Abstract
Developmental brain plasticity involves complex time-dependent dynamic molecular interactions that cannot be observed directly in humans. We propose that the shared evolutionary homology of teeth with the neurosensory system, and the archival nature of dentin microstructure, allow the development of ‘biologic hard drives’ which can characterize perinatal temporal dynamics in neuroplasticity.
Keywords: teeth, critical period, brain plasticity, biomarker
As the brain develops postnatally, particular regions undergo heightened periods of plasticity when their underlying neural circuits are sculpted by experiences to establish normal perceptual and cognitive behaviors, such as sensory processing, language, and emotional processing. Conversely, absent or abnormal experiences, such as sensory or social deprivation, during this critical period can disrupt the establishment of behaviors [1] (Figure 1A). By understanding the fundamental neurobiology of how plasticity and recovery become limited as a function of age after abnormal experience or injury during a critical window, we will be able to develop better therapeutic approaches for developmental disorders and adult brain injuries [1]. However, translating the emerging evidence of critical windows of susceptibility from animal models to humans has been hindered by the lack of appropriate human biomarkers that capture the dynamic, time-dependent nature of molecular interactions during brain development. Here, we propose novel tooth-matrix biomarkers to identify not only critical developmental periods of brain plasticity in humans but also their potential clinical applications.
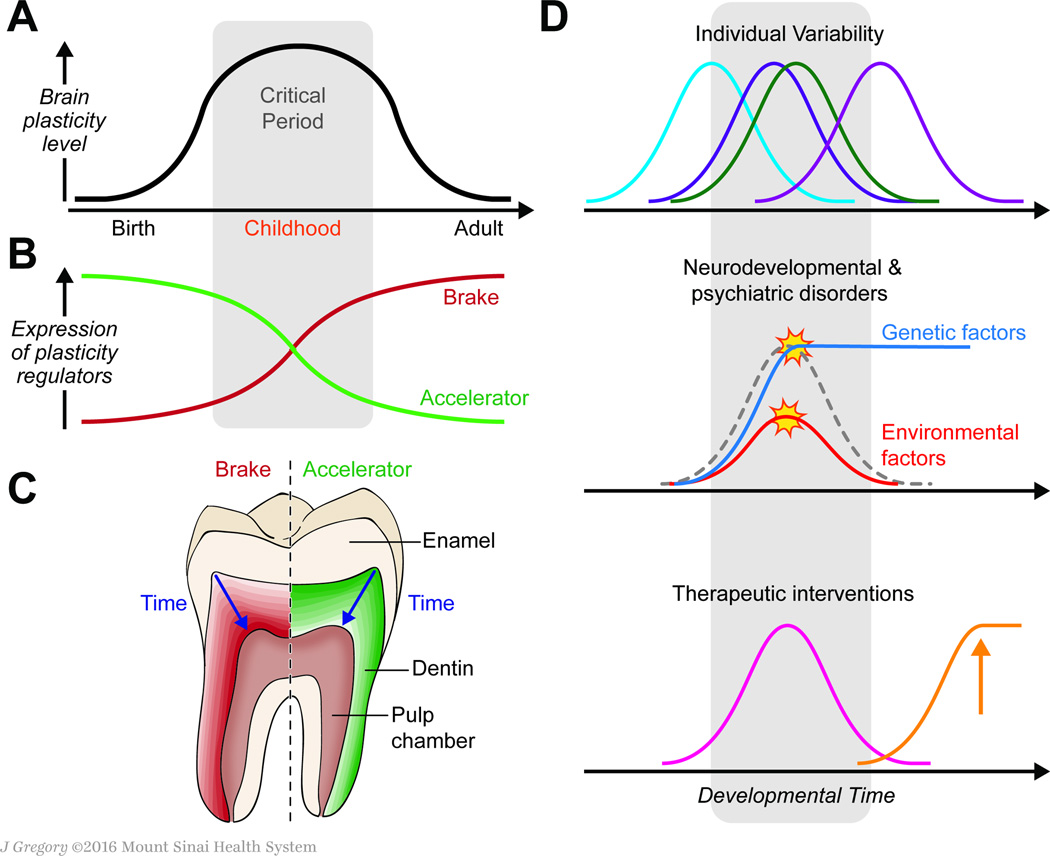
Figure 1. Reconstructing developmental critical period of brain plasticity with toothmatrix biomarker.
(A) The sensitivity of the brain to different environmental factors fluctuates across developmental critical periods. In addition to a prenatal critical period for placenta-mediated environmental influences (e.g. toxins that cross the placenta), there is a postnatal critical period for experience-dependent brain plasticity (gray background). (B) The expression of molecular accelerators and brakes that regulate brain plasticity shapes the critical period for brain plasticity. (C) We propose that the expression of known molecular accelerators and brakes that regulate brain plasticity can be mapped in teeth, revealing their changes in expression level across developmental stages. Expected developmental expression patterns of plasticity regulators in teeth will correlate with those in the brain. (D) Clinical applications of tooth matrix biomarkers. Measurement of molecular regulators of brain plasticity in teeth will allow us to determine (1) individual temporal variability of the critical period, (2) whether disruption of the critical period (e.g., via genetic or environmental factors that augment or restrict plasticity) is involved in the etiology of neurodevelopmental disorders, such as schizophrenia in which the brakes are reduced and (3) if personalized therapeutics based on individual molecular profiles could restore proper balance to brain plasticity regulators.
Molecular mechanisms of postnatal critical period for brain plasticity
One of the best-studied models of a critical period for brain plasticity is the enduring loss of visual responsiveness and anatomic remodeling in primary visual cortex of an eye deprived of vision early in life, resulting in amblyopia [2]. The use of the visual system offers a unique opportunity to examine neuroplasticity from fine-scale molecular and cellular processes to entire systems across species including rodents in which genetic manipulation can be used to dissect the molecular mechanisms. After extensive studies using rodent models, we now know that a natural critical period consists of a sequence of molecular events.. The critical period is triggered by a drastic adjustment of the excitatory-inhibitory (E/I) balance, which is facilitated by the emergence of molecular accelerators of plasticity which collectively contribute to the later maturation of specific inhibitory circuits. Next, anatomic reorganization occurs, which ultimately consolidates plasticity and is mediated by the emergence of several molecular brakes in the adult brain [1] (Figure 1B). Identification of these mechanisms of critical periods in the rodent visual system has led to the preclinical discovery of several pharmacological and behavioral interventions and provided a general conceptual framework for therapeutic intervention for recovery of function in adulthood [1]. It also helped uncover the molecular basis of critical periods in several other brain regions, including the auditory and fear systems. These findings in mice can function as useful molecular models to infer the developmental trajectory of brain maturation in humans.
Tooth-matrix biomarkers as windows into early life development
To successfully translate animal studies of brain plasticity to humans, we need accessible biomarkers that can 1) directly measure the expression of molecular plasticity regulators noninvasively and 2) provide a fine-scale profile of temporal changes in the relative abundance of plasticity regulators during the perinatal period. To accomplish both requirements, we introduce novel tooth-matrix biomarkers. Because teeth develop in an incremental manner that can be linked to circadian rhythms (Box 1), they exhibit an archival property whereby molecular information is stored in the dentine and enamel matrices, with limited remodeling. This allows detailed analyses to be undertaken many years after the tooth has developed. The tooth matrix in this context functions as a ‘biologic hard-drive’ that has stored early-life developmental information [3]. The absence of direct fetal biomarkers that provide information on both the magnitude and timing of fetal exposure to multiple chemicals has been a major barrier [4]. Multiplexed mass-spectrometry methods have been recently applied to the tooth-matrix to reconstruct prenatal and early postnatal exposure to thousands of compounds, including environmental chemicals and their internal reaction products [5]. For example, neurodevelopmental deficits from excessive exposure to manganese were uncovered in children exposed to pesticides high in this metal. Recently, we also showed that a broad range of homeostatic disruptions caused by physical trauma, infections and even psychosocial stressors, such as separation from the mother, are captured in the dentine matrix [5] [6].
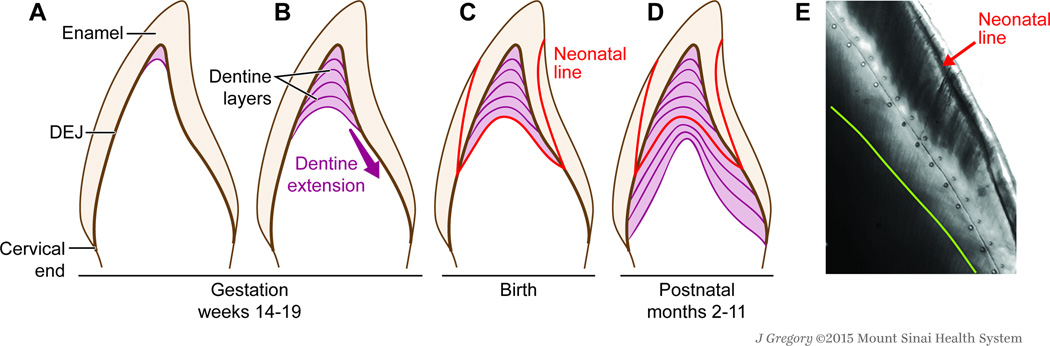
Key aspects of tooth development relevant to this biomarker.
A. Between the 14th and 19th week of intrauterine development, the tooth germ enters the advanced bell stage, which is characterised by the appearance of enamel and dentine at the future dentine-enamel junction (DEJ) on the cusp tip. B. Subsequently, enamel and dentine deposition occurs in a rhythmic manner forming incremental lines—akin to growth rings in a tree—in both enamel and dentine. C. At birth, an accentuated incremental line, the neonatal line, is formed due to disturbances in the secretory cells during protein matrix deposition67. D. A resultant change in crystal orientation and a lower degree of mineralization has been detected at the neonatal line in the enamel of human primary teeth. E. This line forms a clear histological landmark that demarcates pre- and postnatally formed parts of teeth. After birth, teeth continue to manifest daily growth lines, which reflect chronological ages at various positions within the tooth crown and roots. We have validated this biomarker for certain metals (manganese, lead, barium, strontium), and validation for a range of organic targets is underway. We have also shown how early-life homeostatic disruptions are captured in the dentine matrix.
Measuring brain plasticity regulators in human teeth
In light of recent studies of toxicants and markers of homeostatic disruptions [5] [6], we deduced that we could use deciduous teeth to determine perinatal critical periods for brain plasticity. Given that 1) teeth commence development in the prenatal period, extending to early childhood [5], 2) teeth share evolutionary homology with the neurosensory system [7], 3) markers specific to glial cells are expressed in dental tissue [8] and 4) glial cells function as stem cells for neurons in the cerebral cortex [9], it is possible that the timeline of key neurodevelopmental events is imprinted in an individual’s teeth. To uncover the molecular profile of brain plasticity across development, we propose a model in which the two categories of plasticity regulators, accelerators and brakesare studied simultaneously in a single tooth. Figure 1C illustrates an expected profile that accelerators of brain plasticity decline with age, while the expression of molecular brakes, which inhibit plasticity, increase concurrently. The relative balance of the accelerators and brakes determines the temporal profile of a critical period [1]. As proof-of-concept, we demonstrated that the 70-kD heat shock protein (HSP-70), a molecular chaperone that was recently identified as one of top quantitative trait loci associated with visual cortex plasticity [10], is expressed in the tooth matrix [6]. Plasticity brakes, such as Lynx1 [11] have been also detected in human odontoblast (dentine)-based transcriptome databases [12].
Future applications of human tooth-matrix biomarkers
We expect that the use of tooth matrix biomarkers will open up at least three exciting new areas of research and translational applications (Figure 1D). First, tooth biomarkers would be useful tools to assess individual variability of critical period timing across human populations. Gene expression in human postmortem brains of different ages showed that many genes, including those of plasticity regulators, have dissimilar expression levels during development and exhibit variability between samples [9], suggesting considerable inter-individual differences. Tooth biomarkers enable us to directly examine the developmental changes in gene and protein expression of known critical period regulators. Second, analysis of the teeth of patients with neurodevelopmental and psychiatric disorders will enable us to measure noninvasively the temporal profile of brain plasticity regulators. Given that the E/I balance or the expression of molecular brakes that controls the timing of critical periods is disrupted in autism and schizophrenia [1], patients’ teeth will allow us to estimate whether disruption of a critical period (e.g., augmented vs. restricted plasticity) underlies pathophysiology. Lastly, once a patient’s trajectory of brain plasticity has been characterized with his/her teeth, then a therapeutic regimen can be personalised based on the individual’s developmental molecular profiles. Preclinical studies identified two intervention strategies: (1) resetting the E/I balance and (2) removing the brakes, that are tightly linked with physiological mechanisms of critical period regulation to effectively restore brain plasticity [1]. Tooth-matrix biomarkers provide crucial information to achieve both these therapeutic goals.
In summary, the archival nature of enamel and dentine, which captures and preserves important aspects of developmental history, and the shared evolutionary origin of teeth and the neurosensory system together provide a framework to function as a biomarker to study aspects of human brain development and plasticity that were previously concealed.
Acknowledgments
This work was supported by the US National Institutes of Health grants (DP2ES025453, R00ES019597, R01EY024918, R01EY026053, R21MH106919) and a grant from the Mindich Institute of the Icahn School of Medicine at Mount Sinaii. We thank Jill Gregory, Academic Medical Illustration, for her assistance with preparation of the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: Authors declare no competing interests
References
- 1.Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 2.Wiesel TN. Postnatal development of the visual cortex and the influence of environment. Nature. 1982;299(5884):583–591. doi: 10.1038/299583a0. [DOI] [PubMed] [Google Scholar]
- 3.Austin C, et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature. 2013;498(7453):216–219. doi: 10.1038/nature12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 5.Andra SS, et al. The tooth exposome in children's health research. Curr Opin Pediatr. 2016;28(2):221–227. doi: 10.1097/MOP.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin C, et al. Uncovering system-specific stress signatures in primate teeth with multimodal imaging. Sci Rep. 2016;6:18802. doi: 10.1038/srep18802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farahani RM, et al. Blueprint of an ancestral neurosensory organ revealed in glial networks in human dental pulp. J Comp Neurol. 2011;519(16):3306–3326. doi: 10.1002/cne.22701. [DOI] [PubMed] [Google Scholar]
- 8.Kaukua N, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513(7519):551–554. doi: 10.1038/nature13536. [DOI] [PubMed] [Google Scholar]
- 9.Kang HJ, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478(7370):483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimel JA, et al. Genetic control of experience-dependent plasticity in the visual cortex. Genes Brain Behav. 2008;7(8):915–923. doi: 10.1111/j.1601-183X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 11.Morishita H, et al. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330(6008):1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paakkonen V, et al. Effects of TGF-beta 1 on interleukin profile of human dental pulp and odontoblasts. Cytokine. 2007;40(1):44–51. doi: 10.1016/j.cyto.2007.08.003. [DOI] [PubMed] [Google Scholar]