Robust experimental and observational data implicate inflammation as a fundamental driver in the pathogenesis of atherosclerotic cardiovascular disease (CVD).1 Chronic inflammatory diseases provide unique opportunities to gain insight into mechanisms of accelerated atherogenesis in broader populations. Patients with diseases such as psoriasis and rheumatoid arthritis that are characterized by chronically higher levels of systemic inflammation, also have substantially elevated risk of CVD, and this risk is lower when these patients are treated with anti-inflammatory medications. For example, studies of patients with psoriasis, a chronic inflammatory skin disorder, have suggested ~25% reduction in CVD risk with the use of disease modifying agents that suppress systemic inflammation.2 In these populations, traditional risk assessment tools often underestimate the true burden of cardiovascular risk, at least in part because of inadequately capturing the magnitude of inflammatory risk. Hence, there is a need for more reliable inflammatory risk assessment tools.
An important avenue of research that addresses this gap comes from recent technical advances in high performance metabolomics profiling, both nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) technologies that now allow the identification and quantification of systemic inflammation through protein glycosylation signatures. Regulated enzymatic glycosylation involves the post-translational modification of proteins by attaching oligosaccharide (“sugar”) moieties, and is an important step in regulating protein folding, localization, function, and stability. The significance of these glycosylated attachments is exemplified by their role in modulating numerous biological processes, including cell trafficking, signal transduction, regulation of metabolism, and host-pathogen recognition. As they reflect more “downstream” protein phenotypes, characterizing the human glycome has received increasing interest as a novel tool to identify markers and potential mediators of disease pathogenesis.3, 4
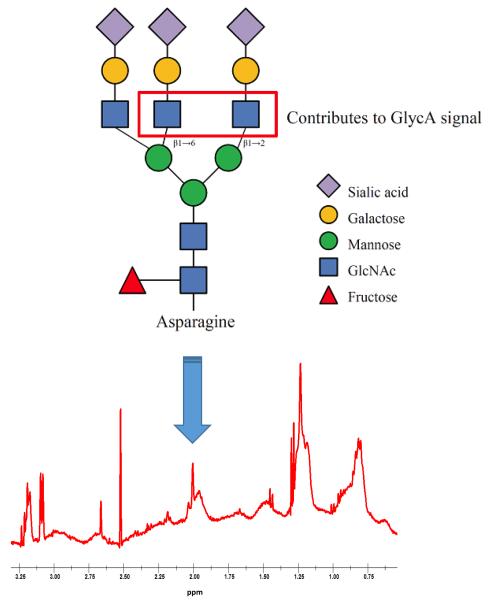
Most secreted proteins undergo N-linkage of glycan moieties to the amide side chains of specific asparagine residues, creating bi-, tri-, and tetra-antennary glycan branches (Figure). In proton (1H) NMR spectra of blood plasma or serum, measured as part of clinical lipoprotein testing5 or for NMR-based metabolomics research,6 a prominent signal appears at 2.00±0.01 ppm arising from the mobile N-acetyl methyl protons of a subset of glycan N-acetylglucosamine residues (specifically those in β1→2 and β1→6 linkages with preceding mannose sugars). This signal been given the name GlycA5 and is contributed to by many circulating glycoproteins, but for reasons of relative abundance reflects primarily the glycan on 5 major acute-phase inflammatory proteins and immune modulators: α1-acid glycoprotein, haptoglobin, α1-antitrypsin, α1-antichymotrypsin, and transferrin. The measured amplitude (size) of the GlycA signal therefore serves as a potential composite marker of systemic inflammation sensing the levels and glycosylation states of multiple inflammatory blood proteins. Indeed, we and others have observed that the GlycA signal is associated with longitudinal risk of CVD,7-9 diabetes,3, 10 and mortality11-13 (both cardiovascular and cancer mortality11), in multiple diverse cohorts.
Figure.
Proton nuclear magnetic resonance spectroscopy is used to measure the methyl protons of N-acetylglucosamine (GlcNAc) residues on the N-linked glycan chains of circulating glycoproteins, most prominently secreted inflammatory and immunologic proteins. (Modified from Otvos et al. Clinical Chemistry 20155 and Lawler et al. Circulation Research 201611, with permission).
Through its putatively summative nature, a potential advantage of GlycA in clinical practice is that it may be less prone to day-to-day fluctuations that could complicate clinical use of an individual biomarker.5 Indeed, the intra-individual coefficient of variation of 4.3% over short-term repeated assessments for this signal has been observed to be lower than that for other inflammatory biomarkers.5 Furthermore, this summative property could imply that GlycA is more likely to provide broader, more integrative profiling of multiple inflammatory pathways, better reflecting the extent of systemic inflammation. GlycA has been correlated with multiple cytokines from various inflammatory pathways, and a relationship with neutrophil activity (through inference form coexpression-based network analysis) has been proposed.14 Additionally, in several of the outcome studies mentioned above,7, 8, 10, 11 comparing patterns of risk associations for GlycA with those for other inflammatory biomarkers supports overlap in the risks captured. In these studies, incremental adjustment for these correlated inflammatory markers in many instances attenuates the magnitude of risk associations. In some contexts, the effect of inclusion of other inflammatory markers in risk models accounts for most or all of the observed risk, whereas in other contexts risk persists despite mutual adjustment.
With this mechanistic and observational background in mind, Joshi et al. sought to better understand the relationship between GlycA and CVD among two well-phenotyped case-control cohorts of patients with psoriasis.15 Strengths of their study include detailed profiling of vascular inflammation by 18-F fluorodeoxyglucose positron emission tomography/computed tomography (18-FDG PET/CT) and coronary artery disease burden by coronary CT angiography. Cross-sectional associations between GlycA and these measures demonstrated robust relationships in this population. Notably, these associations remained significant after adjusting for traditional markers of CVD risk, including another marker of systemic inflammation, hsCRP. The observations were strengthened by the finding of a dose-dependent relationship between GlycA level and severity of psoriasis. Intriguingly, anti-inflammatory agents used to treat psoriasis by inhibiting tumor necrosis factor (anti-TNF) led to reductions in GlycA and vascular inflammation; however, even after therapeutic reduction in systemic inflammation, the relationship between GlycA and vascular inflammation persisted.
The findings have several important implications. First, compelling evidence is provided that GlycA is a robust blood biomarker of inflammation-related CVD risk in this population, and may outperform other traditional risk markers. Second, as previous studies have suggested,5, 11 examination of correlations and patterns of risk associations after adjustment for other inflammatory biomarkers supports the hypothesis that GlycA captures summative risk related to multiple inflammatory pathways. Finally, and perhaps of greatest interest, the observation that GlycA is a marker of anti-TNF therapy suggests that this biomarker could be employed as a marker of treatment response and CVD risk among individuals receiving systemic anti-inflammatory therapies – potentially even beyond only those with psoriasis. By comparison, HMG-CoA reductase inhibitors (statins) reduce high-sensitivity C-reactive protein levels by ~20 to 30%, but do not result in clinically meaningful reductions in GlycA.8 Future prospective studies will be needed to further evaluate this, including in on-going, large-scale clinical trials that are investigating systemic anti-inflammatory therapies among broader, non-rheumatologic populations to reduce residual risk of CVD. The performance of GlycA in reflecting the effects of non-pharmacologic, lifestyle interventions to reduce inflammation and CVD risk also warrants further study.
In conclusion, the study by Joshi et al. provides significant insight into the markers and mechanisms of atherogenesis in a well-phenotyped high-risk population with psoriasis, with plausible implications for atherosclerosis prediction and treatment in broader populations. By studying patients with more extreme phenotypes – as has been undertaken for evaluating lipid-related risk pathways – we gain deeper insight into the mechanisms of atherosclerosis risk. More fundamentally, the findings support ongoing efforts, founded in biologic understanding, to develop innovative, metabolomics-based targeted biomarkers that push the frontier of precision cardiovascular diagnostics.
Acknowledgments
SOURCES OF FUNDING
P.R. Lawler receives support from NIH T32 (HL007575), NIH LRP, and Brigham and Women’s Hospital. S. Mora receives support from the National Institutes of Health (R01HL117861, HL117861-S1, HL117861-S2, R03CA211815-01), as well as from the Molino Family Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
P.R. Lawler: None; S. Mora: Research grant support from Atherotech Diagnostics, consultant to Amgen, Lilly, Pfizer, Quest Diagnostics, co-inventor on a patent for GlycA in predicting risk of colorectal cancer incidence and mortality.
REFERENCES
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Annals of the Rheumatic Diseases. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lauc G. Precision medicine that transcends genomics: Glycans as integrators of genes and environment. Biochimica et Biophysica Acta. 2016;1860:1571–1573. doi: 10.1016/j.bbagen.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Connelly MA, Gruppen EG, Otvos JD, Dullaart RP. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2016;459:177–186. doi: 10.1016/j.cca.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. GlycA: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clinical Chemistry. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 6.Ala-Korpela M. Serum nuclear magnetic resonance spectroscopy: One more step toward clinical utility. Clinical Chemistry. 2015;61:681–683. doi: 10.1373/clinchem.2015.238279. [DOI] [PubMed] [Google Scholar]
- 7.Akinkuolie AO, Buring JE, Ridker PM, Mora S. A novel protein glycan biomarker and future cardiovascular disease events. Journal of the American Heart Association. 2014;3:e001221. doi: 10.1161/JAHA.114.001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akinkuolie AO, Glynn RJ, Padmanabhan L, Ridker PM, Mora S. Circulating N-linked glycoprotein side-chain biomarker, rosuvastatin therapy, and incident cardiovascular disease: An analysis from the JUPITER trial. Journal of the American Heart Association. 2016;5:e003822. doi: 10.1161/JAHA.116.003822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurtz P, Havulinna AS, Soininen P, Tynkkynen T, Prieto-Merino D, Tillin T, Ghorbani A, Artati A, Wang Q, Tiainen M, Kangas AJ, Kettunen J, Kaikkonen J, Mikkila V, Jula A, Kahonen M, Lehtimaki T, Lawlor DA, Gaunt TR, Hughes AD, Sattar N, Illig T, Adamski J, Wang TJ, Perola M, Ripatti S, Vasan RS, Raitakari OT, Gerszten RE, Casas JP, Chaturvedi N, Ala-Korpela M, Salomaa V. Metabolite profiling and cardiovascular event risk: A prospective study of 3 population-based cohorts. Circulation. 2015;131:774–785. doi: 10.1161/CIRCULATIONAHA.114.013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinkuolie AO, Pradhan AD, Buring JE, Ridker PM, Mora S. Novel protein glycan side-chain biomarker and risk of incident type 2 diabetes mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35:1544–1550. doi: 10.1161/ATVBAHA.115.305635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawler PR, Akinkuolie AO, Chandler PD, Moorthy MV, Vandenburgh MJ, Schaumberg DA, Lee IM, Glynn RJ, Ridker PM, Buring JE, Mora S. Circulating N-linked glycoprotein acetyls and longitudinal mortality risk. Circulation Research. 2016;118:1106–1115. doi: 10.1161/CIRCRESAHA.115.308078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer K, Kettunen J, Wurtz P, Haller T, Havulinna AS, Kangas AJ, Soininen P, Esko T, Tammesoo ML, Magi R, Smit S, Palotie A, Ripatti S, Salomaa V, Ala-Korpela M, Perola M, Metspalu A. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: An observational study of 17,345 persons. PLoS Medicine. 2014;11:e1001606. doi: 10.1371/journal.pmed.1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duprez DA, Otvos J, Sanchez OA, Mackey RH, Tracy R, Jacobs DR., Jr. Comparison of the predictive value of GlycA and other biomarkers of inflammation for total death, incident cardiovascular events, noncardiovascular and noncancer inflammatory-related events, and total cancer events. Clinical Chemistry. 2016;62:1020–1031. doi: 10.1373/clinchem.2016.255828. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie SC, Wurtz P, Nath AP, Abraham G, Havulinna AS, Fearnley LG, Sarin AP, Kangas AJ, Soininen P, Aalto K, Seppala I, Raitoharju E, Salmi M, Maksimow M, Mannisto S, Kahonen M, Juonala M, Ripatti S, Lehtimaki T, Jalkanen S, Perola M, Raitakari O, Salomaa V, Ala-Korpela M, Kettunen J, Inouye M. The biomarker GlycA is associated with chronic inflammation and predicts long-term risk of severe infection. Cell Systems. 2015;1:293–301. doi: 10.1016/j.cels.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly M, Remaley AT, Bluemke DA, Playford MP, Gelfand JM, Mehta NN. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circulation Research. 2016 doi: 10.1161/CIRCRESAHA.116.309637. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]