Summary of Viewpoint
Iron chelation rather than supplementation would be beneficial to patients with heart diseases. More effort should be devoted to the development of iron chelators that could target mitochondrial iron.
Keywords: iron, mitochondria, ischemia/reperfusion, heart failure
Iron is an essential trace mineral for normal mammalian physiology. It is incorporated into iron/sulfur (Fe/S) clusters that serve as important cofactors for many enzymes, including the mitochondrial respiratory chain, and it gives blood and muscle their signature red color through its presence in heme.1 Because of its involvement in many important cellular processes, dysregulation of iron often causes disease. There are two broad categories of diseases associated with iron dysregulation– iron overload and iron deficiency. Each category can be further subdivided based on whether the changes in iron occur at the cellular or the systemic level.
Iron Deficiency
Iron is critical for developmental processes, and studies have linked severe systemic iron deficiency to impaired brain development in children.2 However, most of these studies were conducted in developing countries where iron deficiency tends to be more severe. In developed countries, the major disorder associated with iron deficiency is anemia. While systemic iron deficiency and anemia may have been used interchangeably, these two terms refer to different conditions. Systemic iron deficiency refers to low amounts of iron in circulation due to limited iron absorption or excess iron loss. Anemia is defined as low hemoglobin content within red blood cells, and can be caused by low systemic iron, mutations in hemoglobin genes, vitamin B12 deficiency, or underlying chronic diseases. Limited oxygen carrying capacity secondary to anemia can cause fatigue; however, it generally does not cause mortality or major damage to vital organs. Except for high output heart failure (HF) associated with very severe anemia, iron deficiency and anemia are not associated with any other major cardiac disorders.
Cellular iron deficiency is a rare phenomenon, and except for low iron in red blood cells in anemia and impaired neurological development associated with severe iron deficiency, there are no disorders resulting from low cellular iron. This is mainly due to tight cellular iron regulation to ensure adequate supply of this element and to redistribute it to proteins essential for cellular functions. Cells respond to iron restriction first by upregulating iron import proteins and downregulating iron export and storage machineries through the activation of the iron response protein system.1 In case of severe iron deficiency, under which increased iron uptake cannot meet intracellular demand, an iron conservation pathway gets activated to redistribute the limited iron supply remaining within the cell.1
Iron overload
Unlike iron deficiency, both cellular and systemic iron overload are associated with several disorders. Systemic iron levels are determined by iron release from enterocytes and macrophages, which in turn is regulated by hepcidin, a small peptide-hormone synthesized by the liver and secreted into circulation. Mutations in genes regulating hepcidin production or mediating its effects on target cells cause a family of systemic iron overload diseases called hemochromatosis.3 Additionally, many diseases are characterized by iron overload at the cellular but not at the systemic level. For example, Friedreich’s ataxia (FRDA) is a disease associated with mitochondrial iron overload in the heart and the brain. FRDA patients often develop dilated cardiomyopathy and arrhythmia secondary to cardiac iron overload.4 Mutations of other genes involved in Fe/S cluster biosynthesis and maturation also results in mitochondrial iron overload diseases, such as myopathy with ISCU deficiency and X-linked sideroblastic anemia with ataxia.4
Iron accumulation can be detrimental to cells through its catalysis of reactive oxygen species (ROS). Through Fenton chemistry, ferrous ion (Fe2+) reacts with hydrogen peroxide to generate ferric ion (Fe3+) and hydroxyl free radicals (the most toxic form of ROS) that in turn damage DNA, protein and lipids. Additionally, perferryl ion, the unstable intermediate in the Fenton reaction, is also capable of initiating lipid peroxidation. The oxidized ferric ion can ultimately be reduced back to ferrous ion by superoxide anions, allowing the process to propagate cyclically. As iron-catalyzed ROS-production can contribute to the development of HF, cardiac iron accumulation and comorbidities associated with FRDA and hemochromatosis are clinically managed by iron chelation therapy.
In addition, several chronic diseases (such as cardiac, renal and neurological diseases and cancer) are also associated with reduced iron in circulation and/or tissue iron overload. Non-microcytic anemia can occur in many chronic diseases not because of decreased iron intake, but due to underlying inflammation and increased hepcidin production. This subtype of anemia is called anemia of chronic disease (ACD). The underlying mechanism of ACD is believed to be through increased production of hepcidin that destabilizes ferroportin (the major cellular iron exporter) on the plasma membrane and reduced iron efflux from macrophages, enterocytes, and other cells.3 Meanwhile, tissue iron accumulation can occur due to decreased ferroportin protein on the cell surface.5 Increased tissue iron is also associated with aging and a number of neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease, as evidenced by the presence of focal iron deposits in the brain.6
Role of Mitochondrial Iron Overload in Cardiovascular Diseases (CVD)
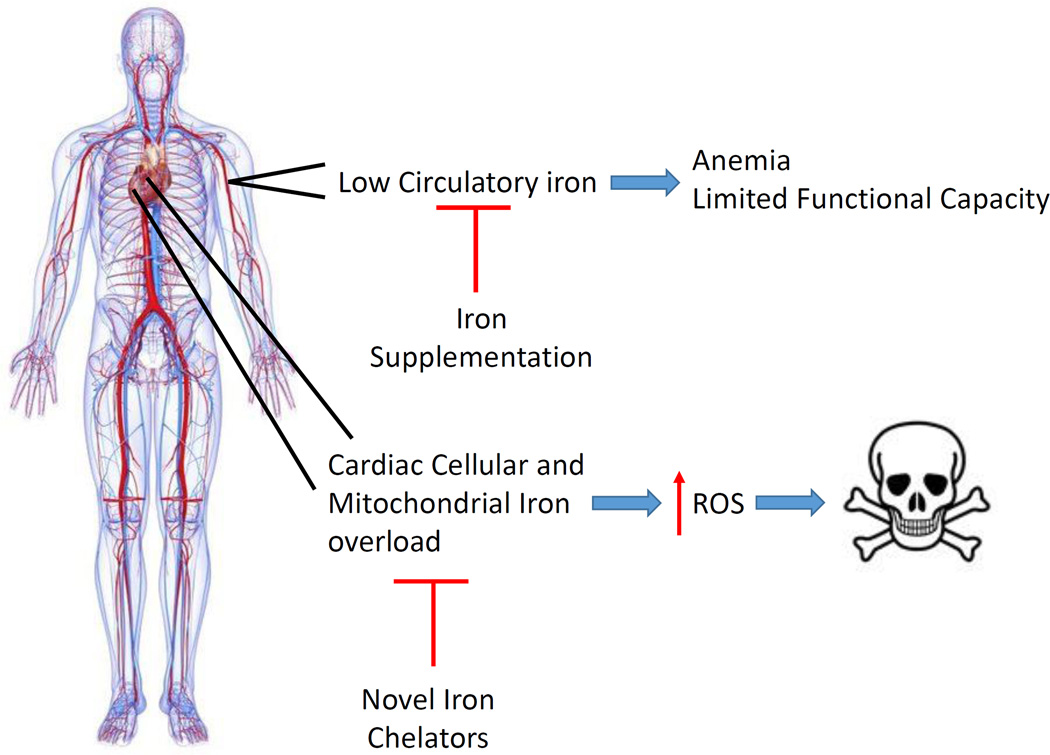
Among all forms of intracellular iron accumulation, mitochondrial iron accumulation is associated with the most detrimental effects. This is because mitochondria are the major site of ROS production and excess iron contributes significantly to free-radical production. In addition to the genetic disorders mentioned above, mitochondrial iron overload is also linked to doxorubicin-induced cardiomyopathy, and is associated with some mouse genetic models of spontaneous cardiomyopathy.7 We recently demonstrated that mitochondrial iron increases during cardiac ischemia/reperfusion (I/R) injury, and that modulating baseline mitochondrial iron (i.e., without underlying iron overload) is sufficient to prevent I/R injury.8 Furthermore, both human and animal studies have provided evidence for cardiomyocyte mitochondrial iron accumulation in HF. Increased mitochondrial iron has been observed in mice with myocardial infarction- or hypertrophy-induced HF,7 and in dog models of HF. In humans, end-stage HF is also associated with increased cardiac mitochondrial heme iron.7 Taken together, these studies suggest that: 1) mitochondrial iron accumulation in cardiomyocytes can contribute to the pathogenesis of HF, 2) HF may be associated with increased mitochondrial iron, and 3) mitochondrial iron chelation may be an effective treatment against the development of HF (even in the absence of iron overload in the heart) (Figure).
Figure. Derangements of Iron Homeostasis in HF.
HF is associated with anemia (low systemic circulatory iron levels) and cardiac tissue iron overload. Low circulatory iron can cause limited functional capacities, so many trials focus on iron supplementation to correct the systemic iron changes. However, HF patients can also have concurrent myocardial iron overload. Increasing cellular and mitochondrial iron contributes to cardiac tissue damage, possibly through a ROS-dependent mechanism. While iron supplementaiton only improves HF symptoms, iron chelation can target tissue iron overload and get to the “heart” of the disease.
Iron in CVD– The Controversy
Patients with HF may suffer from anemia, which could be due to systemic iron deficiency, ACD, concurrent kidney disease, or inhibition of the renin-angiotensin system by pharmacological therapy. As anemic patients often experience fatigue, one school of thought was to replenish iron in order to improve symptoms. This was the rationale behind the Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure (FAIR-HF) and the Ferric CarboxymaltOse evaluatioN on perFormance in patients with IRon deficiency in coMbination with chronic Heart Failure (CONFIRM-HF) trials. These studies demonstrated that iron supplementation in HF patients with iron deficiency results in improved functional capacity, as assessed by six-minute-walk-test, New York Heart Association (NYHA) HF classification, and subjective assessments from patients (Figure).9, 10
While patients with HF may be anemic, they may simultaneously have more iron in their heart tissue, as described in previous human and mouse studies.7 Even if iron levels are not increased in the heart of patients with HF, a reduction in baseline iron may reduce cellular oxidative stress and damage.8 Thus, a number of human clinical studies have investigated the use of iron chelators as treatment for CVD. Infusion of an iron chelator during and after coronary artery bypass surgery is associated with improved cardiac function, and iron chelation has been shown to improve cardiac outcomes in other iron overload diseases such as thalassemia and hemochromatosis.11 Additionally, the Trial to Assess Chelation Therapy (TACT) trial reported a reduction in the risk of adverse cardiovascular outcomes in patients receiving IV infusion of EDTA (which is capable of decreasing circulatory iron) at least six weeks after their previous myocardial infarction.12 In summary, there is conflicting data suggesting that both iron reduction and iron supplementation may be beneficial in CVD.
Concerns with Current Strategies for Targeting Iron
While previous clinical trials demonstrated positive results associated with iron supplementation, the trials rely on subjective measurements such as six-minute-walk-distance, NYHA HF classification, and hospitalization due to “worsening heart failure”, rather than cardiac function. Higher circulatory iron levels due to iron supplementation may improve functional capacity and reduced hospitalizations. However, iron supplementation in any chronic disease with iron deficiency will likely lead to symptom improvement. Nevertheless, a subset of these patients may still have underlying myocardial iron overload, as suggested by previous reports,7, 8 and their cardiac function may continue to deteriorate despite temporary improvement in functional capacities. Therefore, unless trials include patient outcomes and analysis of cardiac function, the use of subjective surrogate endpoints will not determine whether iron modulation therapies are actually beneficial in patients with HF.
Another issue to be considered is the safety of iron supplementation. Dietary iron supplementation reduces life span in lower organisms and is associated with accelerated aging in rats, potentially through a ROS-mediated mechanism. Observational studies have also linked increased iron intake and stores with metabolic and cardiovascular diseases in human.13 Additionally, even baseline myocardial iron levels play an important role in determining the extent of tissue injury when the heart is under stress.8 Without first confirming the safety of iron supplementation in a study of longer duration, its wide use should not be advocated in HF patients.
Iron Chelation versus Antioxidant Therapy
Although both iron chelation therapy and antioxidant therapy target ROS during the injury process, it is important to point out the difference between these two therapies. While studied extensively, various antioxidants have yet to demonstrate therapeutic benefits in CVD. In fact, vitamin C, a commonly studied antioxidant, can promote ROS production in the presence of metal ions. Additionally, antioxidant therapy may decrease ROS both during injury and at baseline, the latter of which is not desirable because basal production of ROS is important for intracellular signaling, cell proliferation and response to injury. Therefore, therapies utilizing strong antioxidants may actually have unintended detrimental effects. In contrast, baseline iron chelation only prevents the conversion of less toxic ROS to more toxic ROS during injury and therefore is unlikely to influence the basal level of ROS production.7 As a result, iron chelation may capture the benefits of antioxidant therapy while avoiding the untoward side effects.
Reducing Mitochondrial Iron- How?
While the reduction of mitochondrial iron may be beneficial in patients with ischemic heart disease and HF, the available iron chelator therapies may be inadequate. The most widely used FDA-approved iron chelator, deferoxamine (DFO), has a strong affinity for iron but poor cellular penetrance. It is used in systemic iron overload diseases such as hemochromatosis because it is very effective in removing iron from circulation, but studies using DFO failed to demonstrate cardio-protection against I/R injury of the heart. This lack of protective effect of DFO against cardiac I/R was attributed to its inability to penetrate the cell and to target mitochondrial iron.8 There are a few iron chelators that have better mitochondrial penetrance and have demonstrated protective effects in preclinical studies.8 Further optimization of these chelators could generate novel clinical therapies for the management of cardiac diseases. Additionally, since Fe2+ is directly involved in hydroxyl radical production, designing and optimizing novel iron chelators with strong affinities for Fe2+ could be a rational drug design approach. However, because iron cycles back and forth between the Fe2+ and Fe3+ states in Fenton chemistry, depleting either Fe2+ or Fe3+ would force the reaction to a halt and can be effective in CVD.
Conclusion and Future Considerations
Evidence suggests that even baseline intracellular iron levels play a significant role in the cardiac injury process, thus supporting the broader use of iron chelators that target intracellular compartments in CVD. Iron chelation may not be appropriate for every patient with HF, as iron chelation in patients with severe anemia may have detrimental effects. However, novel iron chelators that preferentially partition into the intracellular compartments should maximize the impact on mitochondrial iron while sparing circulating iron levels. With a smaller impact on circulating iron levels, these novel chelators should be able to preserve patients’ functional capacities.
Acknowledgments
Sources of Funding
The authors are support in part by the National Institutes of Health R01 HL127646.
Footnotes
Disclosure
H.-A. a consultant to Gerson-Lehrman Group. The other authors declares no conflict of interests.
REFERENCES
- 1.Bayeva M, Chang HC, Wu R, Ardehali H. When less is more: novel mechanisms of iron conservation. Trends in endocrinology and metabolism: TEM. 2013;24:569–577. doi: 10.1016/j.tem.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgieff Michael K. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochemical Society Transactions. 2008;36:1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Disease models & mechanisms. 2012;5:155–164. doi: 10.1242/dmm.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakhal-Littleton S, Wolna M, Carr CA, Miller JJJ, Christian HC, Ball V, Santos A, Diaz R, Biggs D, Stillion R, Holdship P, Larner F, Tyler DJ, Clarke K, Davies B, Robbins PA. Cardiac ferroportin regulates cellular iron homeostasis and is important for cardiac function. Proceedings of the National Academy of Sciences. 2015;112:3164–3169. doi: 10.1073/pnas.1422373112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stankiewicz J, Panter SS, Neema M, Arora A, Batt CE, Bakshi R. Iron in chronic brain disorders: Imaging and neurotherapeutic implications. Neurotherapeutics. 2007;4:371–386. doi: 10.1016/j.nurt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawicki KT, Shang M, Wu R, Chang HC, Khechaduri A, Sato T, Kamide C, Liu T, Naga Prasad SV, Ardehali H. Increased Heme Levels in the Heart Lead to Exacerbated Ischemic Injury. Journal of the American Heart Association. 2015;4 doi: 10.1161/JAHA.115.002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H-C, Wu R, Shang M, Sato T, Chen C, Shapiro JS, Liu T, Thakur A, Sawicki KT, Prasad SVN, Ardehali H. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Molecular Medicine. 2016;8:247–267. doi: 10.15252/emmm.201505748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan B-A, Mori C, von Eisenhart Rothe B, Pocock SJ, Poole-Wilson PA, Ponikowski P. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. New England Journal of Medicine. 2009;361:2436–2448. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 10.Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. European Heart Journal. 2014 doi: 10.1093/eurheartj/ehu385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennell D, Carpenter J, Roughton M, Cabantchik Z. On improvement in ejection fraction with iron chelation in thalassemia major and the risk of future heart failure. Journal of Cardiovascular Magnetic Resonance. 2011;13:1–9. doi: 10.1186/1532-429X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamas GA, Goertz C, Boineau R, et al. Effect of disodium edta chelation regimen on cardiovascular events in patients with previous myocardial infarction: The tact randomized trial. JAMA. 2013;309:1241–1250. doi: 10.1001/jama.2013.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basuli D, Stevens RG, Torti FM, Torti SV. Epidemiological associations between iron and cardiovascular disease and diabetes. Frontiers in Pharmacology. 2014;5 doi: 10.3389/fphar.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]