Abstract
Objectives
This study examined the relationship between salivary cortisol levels and hot flashes during midlife. Previous studies have shown that cortisol levels increase with hot flashes in the laboratory, and higher cortisol levels have been associated with more severe hot flashes. Salivary cortisol levels were also examined in relation to total number of midlife symptoms.
Methods
Women aged 40-60 years (n=109) reported the presence or absence of 23 symptoms, including hot flashes, during the previous 2 weeks. Salivary samples were collected at waking, 30 minutes after waking, 1 hour before bedtime, and at bedtime. The cortisol awakening response (CAR), cortisol daily decline (CDD), log transformed salivary cortisol levels at each time point, and mean cortisol levels were compared by hot flash report using t-tests. Logistic regression analyses were performed to assess the association between each cortisol measure and the presence or absence of hot flashes, after controlling for potential covariates.
Results
Salivary cortisol levels were not significantly associated with hot flashes or sum of symptoms. Hot flash report did not differentiate women who had a positive CAR from those who did not, or women who showed strong CDD from those who did not.
Conclusion
Symptomatic women – defined by hot flash report or symptom total – were not found to have higher salivary cortisol levels.
Keywords: hot flashes, hot flushes, salivary cortisol, stress, menopause
Introduction
Hot flashes are common around the time of the menopausal transition, with over 75% of women reporting some hot flash experience (Gold et al. 2006). Hot flashes involve the subjective sensation of heat with a rise in peripheral body temperature and sweating, followed by a decrease in core body temperature (Freedman 2005). The mechanisms are still not completely understood, but hot flashes appear to be a heat dissipation response (Sievert 2007; Sturdee 2008) resulting from disturbances in temperature regulation in the hypothalamus. Hot flashes are associated with declining estrogen levels associated with the menopausal transition apparently because, as estrogen levels fall, there is a narrowing of the thermoneutral zone between core body temperatures that trigger sweating and shivering (Archer et al. 2011; Freedman and Blacker 2002; Freedman and Krell 1999). Elevated central noradrenergic activation, serotonin levels, and possibly kisspeptin, neurokinin B and dynorphin (KNDy) neurons are also implicated in the etiology of hot flashes (Archer et al. 2011; Freedman and Woodward 1992; Rance et al. 2013).
Cortisol is produced by the adrenal cortex in response to stress-induced activation of the HPA axis (Dickerson and Kemeny 2004). With regard to hot flashes, two early studies examined changes in cortisol levels before, during, and after hot flashes in the laboratory. In the first, 18 postmenopausal women with frequent severe hot flashes showed a significant increase in serum adrenocorticotropic hormone (ACTH) that peaked five minutes after the onset of a hot flash (measured as subjective discomfort, an increase in skin temperature, and a fall in skin resistance over the upper sternum). A significant increase in mean serum cortisol peaked 10 minutes after the peak in ACTH, or 15 minutes after the start of the hot flash. The authors hypothesized that the stimulation of ACTH, followed by an increase in cortisol, could be due to altered hypothalamic neurotransmitters, or may be secondary to the stress of the hot flash (Meldrum et al. 1984). The second study measured changes in finger skin conductance, finger blood flow, and finger temperature in 6 postmenopausal women with frequent and severe hot flashes. Plasma cortisol levels increased during the hot flashes from 134 +/- 41 to 162 +/- 52 ng/ml; however, the increase was not statistically significant given the small number of participants (Cignarelli et al. 1989).
In the Seattle Women’s Health Study, urinary cortisol levels (collected at waking) were examined in relation to symptom frequency and severity recorded in diaries before, during, and after urine collection. Mean cortisol levels were higher for women in the late menopausal transition compared to women in other menopausal stages, and women with increased urinary cortisol had significantly greater hot flash and cold sweat symptom severity compared to women without increased cortisol (Woods et al. 2006). A subsequent analysis grouped women into 4 groups based on the severity of 5 symptoms: hot flashes, mood changes, awakening at night, joint ache, and problem concentrating. The likelihood of being included in the “high hot flashes, joint ache, awakening at night” group was significantly greater in those with higher cortisol levels (Cray et al. 2010). In a study of women attending outpatient services for menopause in Modena, Italy, women with severe hot flashes had significantly higher levels of 24-hour urinary cortisol compared to women with none to moderate vasomotor symptoms. However, vasomotor symptom score was not a significant determinant of 24-hour urinary cortisol in regression analyses (Cagnacci et al. 2011).
A rapid increase in cortisol levels occurs just after awakening, with a peak 30-45 minutes after awakening. After the morning peak, cortisol levels drop rapidly for a few hours, then decline more slowly across the day to reach a low point around midnight (Adam et al. 2006; Clow et al. 2010; Fries et al. 2009). In a recent study of salivary cortisol and self-reported hot flashes, women were divided into groups based on the number of hot flashes per day (≤5.5, >5.5-8.8, and >8.8). Women with the least number of hot flashes had the highest level of cortisol 30 minutes after awakening, and women with the greatest number of hot flashes had the highest cortisol level in the early afternoon. Awakening response, diurnal variation, and other cortisol measures did not vary in relation to the frequency of hot flashes (Reed et al. 2016). A study of 44 women using self-reported hot flashes (electronic diary), hair cortisol, and salivary cortisol, found a flatter diurnal slope in salivary cortisol associated with greater hot flash severity (Gibson et al. 2016). In a study of salivary cortisol and hot flashes measured by an ambulatory monitor, objective, but not subjective, hot flashes were associated with the cortisol awakening response (CAR), and women with objective hot flashes showed significantly higher salivary cortisol levels at 15, 30, and 45 minutes post-waking compared to women without objective hot flashes. Women with objective hot flashes also tended to show higher daily cortisol levels compared to women without objective hot flashes (Rubin et al. 2014).
Hot flashes can be experienced as discrete, bothersome symptoms, or as a chronic stress that affects personal comfort and social experiences. In this study we hypothesized that women who report hot flashes during the past two weeks will exhibit elevated total mean cortisol levels, a higher CAR, and higher cortisol levels at specific time-points throughout the day. Salivary cortisol levels were also examined in relation to the sum of reported symptoms.
Methods
Sample
Data for this study were drawn from a cross-sectional investigation (1999-2003) of race/ethnicity, socioeconomic status, and diurnal blood pressure (BP) patterns. Participants (n=211) aged 18-65, white or black, were recruited from Weill Cornell Medical College, Mount Sinai School of Medicine, and Harlem Hospital, using a common protocol and consent form approved by the institutional review committees for research involving human participants at each institution. At recruitment, women had no major medical problems (e.g., diabetes, stroke, myocardial infarction) other than hypertension. Women taking antihypertensive medication were willing and able to discontinue treatment for two weeks prior to participation.
The analyses focused on women aged 40 to 60 (n=139) at the time of evaluation and/or interview to better capture hot flash experience around the time of the menopausal transition. Of the 139 participants, 30 were excluded from analyses because they did not provide at least one salivary cortisol measurement. These 30 did not differ from the 109 remaining participants in demographic or clinical characteristics, with the exception of body mass index (BMI). Women who gave salivary samples had a higher mean BMI compared with women who did not (30.1 kg/m2 vs. 27.7 kg/m2, p<0.05.)
Data collected
Participants completed a medical history questionnaire that included questions about menstruation and use of hormone therapy (HT). Menopausal status was defined as having had at least 12 months of amenorrhea (WHO 1981). Women who had menstruated within the past 12 months were grouped into a combined pre- and peri-menopausal category.
Menopausal symptoms were queried with a frequently used questionnaire that embeds menopausal symptoms into a list of everyday complaints (Avis et al. 1993; Obermeyer et al. 2007). Each participant was asked whether or not she had been bothered by each of 23 symptoms during the past 2 weeks, e.g., hot flashes, trouble sleeping, or feeling blue or depressed. Answers were recorded as yes/no. The presence of each symptom was added together for a sum of symptom report during the past two weeks that ranged from 0 to 20. In addition, women were asked, “In the past year, how often have you been awakened by sweating or hot flashes?” Answers were recorded as 0=never, 1=rarely, 1 time per month or less, 2=sometimes, 2-4 times per month, 3=often, 5-15 times per month, 4=almost always, 16-30 times per month.
Height and weight were measured twice by a technician. The average of the two measurements was used, and body mass index (BMI) was calculated as weight divided by the square of height (kg/m2).
Participants were provided with four labeled Salivettes and instructed to collect 4 salivary samples for cortisol measures during a 24-hour period. The first sample was collected 1 hour before bedtime, the second sample at bedtime, the third sample at awakening, and the fourth sample 30 minutes after awakening. Women were instructed to record the time of collection for each sample and to delay breakfast until after the second sample. Only participants with recorded wake-up times between 4 AM and 11AM and recorded bedtimes between 8 PM and 2AM were included in the study. Samples were frozen at -20° C until thawed and spun in the laboratory immediately prior to assay. Free cortisol was measured using a time-resolved immunoassay with fluorometric detection (Dressendorfer et al., 1992).
At least one salivary sample was collected by 109 women. Among the women who gave salivary samples, 3 provided only one sample, 11 gave two samples, 21 gave three, and 74 women contributed salivary samples for the measure of cortisol at all four points in time. Table 1 shows the characteristics of these 109 women.
Table 1.
Sample characteristics
| Women aged 40- 60 w/ at least 1 cortisol sample | No hot flashes during past 2 weeks | Hot flashes during past 2 weeks | P-value across hot flash comparison | |
|---|---|---|---|---|
| N=109 | N=58a | N=42a | ||
|
| ||||
| Age Mean (s.d.) | 49.2 (5.7) | 47.5 (6.0) | 51.8 (3.9) | p<0.001 |
|
| ||||
| Race/ethnicity | ||||
| White | 44% | 48% | 38% | |
| Black | 56% | 52% | 62% | p=0.311 |
|
| ||||
| BMI Mean (s.d.) | 30.1 (6.8) | 29.8 (6.4) | 29.8 (6.6) | p=0.987 |
|
| ||||
| Menopausal status % | ||||
| Pre or peri | 56% | 68% | 38.5% | |
| post | 44% | 32% | 61.5% | p=0.005 |
|
| ||||
| Symptom score (scale of 0-20) | ||||
| Mean (s.d.) | 6.2 (4.6) | 4.7 (3.5) | 8.3 (5.1) | p=0.001 |
|
| ||||
| Smoking % | ||||
| yes | 21% | 84% | 71% | |
| no | 79% | 16% | 29% | p=0.124 |
|
| ||||
| Depressed mood % | ||||
| yes | 36% | 28% | 46% | |
| no | 64% | 72% | 54% | p=0.055 |
|
| ||||
| Trouble sleeping % | ||||
| yes | 45% | 39% | 52% | |
| no | 55% | 61% | 48% | p=0.173 |
|
| ||||
| Hormone Therapy use % | ||||
| yes | 11% | 9% | 15% | |
| no | 89% | 91% | 85% | p=0.386 |
Nine women did not answer the symptom survey.
Data were excluded if assay values were > or < 2SD from the sample mean or if the assay value was 0. Of the 109 women who collected salivary samples upon awakening, 89 salivary samples were available for analyses after exclusions, and 30 minutes after awakening 88 salivary samples were available for analyses. One hour before bedtime, 101 salivary samples were available for analyses. At bedtime, 90 salivary samples were available for analyses. (See footnote to Table 2 for further details.)
Table 2.
Hot flashes during the past two weeks in relation to salivary cortisol measures
| Women aged 40-60 | No hot flashes in past two weeks, women aged 40- 60 | Hot flashes in past two weeks among women aged 40-60 | p-value across hot flash report | |
|---|---|---|---|---|
|
| ||||
| At awakening | N=89a | N=49 | N=35 | |
| Mean cortisol level nmol/L (sd) | 11.97 (7.10) | 12.55 (7.89) | 11.33 (6.35) | |
| Mean log transformed (sd) | 2.21 (1.02) | 2.27 (0.90) | 2.09 (1.23) | ns* |
|
| ||||
| 30 minutes after waking | N=88b | N=51 | N=30 | |
| Mean cortisol level nmol/L (sd) | 12.84 (7.63) | 13.38 (7.58) | 11.72 (8.27) | |
| Mean log transformed (sd) | 2.19 (1.18) | 2.30 (1.04) | 1.92 (1.46) | ns* |
|
| ||||
| 1 hour before bed | N=98c | N=53 | N=37 | |
| Mean cortisol level nmol/L (sd) | 3.57 (5.33) | 3.31 (4.92) | 3.05 (3.28) | |
| Mean log transformed (sd) | 0.42 (1.63) | 0.19 (1.85) | 0.56 (1.29) | ns* |
|
| ||||
| Bedtime | N=90d | N=50 | N=34 | |
| Mean cortisol level nmol/L (sd) | 3.15 (4.72) | 4.01 (6.05) | 2.09 (1.75) | |
| Mean log transformed (sd) | 0.40 (1.39) | 0.53 (1.53) | 0.26 (1.18) | ns* |
|
| ||||
| Mean cortisol level | N=67 | N=40 | N=24 | |
| Mean nmol/L (sd) | 7.90 (3.75) | 8.45 (4.16) | 7.14 (3.10) | |
| Mean log transformed (sd) | 1.26 (0.81) | 1.31 (0.93) | 1.18 (0.59) | ns* |
|
| ||||
| Cortisol Awakening Response (CAR) | N=78 | N=46 | N=28 | |
| Mean nmol/L (sd) | 0.05 (1.40) | -0.02 (1.16) | 0.23 (1.80) | ns** |
|
| ||||
| Cortisol Daily Decline from awakening (CDD_awake) | N=78 | N=45 | N=29 | |
| Mean nmol/L (sd) | 1.91 (1.60) | 1.81 (1.58) | 2.02 (1.66) | ns** |
|
| ||||
| Cortisol Daily Decline from 30 minutes after awakening | N=76 | N=44 | N=27 | |
| (CDD_30) Mean nmol/L (sd) | 1.96 (1.78) | 1.81 (1.84) | 2.11 (1.71) | ns** |
A total of 109 women provided saliva for cortisol samples.
Awakening sample: 15 missing, 1 excluded for value of 0, 4 excluded for value > mean + 2SD, thus 89 samples were available for analyses.
30 minutes after awakening: 16 missing, 1 excluded for value of 0, 4 excluded for value > mean + 2SD, thus 88 were available for analyses.
1 hour before bed: 8 missing, 1 excluded for value of 0, 2 excluded for value > mean + 2 SD, thus 98 were available for analyses.
Bedtime: 13 missing, 1 excluded for value of 0, 5 excluded for value > mean + 2 SD, thus 90 were available for analyses.
no significant differences in mean log transformed cortisol levels by t-test, or in median cortisol levels by nonparametric median test (not shown) in relation to hot flash report.
no significant difference in mean by t-test
Analyses
The four timed cortisol measures (awakening, 30 minutes after awakening, 60 minutes before bedtime, and bedtime) were assessed for normal distribution, and were not normally distributed. Untransformed cortisol levels were compared by nonparametric median and Mann-Whitney U distribution tests in relation to hot flash report during the past two weeks. The four timed cortisol levels were log transformed in order to compare means in relation to hot flash report. For those with all four measures of cortisol, mean log-transformed cortisol level was computed.
CAR is a rapid increase in cortisol levels just after awakening, with a peak 30-45 minutes after awakening. For participants with morning measures, the CAR was computed as the log transformed cortisol level at awakening subtracted from the log transformed level 30 minutes after awakening (Adam et al. 2006). After the morning peak, cortisol levels drop rapidly for a few hours, then decline more slowly across the day to reach a low point around midnight. For women with morning and bedtime measures, cortisol daily decline (CDD_30) was computed by subtracting the log transformed cortisol levels measured at bedtime from the levels 30-min after awakening (Karlson et al. 2010). Similarly, CDD_awake was computed by subtracting the log transformed cortisol levels at bedtime from the levels at awakening.
For exploratory purposes, we examined untransformed cortisol patterns in relation to the presence or absence of hot flashes. Untransformed CAR and CDD were approximately normally distributed. We categorized women into two groups: those for whom CAR rose (value at awakening subtracted from the value 30 minutes after awakening, CAR >1), and those for whom CAR levels remained relatively flat or fell (CAR ≤1). We also examined diurnal patterns (CDD_awake and CDD_30) to categorize women with and without flattened diurnal cycles (>5 and ≤5) to compare hot flash report. The cutoff of 5 represented the 25 percentile in CDD values.
Logistic regression analyses were performed to assess the association between each cortisol measure and presence or absence of hot flashes after controlling for the potential covariates of age, race/ethnicity, BMI, menopausal status, HT use, education, current smoking, trouble sleeping, and depressed mood. As a first step, bivariate analyses were performed for each covariate with hot flash status; covariates achieving a p-value of 0.20 or less were retained in the final multivariable model.
The sum of all symptoms was examined by Pearson correlation in relation to measures of cortisol.
Results
Sample characteristics are shown in Table 1. Among the 109 women aged 40-60 who gave at least one saliva sample for cortisol, 100 answered the symptom questionnaire and 58% reported having been bothered by hot flashes during the two weeks prior to interview (Table 1). With regard to being awakened by sweating or hot flashes during the past year, 24% reported awakening often or almost always (2 or more times/month). Forty-four percent of the sample were postmenopausal. Hot flash frequency varied by menopausal status and age (p<0.01), but did not vary significantly in relation to race/ethnicity, BMI, or use of hormone therapy (HT),
Mean salivary cortisol levels were higher at waking and 30 minutes after waking compared to 1 hour before bedtime and bedtime, as shown in Table 2 and Figure 1. As shown in Figure 1, there are only slight differences in mean cortisol levels across the day between symptomatic and asymptomatic women.
Figure 1.
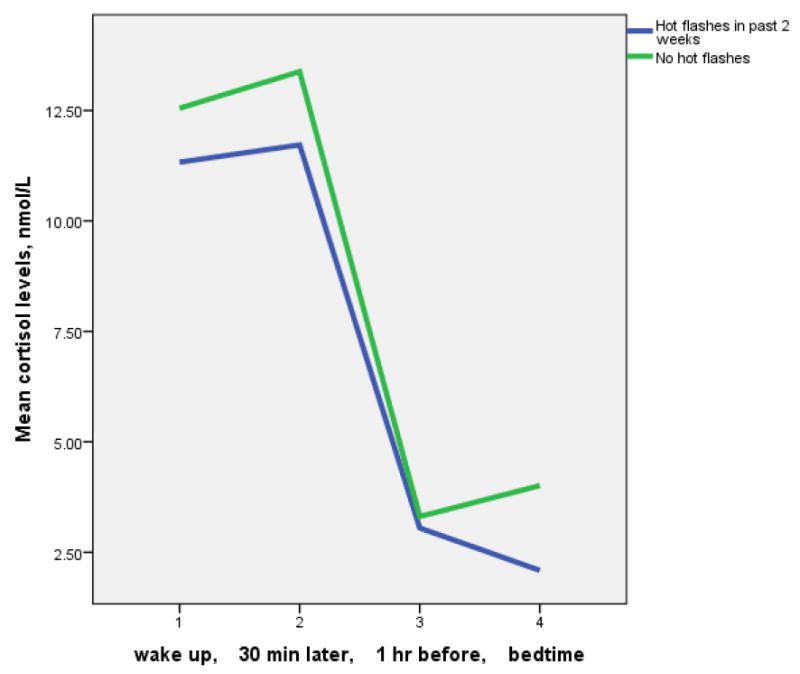
Salivary cortisol levels among women aged 40-60 by hot flash experience during the past 2 weeks.
Table 2 shows that there were no significant differences in mean levels of salivary cortisol between women with and without hot flashes. The same was true for nonparametric assessment of median levels of salivary cortisol in relation to hot flash report, and for analyses of cortisol levels in relation to awakening with hot flashes. The same was also true for analyses limited to pre-menopausal women, and to post-menopausal women (not shown.) In addition, there were no significant correlations between the sum of symptoms by Pearson correlation in relation to measures of cortisol (e.g., mean cortisol level, Figure 2).
Figure 2.
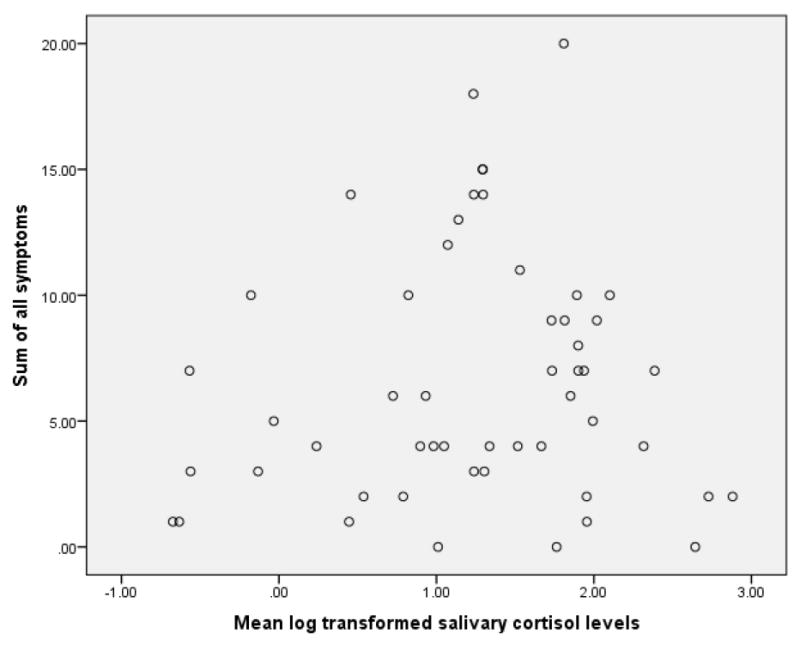
Sum of symptoms in relation to mean log transformed salivary cortisol levels (n=52, r=0.08, n.s.)
Women were categorized as those for whom untransformed CAR rose (n=35), and those for whom CAR levels remained relatively flat or fell (n=39). Hot flash frequency was not significantly different between the two groups. Hot flashes were reported by 34% of those with CAR>1 and 41% of those with CAR≤1 (n.s.). Women were also divided into those with and without flattened diurnal cycles to compare hot flash report. Hot flash report did not differ by the shape of the cortisol diurnal decline (Table 3).
Table 3.
Hot flash frequencies in relation to shape of Cortisol Awakening Response (CAR) and shape of Cortisol Daily Decline (CDD)
| N | Hot flash frequency | P value | |
|---|---|---|---|
| CAR ≤1a | 39 | 41% | ns |
| CAR >1b | 35 | 34% | |
| CDD_awake ≤5c | 17 | 41% | ns |
| CDD_awake >5d | 57 | 39% | |
| CDD_30 ≤5e | 16 | 38% | ns |
| CDD_30 >5f | 54 | 37% |
CAR flat or declines across 30 minutes after awakening
CAR increases across 30 minutes after awakening
CDD_awake fairly flat across the day, from moment of awakening
CDD_awake declines across the day, from moment of awakening
CDD_30 fairly flat across the day, from 30 minutes after awakening
CDD_30 declines across the day, from 30 minutes after awakening
Log transformed salivary cortisol values were not correlated with age (except for 1 hour before bedtime), or BMI (except for CAR). Cortisol levels one hour before bedtime increased with increasing age (r=.203, p<0.05). Cortisol values were not associated with black/white ethnicity, menopausal status, use of hormone therapy, level of education, or depressed symptoms during the past two weeks. Women who smoked at the time of interview had significantly lower CDD_awake and CDD_30 values (0.80 vs. 2.16 and 0.77 vs. 2.19 respectively, p<0.01). Log transformed cortisol levels one hour before bedtime were higher among women who had trouble sleeping during the past two weeks (0.72 nmol/L, s.d. 1.2 vs. 0.06 nmol/L, s.d. 1.9, p<0.05).
Age, menopause status, current smoking, depressed mood, and trouble sleeping were found to be significant in relation to hot flash status in bivariate analyses at the level of p<0.20 (Table 1) and were included in the final multivariable models. Hot flash status was not significantly associated with any measure of salivary cortisol except for CDD_30 (p=0.04) after controlling for covariates (not shown).
Postmenopausal status increased the likelihood of hot flashes in separate multivariable models using each cortisol measure (except for the model with the cortisol measure 1 hour before bedtime). Trouble sleeping was marginally (p<0.1) or significantly related to the likelihood of hot flashes in all models (except for the model with the cortisol measure 1 hour before bedtime).
Discussion
In this study we hypothesized that symptomatic women would exhibit higher levels of salivary cortisol at specific time-points throughout the day, elevated total mean cortisol levels, and a higher CAR. We also hypothesized that cortisol levels could predict hot flash presence or absence. Symptomatic women were defined as those who reported hot flashes during the past two weeks or, in separate analyses, those who reported a higher total number of symptoms. None of our hypotheses were supported by the data. There was no relationship between hot flashes during the past two weeks and any measure of salivary cortisol, except for CDD_30, after controlling for covariates. This significant result may be due to the number of multiple tests. In addition, there was no relationship between sum of symptoms and any measure of salivary cortisol.
In laboratory studies, cortisol levels increased at the moment of a hot flash (Cignarelli et al. 1989; Meldrum et al. 1984); however, we were not able to test concordant hot flashes and cortisol levels. Instead, our question was broader – do symptomatic women differ from asymptomatic women in cortisol levels and diurnal pattern? Higher levels of urinary cortisol have been associated with hot flash severity (Woods et al. 2006); however, Cagnacci et al. (2011) did not find a relationship between the Greene Climacteric Scale subscale for vasomotor symptoms and 24-hour urinary cortisol levels. They did find a significant relationship between the Greene Climacteric total symptom scale and urinary cortisol (Cagnacci et al. 2011). In the study presented here, salivary, not urinary cortisol, was measured and there was no relationship observed between salivary levels of cortisol and either hot flashes or the sum of symptoms.
Salivary cortisol collection has numerous advantages over serum and urinary collection because of the ease in collection (no blood stick, and no need to collect urine overnight or for 24 hours.) In addition, salivary cortisol offers an excellent assessment of free cortisol levels at the time of collection (Hellhammer et al. 2009).
Levels of cortisol can serve as a biomarker of stress, and stress has been identified as a determinant of hot flashes in some (Avis et al. 2015; Freeman et al. 2011; Gold et al. 2004; Kuh et al. 1997; Swartzman 1990; Thurston et al. 2008), but not all (Binfa et al. 2004; Sievert et al. 2007; Thurston et al. 2005) studies.
In a cross-sectional analysis of women aged 42-52 drawn from the multi-ethnic Study of Women’s Health Across the Nation (SWAN), perceived stress was a significant predictor of vasomotor symptoms after adjusting for ethnicity, lifestyle, and other confounding variables, self-reported (Gold et al. 2004). Another analysis of the SWAN data showed that perceived stress was significantly related to longer total duration of vasomotor symptoms and longer post final menstrual persistence of hot flashes into the postmenopausal period (Avis et al. 2015). Also from the SWAN, white and African-American women who reported a history of childhood abuse or neglect, measured retrospectively, had a greater likelihood of hot flashes and night sweats in age-adjusted models (Thurston et al. 2008). In a 13-year longitudinal study, women who experienced moderate or severe hot flashes during the study period had a higher baseline Perceived Stress Scale score compared to women with mild hot flashes or no hot flashes during the study period, p<0.01. Stress was not significantly associated with the duration of hot flushes in a multivariable model (Freeman et al. 2011). Finally, in many, but not all (Gallicchio et al. 2010), studies of BP and hot flashes, women with hot flashes exhibit higher BP levels (Brown et al. 2011; Erkal et al. 2014; Gast et al. 2008; Gerber et al. 2007; James et al. 2004; Reed et al. 2016). Assuming that an increase in BP may, in part, be related to stress, we hypothesized an increase in cortisol among symptomatic women. The data presented in this study did not support that hypothesis.
In the study presented here, symptom experience, including hot flashes, preceded the day of cortisol collection, therefore, retrospective symptom report could be examined in relation to current levels of cortisol to test the hypothesis that cortisol levels differ between symptomatic and asymptomatic women. It may be that episodic acute stress, perhaps associated with increased cortisol levels, or bothersome chronic stress, perhaps associated with a flattened diurnal rhythm (Adam and Kumari, 2009; Desantis et al. 2015; Kumari et al. 2010) are associated with hot flash presence or absence. It is also possible that a different source of stress results in both altered cortisol levels and hot flashes or other symptoms.
Objective hot flashes have been associated with higher CAR measured with salivary cortisol, but in that same study, subjective hot flash reports were not associated with CAR (Rubin et al. 2014). Our results, based on subjective report of hot flashes from questionnaires, were consistent with the latter findings. Although the CAR is not influenced by phase of the menstrual cycle (Kudielka and Kirschbaum, 2003), phase of the menstrual cycle does affect hot flash frequency because pre-menopausal women are more likely to experience vasomotor symptoms during the late luteal phase of their cycle (Hale et al. 2003). In the study presented here, it is not known whether pre-menopausal women were in follicular or luteal phases of their cycles.
Menopausal status and hormone therapy use can affect HPA axis responsivity (Hellhammer et al. 2009). In the study presented here, cortisol levels did not vary by pre/post-menopausal status or by use of HT. In the Seattle Women’s Health Study, mean urinary cortisol levels were higher for women in the late menopausal transition compared to women in other menopausal stages (Woods et al. 2006). We were not able to examine menopausal stages with the same level of detail. Woods et al. (2006) did not find a relationship between increased cortisol and age, BMI, or sleep. Likewise, we did not find a relationship between levels of cortisol and age, BMI, or sleep except in cortisol levels one hour before bedtime. It is a limitation that we did not directly ask about contraceptive use, although four women listed oral contraceptives as a type of medication.
In the study presented here, the sample size (n=109) was somewhat larger than the number of participants in some (but not all, Reed et al. 2016) studies that have examined hot flashes in relation to cortisol. Those samples ranged from 40 (Rubin et al. 2014) to 44 (Gibson et al. 2016), 85 (Cagnacii et al. 2011), 91 (Woods et al. 2006), and 103 (Cray et al. 2010). Although it was possible to ask whether symptomatic women had higher levels of cortisol or a different diurnal pattern of cortisol rise and decline, a limitation of this study is that hot flashes were queried as having occurred during the past two weeks while cortisol levels were sampled over a 24-hour period. In addition, hot flashes were measured by subjective report rather than objective monitor (Carpenter et al. 1999; Sievert 2013). Future work should examine cortisol levels on the same day in which women are asked to keep a diary and/or wear an ambulatory hot flash monitor (Rubin et al. 2014). Another limitation is that the timing of the true peak in CAR for each individual is not known, and this could affect the levels of cortisol measured (Nepomnaschy et al. 2012).
In conclusion, symptomatic women – defined by hot flash report and by total number of symptoms – were not found to have higher levels of cortisol or a different diurnal pattern of cortisol rise and decline.
Highlights.
Salivary cortisol levels were measured in 109 women aged 40-60 years, who reported the presence or absence of 23 symptoms, including hot flashes, during the previous 2 weeks.
Women with hot flashes and other symptoms did not exhibit higher levels of salivary cortisol at specific time points throughout the day.
Neither did they demonstrate a different diurnal pattern of cortisol rise and decline.
Acknowledgments
Funding
The study was supported by grants NIH P01HL47540, R24HL76857, and M01RR00047.
Footnotes
Contributors
LMG designed the study, directed implementation and data collection, assisted in the analysis of data and drafted the manuscript.
LLS participated in analyzing the data and in drafting the manuscript.
JES designed the study, directed implementation and data collection, and provided critical comments on the manuscript.
All authors saw and approved the final version of the paper.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The protocol and consent form were approved by the institutional review committees for research involving human participants at each institution.
Provenance and peer review
This article has undergone peer review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience – cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103(45):17058–63. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Archer DF, Sturdee DW, Baber R, de Villiers TJ, Pines A, Freedman RR, Gompel A, Hickey M, Hunter MS, Lobo RA, Lumsden MA, MacLennan AH, Maki P, Palacios S, Shah D, Villaseca P, Warren M. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14:515–528. doi: 10.3109/13697137.2011.608596. [DOI] [PubMed] [Google Scholar]
- Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, Thurston RC Study of Women’s Health Across the Nation. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis NE, Kaufert PA, Lock M, McKinlay SM, Vass K. The evolution of menopausal symptoms. Bailliere’s Clin Endocrin Metab. 1993;7:17–32. doi: 10.1016/s0950-351x(05)80268-x. [DOI] [PubMed] [Google Scholar]
- Binfa L, Castelo-Branco C, Blumel JE, Cancelo MJ, Bonilla H, Munoz I, Vergara V, Izaguirre H, Sarra S, Rios RV. Influence of psycho-social factors on climacteric symptoms. Maturitas. 2004;48(4):425–31. doi: 10.1016/j.maturitas.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Brown DE, Sievert LL, Morrison LA, Rahberg N, Reza A. Relationship between hot flashes and ambulatory blood pressure: The Hilo Women’s Health Study. Psychosomatic Medicine. 2011;73(2):166–72. doi: 10.1097/PSY.0b013e318205e35b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnacci A, Cannoletta M, Caretto S, Zanin R, Xholli A, Volpe A. Increased cortisol level: a posible link between climacteric symptoms and cardiovascular risk factors. Menopause. 2011;18(3):273–278. doi: 10.1097/gme.0b013e3181f31947. [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Andrykowski MA, Freedman RR. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6(3):209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- Cignarelli M, Cicinelli E, Corso M, et al. Biophysical and endocrinemetabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels. Gynecol Obstet Invest. 1989;27:34–37. doi: 10.1159/000293612. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. Cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35(1):97–103. doi: 10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cray L, Woods NF, Mitchell ES. Symptom clusters during the late menopausal transition stage: observations from the Seattle Midlife Women’s Health Study. Menopause. 2010;17(5):972–977. doi: 10.1097/gme.0b013e3181dd1f95. [DOI] [PubMed] [Google Scholar]
- Desantis AS, Kuzawa CW, Adam EK. Developmental origins of flatter cortisol rhythms: socioeconomic status and adult cortisol activity. 2015 doi: 10.1002/ajhb.22668. early view. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Erkal N, Caglar M, Sahillioglu B, Gulerman C, Guray Y, Korkmaz S. Is there any association between mild hypertension and hot flash experience among women? Clin Exp Obstet Gynecol. 2014;41(4):409–14. [PubMed] [Google Scholar]
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23(2):117–125. doi: 10.1055/s-2005-869479. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Blacker CMSO. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77(3):487–490. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. doi: 10.1016/s0002-9378(99)70437-0. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Woodward S. Elevated alpha-2 adrenergic responsiveness in menopausal hot flushes: pharmacologic and biochemical studies. In: Lomax P, Schonbaum E, editors. Thermoregulation: The Pathophysiological Basis of Clinical Disorders. Basal: Karger; 1992. pp. 6–9. [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095–104. doi: 10.1097/AOG.0b013e318214f0de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Miller SR, Zacur H, Flaws JA. Hot flashes and blood pressure in midlife women. Maturitas. 2010;65(1):69–74. doi: 10.1016/j.maturitas.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gast G-CM, Grobbee DE, Pop VJM, Keyzer JJ, Wijnands-van Gent CJM, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–1498. doi: 10.1161/HYPERTENSIONAHA.107.106526. [DOI] [PubMed] [Google Scholar]
- Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause. 2007;14(2):308–315. doi: 10.1097/01.gme.0000236938.74195.c6. [DOI] [PubMed] [Google Scholar]
- Gibson CJ, Thurston RC, Matthews KA. Cortisol dysregulation is associated with daily diary-reported hot flashes among midlife women. Clinical Endocrinology. 2016;85(4):645–651. doi: 10.1111/cen.13076. [DOI] [PubMed] [Google Scholar]
- Gold EB, Block G, Crawford S, Lachance L, FitzGerald G, Miracle H, Sherman S. Lifestyle and demographic factors in relation to vasomotor symptoms: baseline results from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2004;159(12):1189–99. doi: 10.1093/aje/kwh168. [DOI] [PubMed] [Google Scholar]
- Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women’s health across the nation. Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale GE, Hitchcock CL, Williams LA, Vigna YM, Prior JC. Cyclicity of breast tenderness and night-time vasomotor symptoms in mid-life women: information collected using the Daily Perimenopause Diary. Climacteric. 2003;6(2):128–139. [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34(2):163–71. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- James GD, Sievert LL, Flanagan E. Ambulatory blood pressure and heart rate in relation to hot flash experience among women of menopausal age. Annals of Human Biology. 2004;31(1):49–58. doi: 10.1080/03014460310001636561. [DOI] [PubMed] [Google Scholar]
- Karlson B, Eek F, Hansen AM, Garde AH, Orbaek P. Cortisol variability and self-reports in the measurement of work-related stress. Stress and Health. 2011;27(2):e11–e24. doi: 10.1002/smi.1330. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28(1):35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kuh DL, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol. 1997;104(8):923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- Kumari M, Badrick E, Chandola T, Adler NE, Epel E, Seeman T, Kirschbaum C, Marmot MG. Measures of social position and cortisol secretion in an aging population: findings from the Whitehall II study. Psychosomatic Medicine. 2010;72:27–34. doi: 10.1097/PSY.0b013e3181c85712. [DOI] [PubMed] [Google Scholar]
- Meldrum DR, Defazio JD, Erlik Y, et al. Pituitary hormones during the menopausal hot flash. Obstet Gynecol. 1984;64:752–756. [PubMed] [Google Scholar]
- Nepomnaschy PA, Lee TCK, Zeng L, Dean CB. Who is stressed? Comparing cortisol levels between individuals. Am J Human Biol. 2012;24:515–525. doi: 10.1002/ajhb.22259. [DOI] [PubMed] [Google Scholar]
- Obermeyer CM, Reher D, Saliba M. Symptoms, menopausal status, and country differences: a comparative analysis from the DAMeS project. Menopause. 2007;14:788–797. doi: 10.1097/gme.0b013e318046eb4a. [DOI] [PubMed] [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynophin) neurons: A novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):1–42. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, Newton KM, Larson JC, Booth-LaForce C, Woods NF, Landis CA, Tolentino E, Carpenter JS, Freeman EW, Joffe H, Anawalt BD, Guthrie KA. Daily salivary cortisol patterns in midlife women with hot flashes. Clin Endocrinol. 2016;84(5):672–679. doi: 10.1111/cen.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Drogos LL, Kapella MC, Geller SE, Maki P. Cortisol awakening response differs for midlife women with objective vasomotor symptoms versus without vasomotor symptoms. Menopause. 2014;21(12):1362. [abstract] [Google Scholar]
- Sievert LL. Variation in sweating patterns: implications for studies of hot flashes through skin conductance. Menopause. 2007;14(4):742–751. doi: 10.1097/gme.0b013e3180577841. [DOI] [PubMed] [Google Scholar]
- Sievert LL. Subjective and objective measures of hot flashes. Am J Human Bio. 2013;25(5):573–580. doi: 10.1002/ajhb.22415. [DOI] [PubMed] [Google Scholar]
- Sievert LL, Obermeyer CM, Saliba M. Symptom groupings at midlife: cross-cultural variation and association with job, home, and life change. Menopause. 2007;14(4):798–807. doi: 10.1097/gme.0b013e31804f8175. [DOI] [PubMed] [Google Scholar]
- Sturdee DW. The menopausal hot flush – anything new? Maturitas. 2008;60(1):42–9. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Swartzman LC. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychology. 1990;9(5):529–545. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Blumenthal JA, Babyak MA, Sherwood A. Emotional antecedents of hot flashes during daily life. Psychosom Med. 2005;67(1):137–146. doi: 10.1097/01.psy.0000149255.04806.07. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Bromberger J, Chang Y, Goldbacher E, Brown C, Cyranowski JM, Matthews KA. Childhood abuse or neglect is associated with increased vasomotor symptom reporting among midlife women. Menopause. 2008;15:16–22. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO Technical Report Series No 670. Geneva: WHO; 1981. Research on the Menopause. [PubMed] [Google Scholar]
- Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopause transition. Menopause. 2006;13(2):212–221. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES, Smith-DiJulio K. Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2009;16(4):708–718. doi: 10.1097/gme.0b013e318198d6b2. [DOI] [PMC free article] [PubMed] [Google Scholar]


