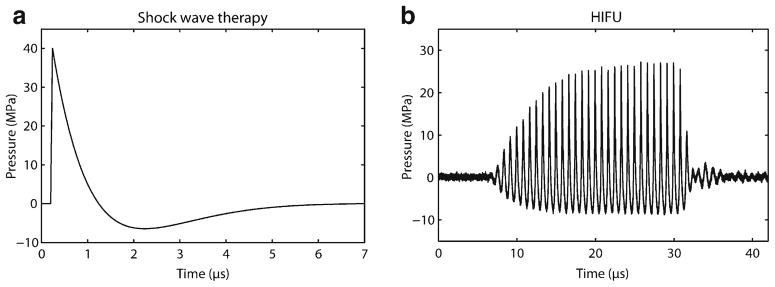
Two main therapeutic US delivery devices have been developed for cardiac applications based on their predominating biomechanical effects: extracorporeal shock wave therapy (ECWT) and high-intensity focused ultrasound (HIFU) (Table 2). Well-known for its use in lithotripsy of kidney stones, ECWT transducers send individual waveforms with approximately 1 ms-long, high-pressure/amplitude positive pulse (usually 10–100 MPa) followed by a longer (~5 ms), lower-amplitude negative pressure tail (2–10 MPa) (Fig. 2a). Numerous (on the order of 100 to 1,000 s) separate pulses are applied to the area of interest. Given its high amplitude, and thus ability to potentiate non-thermal biomechanical effects of US, ECWT has mainly been investigated for the treatment of IHD.
Table 2.
Comparison of ECWT and HIFU: biomechanical effects and clinical applications
| Ultrasound parameters | Predominant biomechanical effects | Delivery | FDA-approved clinical applications | Potential cardiovascular applications | |
|---|---|---|---|---|---|
| ECWT | High amplitude, separate pulses | Non-thermal (cavitation, streaming) | Linear, absorbed by tissue between transducer and target | Lithotripsy, plantar fasciitis, epicondylitis | Ischemic heart disease, peripheral arterial disease |
| HIFU | High frequency, continuous wave | Thermal (heating, liquefactive necrosis) | Focused, minimal off-target effects | Uterine fibroids, laparoscopic tissue ablation, venous and arterial thromboses | AF and VT ablation, ASD/VSD creation, AVN ablation, functional MR, peripheral vascular disease, ischemic heart disease |
Fig. 2.
Comparison of ECWT (a) and HIFU (b) waveforms. Note the continuous HIFU waveform with a dedicated frequency, compared with the higher pressure/amplitude, single waveform of ECWT
Unlike ECWT, HIFU applies continuous US waves with a dedicated frequency (usually 1–10 MHz) and amplitude (~10 MPa) (Fig. 2b). Whereas ECWT delivers shock waves in a linear fashion and is absorbed by all tissue between the transducer-tissue interface and target tissue, HIFU signal generators are typically mounted on a concave transducer and focus the US at a fixed focus length. This allows HIFU beams to converge energy onto a target tissue, at a pre-determined depth from the tissue surface, with a resolution of a few millimeters, and minimal effect on tissue between the surface and the target [6]. This ability to converge higher power at a higher resolution makes HIFU an ideal technology for clinical applications requiring the thermal effects of US.
4 Cardiovascular applications
4.1 Ischemic heart disease
The potential of ECWT to treat IHD by promoting angiogenesis was first shown by Nishida and colleagues in a porcine model of chronic ischemic cardiomyopathy, in which the therapy was shown to increase ejection fraction, wall thickening, myocardial blood flow and capillary density [16]. Similar results were seen in porcine models of acute myocardial infarction [21] and ischemia–reperfusion injury [22].
This technology has since been adapted to humans, and is delivered in a non-invasive manner with a shock wave generator gently applied to the chest wall. A diagnostic echocardiogram transducer is mounted in-line with the generator to provide direct echocardiographic guidance to the desired treatment area. There is no need for sedation or general anesthesia. Approximately one tenth the total power of energy used for lithotripsy is applied to the tissue (0.09 mJ/mm2). As exogenous stimulation by therapeutic US could promote atrial or ventricular ectopy—indeed one study showed that HIFU therapy could cause premature ventricular contractions [23]—shock waves are electrocardiogram-gated to prevent R-on-T phenomena.
The first published experience of ECWT in humans was reported by Fukumoto et al., who described a series of nine patients with coronary artery disease and stable angina who were not eligible for percutaneous coronary intervention or coronary artery bypass graft surgery and underwent ECWT three times a week, for up to 3 weeks [24]. Decreased angina was noted, with decrease in Canadian Cardiovascular Society score and nitroglycerine use, and improvements in myocardial perfusion by thallium scintigraphy. Effects persisted for up to 12 months after therapy. The first randomized, double-blinded trial of ECWT was performed by the same group; eight patients were randomized in a cross-over manner to either ECWT or placebo therapy [25]. Similar improvements in angina symptoms were seen, as well as improvement in the 6-min walk test and ejection fraction. Other small series [26, 27], case–control trials [28], and randomized trials [29, 30] of ECWT for this indication have shown similar improvements compared with pre-treatment baseline and placebo therapy (Table 3). No procedural complications or adverse effects occurred in these studies, although the degree and duration of monitoring was variable. There is a theoretical concern that exposure of epicardial coronary arteries to the ECWT treatment field could lead to endothelial damage or plaque rupture. However, serial measurement of cardiac bio-markers after ECWT showed no difference compared with untreated placebo patients [30].
Table 3.
Summary of clinical trials of ECWT for the treatment of ischemic heart disease
| Trial | Patients (number) | Patient population | Trial type | CCS class | NTG use (per week) | EF (%) | 6MWT (meters) | NYHA class | Nuclear imaging outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Fukumoto, et al. 2006 (26) | 9 | End-stage CAD | Series | 2.7 to 1.8** | 5.4 to 0.3* | n/a | n/a | n/a | Improved SSS* but not SRS (NS) by stress thallium scan |
| Gutersohn, et al. 2006 (29) | 14 | End-stage CAD | Series | 3.4 to 2.4 *** | n/a | n/a | n/a | n/a | Improved perfusion by SPECT |
| Kikuchi, et al. 2010 (27) | 8 | End-stage CAD | Randomized, placebo-controlled, cross-overa | 3.0 to 2.3** | 4.1 to 0.9** | 51 to 56* | 200 to 330** | n/a | n/a |
| Vasyuk, et al. 2010 (28) | 24 | Ischemic cardiomyopathy (EF<40 %) | Seriesb | 2.6 to 1.9 ** | n/a | 32 to 38* | 414 to 538** | 2.2 to 1.7** | Improved SRS* and SSS* by stress SPECT |
| Wang, et al. 2010 (30) | 25 (and 10 controls) | CAD with chronic stable angina | Case–control | Improved | Improved | n/a | Improved | Improved* | Improved perfusion on rest SPECT** |
| Peng, et al. 2012 (31) | 50 | Ischemic cardiomyopathy | Randomized, placebo-controlledc | Improved** | Improved** | 45 to 47** | Improved* | Improved** | Improved perfusion** and viability* on rest SPECT/PET |
| Yang, et al. 2012 (32) | 25 | CAD without prior revascularization | Randomized, placebo-controlledc | 2.0 to 1.0* | 2.0 to 1.0** | 51 to 56* | 383 to 438* | 2.0 to 1.0* | Improved perfusion*** and viability** on rest SPECT/PET |
CCS Canadian Cardiovascular Society (angina class), NTG nitroglycerine, EF ejection fraction, 6MWT 6-minute walk test, NYHA New York Heart Association, CAD coronary artery disease, NS p >0.05, SSS summed stress score, SRS summed rest score, SPECT single photon emission computed tomography, PET positron emission tomography
p <0.05
p <0.01
p <0.001
No significant change in endpoints pre- and post-treatment in control group
No significant change in endpoints pre- and post-placebo treatment
Comparison of all endpoints between post-treatment ECWT and placebo groups p <0.05
To date, there have been no large, randomized clinical trials of ECWT for this indication, nor have any trials demonstrated a reduction in clinical outcomes beyond decreased angina. Clinical trials are ongoing in Japan, China, Pakistan, Netherlands, Germany, and Israel, but none have enrolled more than 100 patients [31]. An ECWT device (Storz Medical; Tägerwilen, Switzerland) has received a European Union CE marking in 2003, was approved by the Japanese Ministry of Health, Labor and Welfare for treatment of refractory angina in 2010, and is being used clinically in over ten countries [32]. Another ECWT device (Medispec, Germantown, Maryland, USA) is currently under development. ECWT has not yet been approved for use in the USA.
4.2 Arrhythmias
HIFU has been used in the treatment of arrhythmias, most commonly AF (Table 4). HIFU was used to treat AF in a series of 103 patients undergoing cardiac surgery [33]. A linear HIFU catheter was used epicardially during cardiac surgery to electrically isolate the pulmonary veins and to create a linear ablation line between the left lower pulmonary vein and mitral valve annulus using the UltraCinch and UltraWand devices (St. Jude Medical, St. Paul, MN). This study showed excellent safety results, and 80 % of patients were free of AF after six months; other trials [34, 35] had similar results. This device was also used in a minimally invasive approach with video-associated thoracoscopic surgery, albeit with only 40 % freedom of AF after 6 months and increased complications in one series [36]. However, the UltraCinch and UltraWand devices entered a surgical AF treatment market largely ingrained in the traditional “cut-and-sew” technique, and with recent competition from epicardial radiofrequency and cryoenergy technologies. Thus, there has been difficulty in marketing and training surgeons to use this device, and St. Jude Medical has ceased production of these devices.
Table 4.
Comparison of clinical and pre-clinical therapeutic ultrasound devices
| Application | Delivery device | Frequency | Acoustic energy/power | Delivery protocol |
|---|---|---|---|---|
| ECWT for ischemic heart disease [27] | Extracorporeal shock wave generator mounted in-line with trans-thoracic echocardiogram | N/A (individual shock waves) | 0.9 mJ/mm2 | 200 shock waves per spot, 40–60 spots per session, three sessions per week for three months |
| HIFU epicardial pulmonary vein isolation for AF [35–37] | UltraCinch and UltraWand devices secured epicardially around pulmonary veins | 3.8–6.4 MHz | 15–130 W | Three sequential stages of ablation corresponding to different depths: endocardial to epicardial. Ten minutes total for UltraCinch, one minute for UltraWand |
| HIFU endocardial pulmonary vein isolation for AF [41] | 12 French balloon-tipped endocardial catheter | 9 MHz | 45 W | Circumferential ablations, 40–90 s per vein, multiple ablations may be required |
| HIFU epicardial VT ablation [46]a | 14 French epicardial catheter | 6.4 MHz | 6 W | 60 s per ablation point, multiple points per animal |
Animal studies only
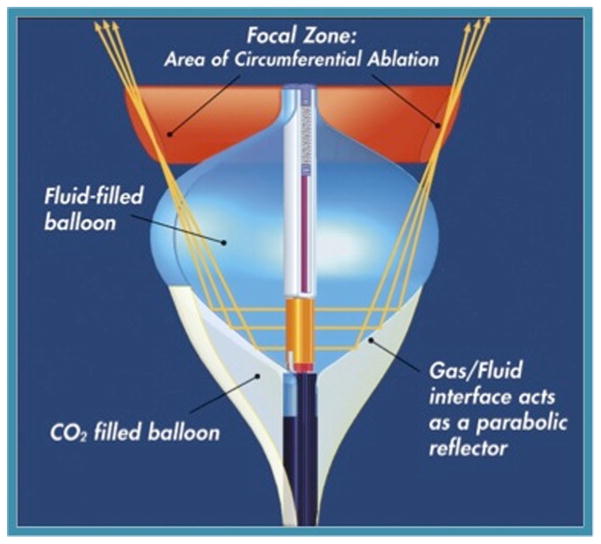
Further advances led to an endocardial, balloon catheter-based approach. The first ultrasound catheter used an ultrasound crystal mounted on a liquid CO2-filled balloon (Atrionix, Palo Alto, CA, USA). The advantage of this approach, compared to standard RF, catheter-based pulmonary vein isolation was that the entire circumference of each pulmonary vein could be treated all at once, as opposed to the “connect-the-dots” strategy of RF ablation. This was anticipated to lead to a much more rapid, safe, and effective procedure with fewer gaps around the pulmonary vein. However, because the balloon needed to be seated inside the pulmonary vein, the lesions were typically too distal to completely treat the arrhythmia. This and other technical problems led to abandonment of this technology. However, another company developed a similar balloon based approach, but directed the HIFU energy forward and took advantage of the interface between H2O and CO2 to reflect the energy more proximally around the pulmonary vein (HIFU-BC; ProRhythm, Ronkonkoma, NY, USA; Fig. 3). In an initial study of 27 patients, 87 % of pulmonary vein ostia were successfully isolated, and 59 % of patients were free of AF at 12 months [37]. Longer-term follow-up of a cohort of 32 patients showed 56 % freedom of AF at approximately 4 years [38]. Third-generation, steerable catheters decreased procedure time (to 166 min) and left atrial dwelling time (58 min), with 71 % of patients free of AF at approximately 1 year follow-up [39]. However, the major downfall of this technology was the complication of atrio-esophageal fistula [40]. Because of the uniformity and depth of the HIFU lesion, this complication could not be avoided, leading to several deaths and eventual termination of the pivotal US trial and dissolution of the company developing this technology. Unfortunately, this has impeded the development of HIFU for catheter ablation applications despite the promise of uniform deep lesions. It may be that use of lower or variable HIFU energy could have avoided the development of atrio-esophageal fistulae, however after the failure of the clinical trial development of this promising technology was abandoned.
Fig. 3.
HIFU-BC (balloon catheter) developed by ProRhythm (Ronkonkoma, NY, USA) for circumferential pulmonary vein isolation
HIFU catheters have also been developed for ablation of ventricular tachycardia (VT), and HIFU may be of particular use for ablation of epicardial VT [41]. Application of RF energy in the epicardium is often limited by epicardial fat, or proximity to the phrenic nerve or coronary arteries [42]. Epicardial fat impedes delivery of RF energy to the underlying myocardium, and ablation near epicardial coronary vessels may lead to acute or chronic vessel thrombosis and acute myocardial infarction [43]. Thus far, one study has assessed the feasibility of HIFU ablation in an open-chest swine model, demonstrating deep (16 mm) necrotic lesions up to 8 weeks after ablation [44]. Given its ability to focus energy at a specific tissue depth, HIFU may also be useful for VTs that originate deep in the wall between the right and left lower chambers (septum), which is difficult to reach with standard radiofrequency energy.
4.3 Other cardiovascular applications
The thermal effects of HIFU have also been investigated for non-invasive ligation of mitral chordae (for treatment of functional mitral regurgitation) [45], atrial septostomy [46, 47], creation of ventricular septal defects (for palliation of cyanotic congenital heart disease) [48], and atrio-ventricular nodal ablation [49] in dog and ex vivo models.
Intravascular therapeutic US has also been used in a catheter-based approach for thrombolysis of venous and arterial clots (EKOS Corporation, Bothell, WA, USA), with promising results. This catheter-based approach can be used alone, or as an adjunct to enhance the effects of pharmacologic thrombolytics [50]. While US-accelerated thrombolysis has been approved by the FDA, it has yet to gain widespread clinical use.
Ultrasound has been used in combination with contrast agent microbubbles to mediate localized drug or gene delivery, either by increasing local vascular permeability, or by releasing drugs or plasmids from within the microbubbles upon cavitation. In the recent CELLWAVE study [51], adjunctive ECWT was shown to modestly potentiate the effects of autologous bone marrow-derived mononuclear cells in patients with chronic ischemic cardiomyopathy, leading to mild improvements in ejection fraction (+3.5 %) compared with placebo ECWT (+0.8 %).
Finally, ECWT has been investigated in animal models [52] of and patients [53] with peripheral arterial disease in order to promote angiogenesis. Initial findings were promising, but future studies are needed. The mechanisms underlying this benefit include the cavitation effects leading to increased vascular permeability and thus angiogenesis, as well as the VEGF- and stem cell-mediated effects discussed above, but need to be clearly demonstrated.
5 Conclusions
US technology has progressed from a predominantly diagnostic technology to a promising therapeutic modality for many cardiovascular applications. Its thermal effects have been investigated to develop HIFU as an ablative treatment for cardiac arrhythmias. Its non-thermal properties, with possible effects on stem cell differentiation, angiogenesis and inflammation, have been utilized to develop ECWT as a treatment of IHD. Further proliferation and FDA approval of ECWT for IHD will require large, randomized, blinded, placebo-controlled trials with long-term clinical endpoints, as well as close peri-procedural and long-term safety monitoring. It is also important to elucidate further the cellular and molecular mechanisms by which US leads to improvements in IHD. The use of HIFU for AF ablation has been hampered by safety concerns, notably atrio-esophageal fistulas. Other cardiac uses of HIFU are under development. Finally, therapeutic US may also be a treatment for ventricular tachycardia, peripheral arterial disease, and congenital/structural heart disease, but these indications require further investigation.
Acknowledgments
The authors would like to acknowledge Dr. Hong Chen for her hard work obtaining microscopic images of cavitation phenomena. The authors would also like to thank Camilo Perez and Dr. Wayne Kreider for their help with HIFU and SWT illustrations.
Contributor Information
Babak Nazer, Division of Cardiology, University of California San Francisco, 505 Parnassus Avenue, Room M1184, San Francisco, CA 94143-0124, USA.
Edward P. Gerstenfeld, Division of Cardiology, University of California San Francisco, 505 Parnassus Avenue, Room M1184, San Francisco, CA 94143-0124, USA.
Akiko Hata, Cardiovascular Research Institute, University of California San Francisco, San Francisco, USA.
Lawrence A. Crum, Applied Physics Laboratory, University of Washington, Seattle, USA.
Thomas J. Matula, Applied Physics Laboratory, University of Washington, Seattle, USA.
References
- 1.Edler I, Hertz CH. The use of ultrasonic reflectoscope for the continuous recording of the movements of heart walls. 1954. Clinical Physiology And Functional Imaging. 2004;24(3):118–136. doi: 10.1111/j.1475-097X.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 2.Edler I, Lindström K. The history of echocardiography. Ultrasound In Medicine & Biology. 2004;30(12):1565–1644. doi: 10.1016/S0301-5629(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 3.Donald I, Macivar J, Brown T. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1(7032):1188–1195. doi: 10.1016/s0140-6736(58)91905-6. [DOI] [PubMed] [Google Scholar]
- 4.Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. The Journal Of General Physiology. 1942;26(2):179–193. doi: 10.1085/jgp.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyers R, Fry WJ, Fry FJ, Dreyer L, Schultz D, Noyes R. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. Journal of Neurosurgery. 1959;16(1):32–54. doi: 10.3171/jns.1959.16.1.0032. [DOI] [PubMed] [Google Scholar]
- 6.Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IRS. Overview of therapeutic ultrasound applications and safety considerations. Journal of Ultrasound in Medicine. 2012;31(4):623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fowlkes JB. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: executive summary. Journal Of Ultrasound In Medicine. 2008;27(4):503–515. doi: 10.7863/jum.2008.27.4.503. [DOI] [PubMed] [Google Scholar]
- 8.Wu J. Shear stress in cells generated by ultrasound. Progress In Biophysics And Molecular Biology. 2007;93(1–3):363–373. doi: 10.1016/j.pbiomolbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Brayman Aa, Kreider W, Bailey MR, Matula TJ. Observations of translation and jetting of ultrasound-activated microbubbles in mesenteric microvessels. Ultrasound In Medicine & Biology. 2011;37(12):2139–2148. doi: 10.1016/j.ultrasmedbio.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Physical Review Letters. 2011;106(3):34301. doi: 10.1103/PhysRevLett.106.034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49(25):2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Brayman AA, Bailey MR, Matula TJ. Blood vessel rupture by cavitation. Urological Research. 2010;38(4):321–326. doi: 10.1007/s00240-010-0302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanBavel E. Effects of shear stress on endothelial cells: possible relevance for ultrasound applications. Progress In Biophysics And Molecular Biology. 2007;93(1–3):374–383. doi: 10.1016/j.pbiomolbio.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Belcaro G, Nicolaides A, Marlinghaus E, Cesarone M, Incandela L, DeSanctis M, et al. Shock waves in vascular diseases. An in-vitro study. Angiology. 1998;49(10):100–101. doi: 10.1177/000331979804900901. [DOI] [PubMed] [Google Scholar]
- 16.Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia-induced myocardial dysfunction in pigs in vivo. Circulation. 2004;110(19):3055–3061. doi: 10.1161/01.CIR.0000148849.51177.97. [DOI] [PubMed] [Google Scholar]
- 17.Ciampa AR, de Prati AC, Amelio E, Cavalieri E, Persichini T, Colasanti M, et al. Nitric oxide mediates anti-inflammatory action of extracorporeal shock waves. FEBS letters. 2005;579(30):6839–6845. doi: 10.1016/j.febslet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, et al. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric oxide: Biology and Chemistry. 2005;12(2):89–96. doi: 10.1016/j.niox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Nurzynska D, Di Meglio F, Castaldo C, Arcucci A, Marlinghaus E, Russo S, et al. Shock waves activate in vitro cultured progenitors and precursors of cardiac cell lineages from the human heart. Ultrasound In Medicine & Biology. 2008;34(2):334–342. doi: 10.1016/j.ultrasmedbio.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Di Meglio F, Nurzynska D, Castaldo C, Miraglia R, Romano V, De Angelis A, et al. Cardiac shock wave therapy: assessment of safety and new insights into mechanisms of tissue regeneration. Journal Of Cellular And Molecular Medicine. 2012;16(4):936–942. doi: 10.1111/j.1582-4934.2011.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uwatoku T, Ito K, Abe K, Oi K, Hizume T, Sunagawa K, et al. Extracorporeal cardiac shock wave therapy improves left ventricular remodeling after acute myocardial infarction in pigs. Coronary Artery Disease. 2007;18(5):397–404. doi: 10.1097/MCA.0b013e328089f19b. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi GJ, Takeda M, et al. Cardiac shock wave therapy ameliorates left ventricular remodeling after myocardial ischemia–reperfusion injury in pigs in vivo. Coronary Artery Disease. 2010;21(5):304–311. doi: 10.1097/mca.0b013e32833aec62. [DOI] [PubMed] [Google Scholar]
- 23.Hersch A, Adam D. Premature cardiac contractions produced efficiently by external high-intensity focused ultrasound. Ultrasound In Medicine & Biology. 2011;37(7):1101–1110. doi: 10.1016/j.ultrasmedbio.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto Y, Ito A, Uwatoku T, Matoba T, Kishi T, Tanaka H, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial ischemia in patients with severe coronary artery disease. Coronary Artery Disease. 2006;17:63–70. doi: 10.1097/00019501-200602000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi Y, Ito K, Ito Y, Shiroto T, Tsuburaya R, Aizawa K, et al. Double-blind and placebo-controlled study of the effectiveness and safety of extracorporeal cardiac shock wave therapy for severe angina pectoris. Circulation Journal. 2010;74(3):589–591. doi: 10.1253/circj.cj-09-1028. [DOI] [PubMed] [Google Scholar]
- 26.Vasyuk YA, Hadzegova AB, Shkolnik EL, Kopeleva MV, Krikunova OV, Iouchtchouk EN, et al. Initial clinical experience with extracorporeal shock wave therapy in treatment of ischemic heart failure. Congestive Heart Failure. 2010;16(5):226–230. doi: 10.1111/j.1751-7133.2010.00182.x. [DOI] [PubMed] [Google Scholar]
- 27.Gutersohn Achim, Caspari Guido H, Marlinghaus Ernst, Haude M. Comparison of cardiac shock wave therapy and percutaneous laser revascularization therapy in endstage CAD patients with refractory angina. World Congress of Cardiology and ESC Conference.2006. [Google Scholar]
- 28.Wang Y, Guo T, Cai HY, Ma TK, Tao SM, Chen MQ, et al. Extracorporeal cardiac shock wave therapy for treatment of coronary artery disease. Chinese Journal of Cardiovascular Diseases. 2010;38(8):711–715. [PubMed] [Google Scholar]
- 29.Peng Y, Guo T, Yang P, Yang H, Zhou P, Wang Y, et al. Effects of extracorporeal cardiac shock wave therapy in patients with ischemic heart failure. Chinese Journal of Cardiovascular Diseases. 2012;40(2):141–146. [PubMed] [Google Scholar]
- 30.Yang P, Guo T, Wang W, Peng YZ, Wang Y, Zhou P, et al. Randomized and double-blind controlled clinical trial of extracorporeal cardiac shock wave therapy for coronary heart disease. Heart and vessels. 2013;28(3):284–291. doi: 10.1007/s00380-012-0244-7. [DOI] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov. Retrieved June 2, 2013, from www.clinicaltrials.gov.
- 32.Taylor J. Recent pioneering cardiology developments in Japan: Japanese cardiologists have discovered Waon therapy for severe or refractory heart failure and extracorporeal cardiac shock wave therapy for severe angina pectoris. European heart journal. 2011;32(14):1690–1691. [PubMed] [Google Scholar]
- 33.Ninet J, Roques X, Seitelberger R, Deville C, Pomar JL, Robin J, et al. Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial. The Journal Of Thoracic And Cardiovascular Surgery. 2005;130(3):803–809. doi: 10.1016/j.jtcvs.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Mitnovetski S, Almeida AA, Goldstein J, Pick AW, Smith JA. Epicardial high-intensity focused ultrasound cardiac ablation for surgical treatment of atrial fibrillation. Heart, Lung & Circulation. 2009;18(1):28–31. doi: 10.1016/j.hlc.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Schopka S, Schmid C, Keyser A, Kortner A, Tafelmeier J, Diez C, et al. Ablation of atrial fibrillation with the Epicor system: a prospective observational trial to evaluate safety and efficacy and predictors of success. Journal of Cardiothoracic Surgery. 2010;5:34. doi: 10.1186/1749-8090-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinkenberg TJ, Ahmed S, Ten Hagen A, Wiesfeld ACP, Tan ES, Zijlstra F, et al. Feasibility and outcome of epicardial pulmonary vein isolation for lone atrial fibrillation using minimal invasive surgery and high intensity focused ultrasound. Europace. 2009;11(12):1624–1631. doi: 10.1093/europace/eup299. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa H, Antz M, Wong T, Schmidt B, Ernst S, Ouyang F, et al. Initial experience using a forward directed, high-intensity focused ultrasound balloon catheter for pulmonary vein antrum isolation in patients with atrial fibrillation. Journal of Cardiovascular Electrophysiology. 2007;18(2):136–144. doi: 10.1111/j.1540-8167.2006.00715.x. [DOI] [PubMed] [Google Scholar]
- 38.Metzner A, Chun KRJ, Neven K, Fuernkranz A, Ouyang F, Antz M, et al. Long-term clinical outcome following pulmonary vein isolation with high-intensity focused ultrasound balloon catheters in patients with paroxysmal atrial fibrillation. Europace. 2010;12(2):188–193. doi: 10.1093/europace/eup416. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt B, Chun KRJ, Metzner A, Fuernkranz A, Ouyang F, Kuck K-H. Pulmonary vein isolation with high-intensity focused ultrasound: results from the HIFU 12F study. Europace. 2009;11(10):1281–1288. doi: 10.1093/europace/eup208. [DOI] [PubMed] [Google Scholar]
- 40.Neven K, Schmidt B, Metzner A, Otomo K, Nuyens D, De Potter T, et al. Fatal end of a safety algorithm for pulmonary vein isolation with use of high-intensity focused ultrasound. Circulation: Arrhythmia and Electrophysiology. 2010;3(3):260–265. doi: 10.1161/CIRCEP.109.922930. [DOI] [PubMed] [Google Scholar]
- 41.Haqqani HM, Tschabrunn CM, Tzou WS, Dixit S, Cooper JM, Riley MP, et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8(8):1169–1176. doi: 10.1016/j.hrthm.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Tung R, Michowitz Y, Yu R, Mathuria N, Vaseghi M, Buch E, et al. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013;10(4):490–498. doi: 10.1016/j.hrthm.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 43.D’Avila A, Gutierrez P, Scanavacca M, Reddy V, Lustgarten DL, Sosa E, et al. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implications for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing and Clinical Electrophysiology. 2002;25(10):1488–1495. doi: 10.1046/j.1460-9592.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- 44.Koruth JS, Dukkipati S, Carrillo RG, Coffey J, Teng J, Eby TB, et al. Safety and efficacy of high-intensity focused ultrasound atop coronary arteries during epicardial catheter ablation. Journal of cardiovascular electrophysiology. 2011;22(11):1274–1280. doi: 10.1111/j.1540-8167.2011.02084.x. [DOI] [PubMed] [Google Scholar]
- 45.Abe Y, Otsuka R, Muratore R, Fujikura K, Okajima K, Suzuki K, et al. In vitro mitral chordal cutting by high intensity focused ultrasound. Ultrasound In Medicine & Biology. 2008;34(3):400–405. doi: 10.1016/j.ultrasmedbio.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Takei Y, Muratore R, Kalisz A, Okajima K, Fujimoto K, Hasegawa T, et al. In vitro atrial septal ablation using high-intensity focused ultrasound. Journal of the American Society of Echocardiography. 2012;25(4):467–472. doi: 10.1016/j.echo.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 47.Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010;121(6):742–749. doi: 10.1161/CIRCULATIONAHA.109.889071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owens GE, Miller RM, Ensing G, Ives K, Gordon D, Ludomirsky A, et al. Therapeutic ultrasound to noninvasively create intracardiac communications in an intact animal model. Catheterization And Cardiovascular Interventions. 2011;77(4):580–588. doi: 10.1002/ccd.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strickberger SA, Tokano T, Kluiwstra JU, Morady F, Cain C. Extracardiac ablation of the canine atrioventricular junction by use of high-intensity focused ultrasound. Circulation. 1999;100(2):203–208. doi: 10.1161/01.cir.100.2.203. [DOI] [PubMed] [Google Scholar]
- 50.Doomernik DE, Schrijver AM, Zeebregts CJ, de Vries JPPM, Reijnen MM. Advancements in catheter-directed ultrasound-accelerated thrombolysis. Journal of Endovascular Therapy. 2011;18(3):418–434. doi: 10.1583/10-3362.1. [DOI] [PubMed] [Google Scholar]
- 51.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, et al. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. Journal of the American Medical Association. 2013;309(15):1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 52.Oi K, Fukumoto Y, Ito K, Uwatoku T, Abe K, Hizume T, et al. Extracorporeal shock wave therapy ameliorates hindlimb ischemia in rabbits. The Tohoku Journal Of Experimental Medicine. 2008;214(2):151–158. doi: 10.1620/tjem.214.151. [DOI] [PubMed] [Google Scholar]
- 53.De Sanctis M, Belcaro G, Nicolaides A, Cesarone M, Incandela L, Marlinghaus E, et al. Effects of shock waves on the microcirculaation in critical limb ischemia (CLI) (8-week study) Angiology. 2000;51(8):83–84. doi: 10.1177/000331970005100809. [DOI] [PubMed] [Google Scholar]