Abstract
All living cells require membrane proteins that act as conduits for the regulated transport of ions, solutes and other small molecules across the cell membrane. Ion channels provide a pore that permits often rapid, highly selective, and tightly regulated movement of ions down their electrochemical gradient. In contrast, active transporters can move moieties up their electrochemical gradient. The secondary active transporters (such as SLC superfamily solute transporters) achieve this by coupling uphill movement of the substrate to downhill movement of another ion, such as sodium. The primary active transporters (including H+/K+-ATPases and Na+/K+-ATPases) utilize ATP hydrolysis as an energy source to power uphill transport. It is well known that proteins in each of these classes work in concert with members of the other classes to ensure, for example, ion homeostasis, ion secretion, and restoration of ion balance following action potentials. More recently, evidence is emerging of direct physical interaction between true ion channels, and some primary or secondary active transporters. Here, we review the first known members of this new class of macromolecular complexes that we term “chansporters”, explore their biological roles, and discuss the pathophysiological consequences of their disruption. We compare functional and/or physical interactions between the ubiquitous KCNQ1 potassium channel and various active transporters, and examine other newly discovered chansporter complexes that suggest we may be seeing the tip of the iceberg in a newly emerging signaling modality.
Keywords: active transport, ATPase, KCNQ1, NIS, SMIT1, voltage-gated potassium channel
INTRODUCTION
Ion channels constitute a numerous and eclectic class of membrane proteins that are essential for the function of most if not all cell types from the archaebacteria through to the plethora of specialized cells in higher mammals. Ion channels facilitate passive yet often rapid (e.g., tens of thousands of ions per second per channel pore) movement of aqueous ions across the otherwise forbidding barrier of the hydrophobic plasma membrane that evolved at least partly to prevent such leakage. Thus, ion channels rely on an electrochemical gradient for their activity. This property, together with their often remarkable ion selectivity (K+ channels can readily permit K+ movement but are proficient at limiting Na+ leak, which bears similar charge to K+ but is slightly smaller), has endowed cells with the ability to communicate on the millisecond timescale using action potentials. Conversely, some ion channels exist in non-excitable cells where their role is to ensure ion and fluid homeostasis or supply the necessary ingredients or conditions for biological functions such as hormone synthesis or mucous secretion. Another class of channels that primarily provides a conduit for water across the plasma membrane are the water channels, or aquaporins (Morelle et al. 2015).
Other types of membrane proteins can also mediate movement of ions or other moieties across cell membranes but do not fall into the category of ion channels, and are referred to as “transporters”. The transporters that are perhaps furthest removed conceptually from the channels are the primary active transporters, some of which are also referred to as “pumps”. Rather than relying on passive diffusion, the pumps can move molecules such as ions up an electrochemical gradient because they can utilize energy generated, for example, from hydrolysis of ATP (Pedersen & Carafoli 1987a; Pedersen & Carafoli 1987b).
Lying somewhere in the middle of the channels and the pumps are members of the Solute Carrier (SLC) superfamily that comprises facilitative transporters, which as the name suggests facilitate movement of solutes across the cell membrane down an electrochemical gradient (“downhill”), and the secondary active transporters, which can move their cargo uphill by coupling this transport to downhill movement of another solute.
Ion channels, solute carriers and pumps move ions and other solutes in and out of cells, covering a wide range of specificities, transport rates, and biological processes. Many of these functions are crucial production and secretion processes, others are events essential to intercellular communication. One would therefore expect a high degree of crosstalk between the different proteins in these classes. Recently, we and others have discovered a higher degree of crosstalk than expected, with some involving physical interaction between ion channels and transporters, with the formation of what we term “chansporter” complexes. Here, we review the current state of knowledge surrounding this newly-discovered biophysical phenomenon and distill what is known about the regulatory crosstalk between these two very different types of plasma membrane proteins. The focus is largely on a voltage-gated potassium (Kv) channel pore-forming (α) subunit, KCNQ1, that has been found to be particularly active in crosstalk with various transporters, but we discuss other examples that were also recently uncovered.
K+ CHANNELS AND SECONDARY ACTIVE TRANSPORTERS
The majority of the channel-transporter interactions identified to date are between K+ channels and secondary active transporters that rely on coupling uphill movement of the desired solute to downhill movement of sodium ions (sodium-coupled solute transporters). In this category of interactions, KCNQ1 is the most often identified K+ channel. Here, we first introduce the proteins involved before discussing their interaction.
Kv channels and Ca2+-activated K+ channels
Voltage-gated potassium channel (Kv) pore-forming (α) subunits are encoded by genes of a 40-member family in humans. Conserved features among Kv α include a six transmembrane domains (S1-S6), a K+-selective pore region formed by residues between S5 and S6, and a voltage sensor domain (VSD) comprising the S1-S4 segments, which moves in response to changes in membrane voltage (Vm) (Figure 1 A). The movement of the VSD controls channel opening by tugging on the intracellular S4-S5 linker region and inducing a conformational change to open the pore and permit K+ passage (Labro et al. 2011). Kv channels are especially well known for their essential functions in numerous excitable cell types including neurons, cardiac myocytes and skeletal muscle (Abbott et al. 2001; Jentsch 2000; Bellocq et al. 2004). KCNQ1, the primary Kv channel discussed in this review, is notable for its diverse functions in tissues including the heart, inner ear, thyroid, stomach, pancreas, lower gastrointestinal tract and choroid plexus (Lee et al. 2000; Harchi et al. 2010; Purtell et al. 2012; Warth et al. 2002; Abbott et al. 2014).
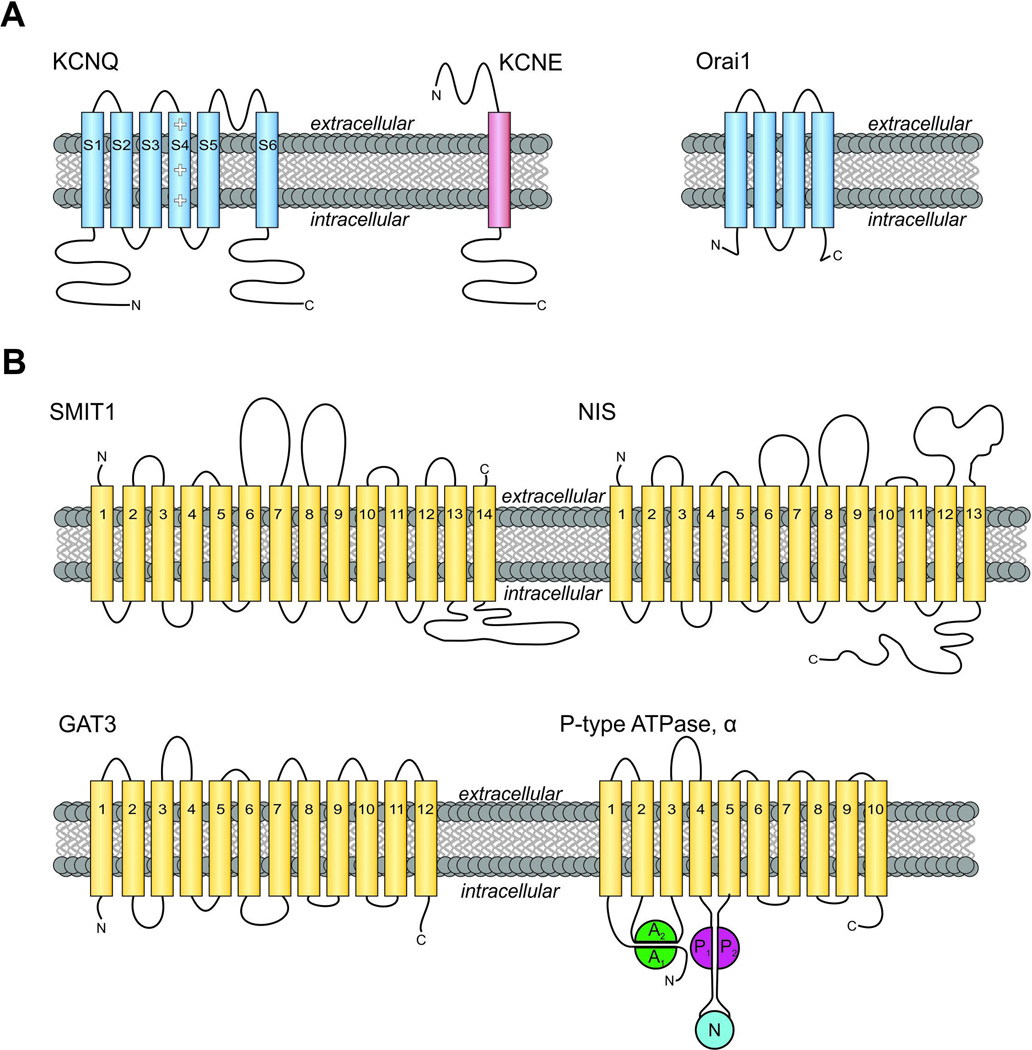
Figure 1. Membrane topology of channel subunits and transporters discussed in this review.
A. Left, transmembrane topology of KCNQ voltage-gated potassium (Kv) channel α subunits and KCNE β subunits. Kv pore-forming loop pictured between S5-S6. Right, topology of Orai1. Intracellular N- and C-terminals labeled accordingly.
B. Upper, transmembrane topology of two SLC5A transporters. (Left) Sodium/myo-inositol co-transporter SMIT1, with extracellular N and C-terminals labeled accordingly. SMIT1 features a large intracellular loop between segments 13 and 14, as well as two large extracellular loops between segments 6–7 and 8–9. (Right) The sodium/iodide symporter NIS. Lower, transmembrane topology of GAT3 (SLC6A11) and a P-type ATPase α subunit such as the gastric H+/K+-ATPase. Extracellular N-terminus and intracellular C-terminus are labeled.
Note that topology maps are shown for clarity and simplicity. High-resolution crystal structures have been solved for Orai (Hou et al. 2012), Kv1.2 (a mammalian relative of KCNQ channels) (Long et al. 2005), and vSGLT (a prokaryotic sodium galactose transporter related to mammalian SLC5A transporters) (Faham et al. 2008). Actual structures may deviate from predicted topologies with respect to, e.g., broken helices and in some cases actual transmembrane disposition.
A color version of the figure is available online.
The KCNQ1 gene encodes a protein spanning six transmembrane segments, and a tetramer of four such proteins forms a functional K+-selective pore. KCNQ1 is best known for its crucial requirement in maintaining normal cardiac rhythm, arising from its role in repolarizing ventricular and atrial myocytes, responding to cellular depolarization by opening relatively slowly to permit K+ efflux and cellular repolarization to help end each heartbeat. Human KCNQ1 perturbation by, e.g., gene mutation, is associated with cardiac arrhythmias including Long QT syndrome (LQTS) (Tester et al. 2005) and atrial fibrillation (AF) (Chen et al. 2003; Bellocq et al. 2004), and has also been associated with extracardiac effects such as increased susceptibility to type II diabetes mellitus (T2DM) (Unoki et al. 2008; Yasuda et al. 2008; Zhou et al. 2010) and gastric cancer (Roepke et al. 2010; Than et al. 2014). In mice, Kcnq1 gene deletion causes deafness, loss of gastric acid secretion, altered insulin sensitivity, and hypothyroidism (Lee et al. 2000; Frohlich et al. 2011; Grahammer et al. 2001; Boini et al. 2009). These extracardiac defects arise from perturbation of the role of KCNQ1 in non-excitable cells such as epithelial cells of the inner ear, stomach and thyroid (Lee et al. 2000; Frohlich et al. 2011)– highlighting the versatility of KCNQ1 and its necessity for many diverse biological processes.
Kv α subunits, including KCNQ1, can interact with smaller β subunits which drastically alter their function. KCNQ1 is most well-known for its interactions with β subunits of the KCNE family (Figure 1 A) (Abbott 2014). Each of the KCNE β-subunits alters KCNQ1 function differently. KCNE1 slows and right-shifts activation, increases conductance and removes inactivation (Tristani-Firouzi & Sanguinetti 1998; Sesti & Goldstein 1998; Sanguinetti et al. 1996; Barhanin et al. 1996); KCNE2 reduces peak outward current but makes KCNQ1 constitutively active (Tinel 2000); KCNE3 maintains peak outward current and makes KCNQ1 constitutively active (Schroeder et al. 2000); KCNE4 inhibits KCNQ1 (Grunnet et al. 2002); KCNE5 slows KCNQ1 activation and right-shifts the channel’s voltage-dependence of activation so strongly as to render the channel inactive at physiological membrane potentials (Angelo et al. 2002).
Studies utilizing Kcne2 knockout mice in particular have revealed the role of heteromeric KCNQ1-KCNE2 channels in supporting function of the thyroid epithelial cell sodium-iodide symporter (NIS) and, in the choroid plexus epithelium, the sodium-dependent myo-inositol transporter (SMIT1)(Roepke et al. 2009; Roepke, Kanda, et al. 2011; Abbott et al. 2014). Investigation into the novel concept of KCNQ1-transporter complexes therefore has the potential to greatly improve our mechanistic understanding of KCNQ1- and KCNE2-linked human diseases as well as the generalizable mechanisms by which ion channels and transporters operate, signal to one another, and are regulated.
Calcium-activated K+ (KCa) channels also play a critical role in many realms of physiology, including smooth muscle tone, action potential regulation, and cell metabolism (Rundén-Pran et al. 2002; Marrion & Tavalin 1998). KCa channels are generally grouped into three categories: large conductance (BK), small conductance (SK), and intermediate conductance (IK) (Vergara et al. 1998). MaxiK, a member of the BK subfamily of KCa channels and the primary KCa discussed in this review, is a seven transmembrane domain (S0-S6) protein with an extracellular N-terminus and large intracellular C-terminus, and must tetramerize to form a functional channel (Toro et al. 2014). MaxiK is involved in diverse protein-protein interactions, with proteins including tubulin (Ou et al. 2009), and voltage-gated calcium channel Cav2.1 (Indriati et al. 2013).
SLC-superfamily secondary active transporters
Sodium-dependent myo-inositol transporters
Both SMIT1 (SLC5A3) and SMIT2 (SLC5A11) are large transmembrane proteins of the Solute Carrier Family 5 (SLC5A) gene family, and they exhibit similar tissue expression patterns. These proteins, each spanning 14 transmembrane segments (Figure 1 B), function as sodium-coupled symporters and actively transport myo-inositol uphill into the cell by coupling to downhill transport of Na+ ions. SMIT1 is additionally capable of transporting scyllo-inositol, l-fucose, l-xylose, l-glucose, d-glucose, α-methyl-d-glucopyranoside, and l-fucosea. SMIT2 transports myo-inositol, d-chiro-inositol, and d-xylose (Schneider 2015). The myo-inositol transported by each of these proteins functions as a crucial cellular osmolyte, is also a substrate of the phosphatidylinositol 4,5-bisphosphate (PIP2) signaling pathway (Buccafusca et al. 2008; Holub 1986), and may also be converted to phosphatidylinositol (PI) – a crucial membrane component (Schneider 2015) (Figure 2). These functions are particularly pertinent to ion channel function, and especially for KCNQ1, because it (and to a greater or lesser degree many other channels) is highly sensitive to changes in both cellular volume and PIP2 (Hammami et al. 2009; G Loussouarn 2003). Perturbations to the transporters’ functions are therefore predicted to alter PIP2-dependent processes such as KCNQ1 channel function as well as cellular responsiveness to osmotic stress, and have been implicated in pathologies including Down syndrome (Berry et al. 1995; Berry et al. 1999) and bipolar disorder (Davanzo et al. 2001; Harwood 2005; Willmroth et al. 2007).
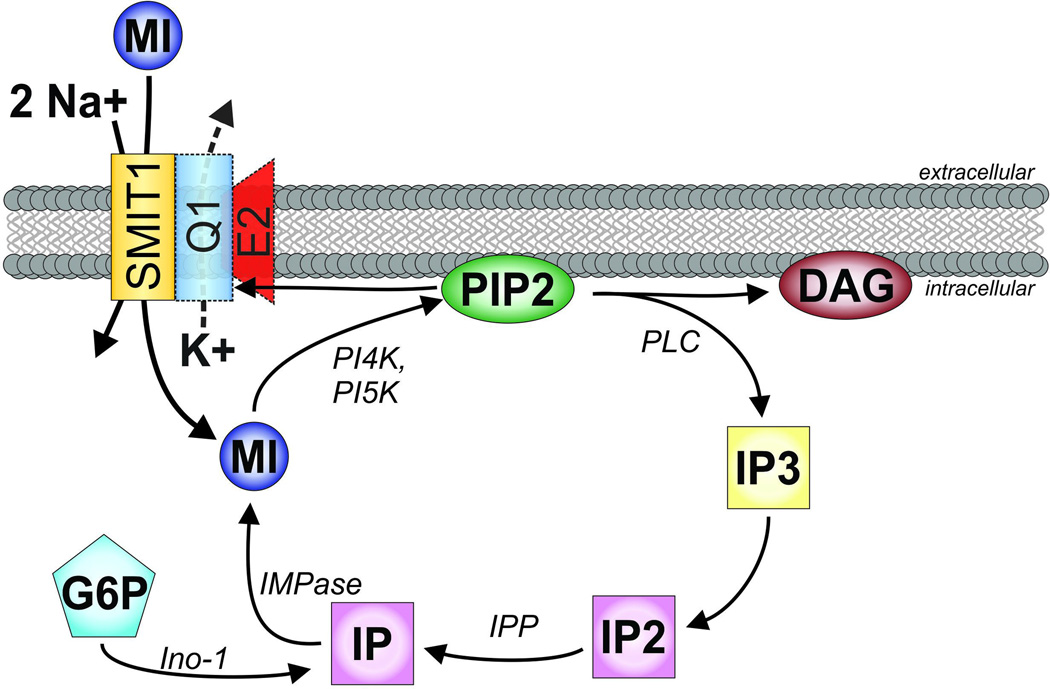
Figure 2. The myo-inositol pathway in the context of SMIT1 and KCNQ1-KCNE2.
SMIT1, orange, transports sodium (Na+) and myo-inositol (MI) with a stoichiometry of 2:1. Phosphatidylinositol 4-kinase (PI4K), followed by phosphatidylinositol 5-kinase (PI5K), add phosphates to MI to form phosphatidylinositol 4,5-bisphosphate (PIP2). When activated, commonly by Gq-coupled receptors, phospholipase C (PLC) cleaves PIP2 into constituent components diacylglycerol (DAG) and inositol(1,4,5)-trisphosphate (IP3). IP3 is then recycled by dephosporylation to inositol(1,4)-bisphosphate (IP2) and inositol monophosphate (IP) by Inositol-1,4 bisphosphate 1-phosphatase (IPP), and back to (myo)inositol by inositol- 1(or 4)-monophosphatase (IMPase). IP can also be synthesized from glucose 6-phosphate (G6P) by inositol synthase (Ino-1). KCNQ1 (blue) with and without KCNE2 (red) can form complexes with SMIT1, although this interaction is not required for basic SMIT1 function. KCNQ1 gating is heavily regulated by PIP2.
A color version of the figure is available online.
The myo-inositol transported by the SMIT proteins serves many important functions within the cell, perhaps the most notable of which occurs after the conversion of myo-inositol to PI and PIP2 by phosphatidylinositol 4-kinase (PI4K), followed by phosphatidylinositol 5-kinase (PI5K). Without PIP2, many channels and cellular processes effectively shut off, including KCNQ channels, transient receptor potential (TRP) channels, inwardly-rectifying potassium (Kir) channels, plasmalemmal calcium pumps (PMCA), cardiac sodium-calcium exchangers (NCX1), sodium-proton exchangers (NHEx), epithelial sodium channels (ENaC), and ryanodine-sensitive calcium channels (RyR). KCNQ channels, along with hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, are notable among the voltage-gated channels for their robust responses to PIP2 (Suh & Hille 2008; Hilgemann et al. 2001; G. Loussouarn 2003). PI also plays a critical role in cellular machinery including activation of the Na+/K+-ATPase, modulation of catecholamine synthesis by tyrosine hydroxylase, and physical association with alkaline phosphatase and acetylcholinesterase (Holub 1986), and may also play a cooperative role in the actin cytoskeleton (Suh & Hille 2008).
PIP2 also plays a prominent role as secondary messenger. When a Gq-coupled receptor is stimulated, phospholipase C (PLC) is activated and cleaves PIP2 into constitutive components: inositol(1,4,5)-triposphate (IP3), and diacylglycerol (DAG) (Figure 2). DAG and IP3 each play a role in activating protein kinase C (PKC) leading to phosphorylation and altered activity of myriad cellular processes. IP3, then released into the cytosol, is free to bind to IP3 receptors, triggering calcium release from intracellular stores.
Of some level of controversy, inositol status has been implicated in various cognitive disorders. Lithium, valproic acid (VPA), and carbamazepine (CBZ) are each potent mood stabilizers which also alter inositol signaling. Lithium treatment reduces MI concentrations in the brain by 30% and concurrently elevates serum MI levels by 50% (Allison & Stewart 1971), and potently inhibits MI synthesis by inhibiting inositol-1 and inositol-4-monophosphatases in a substrate-dependent manner (Harwood 2005). These findings appear to be consistent with later work demonstrating a lithium-induced reduction in both manic symptoms and MI levels in human brain (anterior cingulate cortex) (Davanzo et al. 2001). VPA also inhibits inositol levels in the brain, although by different mechanism. Here, the treatment inhibits myo-inositol-1-phosphate (MIP) synthase activity, thereby preventing conversion of D-glucose-6-phosphate (G6P) to myo-inositol (Shaltiel et al. 2004). It comes as no surprise, then, that lithium and VPA are sometimes clinically co-administered in treatment of biopolar disorder (Harwood 2005). Interestingly, all three of these mood stabilizers (lithium, VPA, and CBZ) inhibit SMIT1 activity and mRNA transcript levels (Lubrich & van Calker 1999; Willmroth et al. 2007).
Even though inositol can be synthesized by some tissues, dietary restriction or supplementation of inositol raises/lowers inositol concentrations in most tissues, sometimes to deleterious effect (Holub 1986). In the liver, an inositol-deficient diet produces elevated triglycerides and fatty liver (Hayashi et al. 1974). In the intestine, an inositol-deficient high-fat diet produces lipodystrophy and altered gross morphology (Hegsted et al. 1973). Interestingly, each of these syndromes resolved upon addition of inositol to the experimental animals’ diets.
Nomenclature of the SMITs can be a source of confusion. Cloned from Madin-Darby canine kidney (MDCK) cells in 1992, SMIT1 was originally known simply as SMIT (Kwon et al. 1992). In 1994 rkST1 was cloned from rabbit (Hitomi & Tsukagoshi 1994), although it was not until nearly a decade later that this protein was renamed to the more suitable “SMIT2” (Coady et al. 2002). Last, SMIT1 is purported to have two splice variants titled “SMIT1-2” and “SMIT1-3” each of which exclude the 14th transmembrane segment of SMIT1 and possess differing c-termini (Schneider 2015).
In mice, deletion of the Slc5a3 gene proves fatal shortly after birth, due to hypoventilation, although the phenotype can be rescued by supplementing the drinking water of both pup and dam with additional myo-inositol (Berry et al. 2003; Chau et al. 2005). Interestingly, SLC5A3 shares exon 1 with mitochondrial ribosomal protein subunit 6 (MRPS6) with the entirety of the SLC5A3 coding region contained by MRPS6 exon 2. Deletion of SLC5A3 does not alter MRPS6 expression, however. The implications of this exon-sharing are not yet clear, although there is some evidence to suggest the presence of a chimeric SMIT1/MRPS6 protein in human tissue (Buccafusca et al. 2008; Porcellati et al. 1999; Porcellati et al. 1998).
The sodium-dependent iodide symporter
The Na+/I− symporter (NIS), encoded by SLC5A5, is a large transmembrane protein containing 13 putative transmembrane segments, an extracellular N-terminal, and a cytosolic C-terminal (Figure 1 B). Like other members of the SLC5A family, NIS exhibits a high sequence similarity to other members of the SLC5A gene family, particularly the sodium-multivitamin transporter (SMVT, SLC5A6), with the most distinct sequence differences occurring near the C-terminus. Although the prototypical function of NIS may be associated with the thyroid, the protein is also expressed and functional in the mammary glands, placenta, gastrointestinal (GI) tract (Dohán et al. 2003), and potentially kidney (Vayre et al. 1999; Spitzweg et al. 2001).
In the thyroid, NIS enables active I− uptake against the electrochemical gradient and into follicular cells - this process is the rate-limiting step in thyroid hormone (TH) biosynthesis. The I− is then passed through to the colloid by another channel or transporter, the identity of which is still under debate. Once in the colloid, I− undergoes oxidation by thyroid peroxidase and is incorporated into the thyroglobulin (Tg) molecule to produce monoiodotyrosine (MIT) and diiodotyrosine (DIT) in a process known as organification. The organified Tg is then resorbed by the follicular cells where, when stimulated by thyroid stimulating hormone (TSH), the iodinated Tgs are cleaved to form either thyroxine (T4), generated from a pair of DIT, or triiodothyronine (T3), produced from one MIT and one DIT molecule (Figure 3). These THs are critical for nervous system and lung development, as well as normal metabolic function (Morreale de Escobar et al. 2004). Perturbations to NIS function, accordingly, cause hypothyroidism (Carrasco 1993).
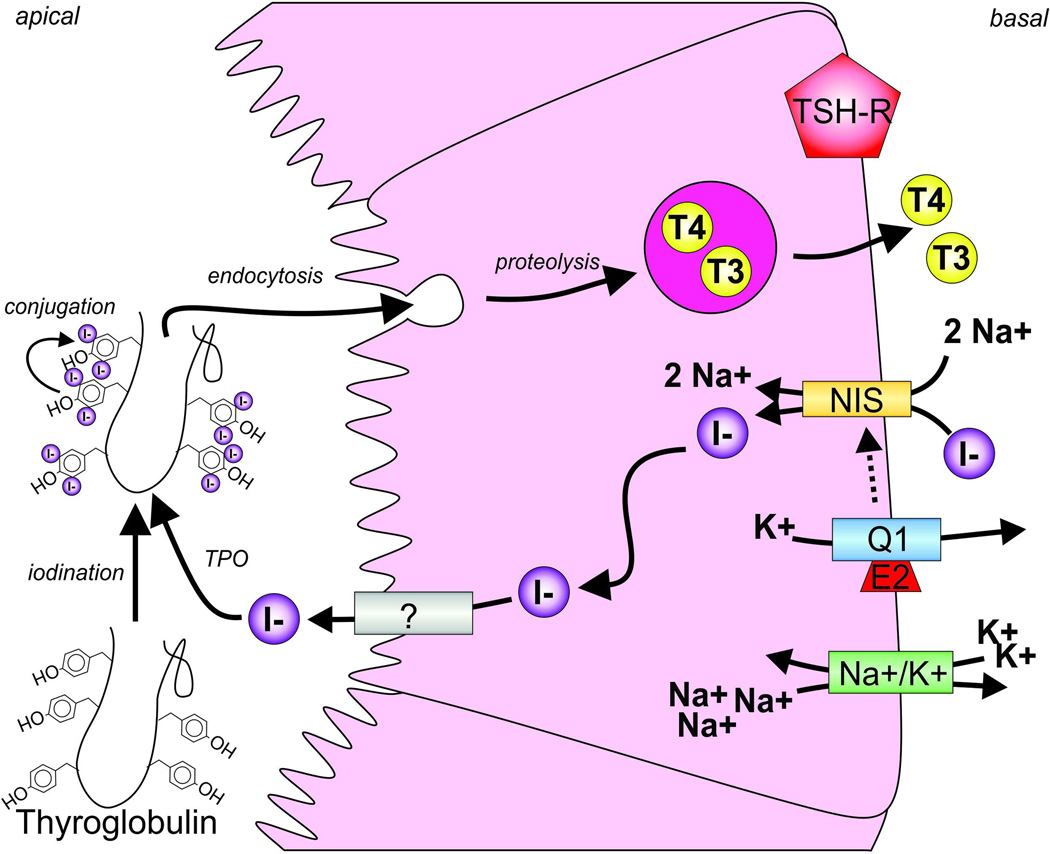
Figure 3. Channel-transporter crosstalk in the thyroid.
The sodium-iodide symporter (NIS, orange) transports Na+ and I− (purple) into the thyrocyte. This process is facilitated, via an incompletely resolved mechanism, by potassium ion efflux through KCNQ1-KCNE2 (Q1, blue; E2, red) channels and also involves active transport by the Na+/K+-ATPase (Na+/K+, green). I− is then passed through to the colloid by another protein (?) whose identity is still under debate. I− undergoes oxidation by thyroid peroxidase (TPO) and is incorporated into the thyroglobulin (Tg) molecule to produce monoiodotyrosine (MIT) and diiodotyrosine (DIT) by organification. The organified Tg is then resorbed by the follicular cells where, when the thyroid stimulating hormone receptor is activated (TSH-R, red), the iodinated Tgs are cleaved to form either thyroxine (T4), generated from a pair of DIT, or triiodothyronine (T3), produced from one MIT and one DIT molecule.
A color version of the figure is available online.
Sodium-dependent GABA transporters
In the brain, the inhibitory neurotransmitter GABA is transported across membranes by GABA transporters (GATs) GAT1, GAT2, GAT3 (Figure 1 B), and the betain/GABA transporter type 1 (BGT1). At inhibitory neuronal synapses, removal of GABA from the synaptic cleft by GATs acts to terminate the GABA signaling process and is accordingly a critical component of inhibitory neuron regulation (Jin, Galvan, et al. 2011). GAT3, a 12 transmembrane domain Na+/Cl−-coupled transporter preferentially expressed in glia (Jin, Paré, et al. 2011; Jin, Galvan, et al. 2011; Borden & Caplan 1996), is here highlighted for its newly-discovered role in ion channel-transporter complex formation (see below) (Singh et al. 2016).
K+ channel-SLC transporter interactions
Functional interaction between KCNQ1-KCNE2 and NIS
We first reported the importance of KCNQ1-KCNE2 complexes for efficient thyroid hormone synthesis in 2009 when, together with the Carrasco lab, we discovered that Kcne2−/− mice are hypothyroid (Roepke et al. 2009). Using positron emission tomography we found that disruption of KCNQ1-KCNE2 complexes, which we discovered in thyroid epithelial cells, impairs iodide accumulation in the thyroid gland (Roepke et al. 2009). Subsequently, the Lang group confirmed this, using Kcnq1−/− mice (Frohlich et al. 2011). We later delineated which of the processes involved in thyroid iodide accumulation (focusing on iodide uptake versus organification) was disrupted by Kcne2 deletion, discovering it to be iodide uptake by NIS (Purtell et al. 2012).
In the thyroid, I− uptake is mediated by NIS (Figure 3) (Dai et al. 1996). We found that NIS-mediated I− uptake can be strongly inhibited by the KCNQ1-selective antagonist [3R,4S]-chromanol 293B. This effect was selective to I− transport, without directly altering organification, and occurred both in vivo (in the mouse), and in vitro (in the rat thyroid cell line, FRTL-5) (Purtell et al. 2012). The effects of chromanol 293B on I− transport are only apparent when KCNQ1-KCNE2 and NIS are co-expressed, providing strong support for KCNQ1-KCNE2 mediation of NIS function.
Regarding the mechanism of KCNQ1-NIS interaction, we do not yet have confirmation of a physical interaction, although this is suspected. The KCNQ1-KCNE2 channel is constitutively active at weakly negative potentials, because KCNE2 modifies the voltage-dependent gating of KCNQ1 such that its current voltage relationship is linear rather than outwardly rectifying (Tinel 2000). The related β subunit KCNE3 achieves a similar effect on KCNQ1 by directly locking its VSD in the activated position via acidic residues in the membrane proximal KCNE3 extracellular segment (Choi & Abbott 2010; Panaghie & Abbott 2007), but this mechanism has not yet been confirmed for KCNQ1-KCNE2 (which is a more difficult channel to study, because KCNE2 also shrinks macroscopic current density). One might predict that the relatively constant outward K+ flux that KCNQ1-KCNE2 would be predicted to provide in thyroid cells at weakly negative potentials might facilitate NIS function by enhancing the electrical gradient required for sodium entry (with other pumps etc. redistributing Na+ and K+ ions to take care of the chemical part of the gradient), but we found that the NIS-related sodium-dependent monocarboxylate transporter (SMCT) was not affected by KCNQ1-KCNE2 status (Purtell et al. 2012), possibly suggesting against the electrical gradient hypothesis. However, it may be that for the outward movement of K+ through KCNQ1-KCNE2 to have any effect on sodium-dependent transporters, they need to be localized close to one another, or even tightly coupled; a hypothesis to be tested in the future. Either way, KCNQ1-KCNE2 is critical in the thyroid for its regulation of NIS activity, and an excellent example of biologically important functional interaction between an ion channel and a transporter. Accordingly, disruption of Kcnq1 or Kcne2 genes causes hypothyroidism, and in the case of Kcne2 other phenotypic hallmarks of insufficient THs including dwarfism, alopecia, and cardiomegaly (Roepke et al. 2009; Frohlich et al. 2011).
Physical interaction between KCNQ1-KCNE2 and SMIT1
Additional phenotypic assessment of the Kcne2 knockout mouse line lead to the discovery that Kcne2 disruption in mice reduces the seizure threshold and causes behavioral abnormalities (Abbott et al. 2014). We had previously found that in the CNS, KCNE2 is most highly expressed in the choroid plexus epithelium, the primary site of cerebrospinal fluid (CSF) production and secretion (Roepke, Kanda, et al. 2011), where it regulates KCNQ1 and KCNA3 (Kv1.3) channels. We suspected a role for KCNQ1-KCNE2 in regulating CSF composition. After exhausting other hypothesis-based alternatives, we performed unbiased mass spectrometry-based metabolomics analysis of the CSF and found that Kcne2 deletion altered the concentration of just one identifiable CSF metabolite: myo-inositol. We discovered that under normal conditions, KCNQ1-KCNE2 channels physically interact with the sodium-dependent myo-inositol transporter (SMIT1) to form chansporter complexes in which the two protein classes reciprocally regulate one another (Figure 2). KCNQ1 doubled the myo-inositol uptake activity of SMIT1, but addition of KCNE2 to the complex had the opposite effect, inhibiting SMIT1. SMIT1 augmented the activity of both KCNQ1 channels and KCNQ1-KCNE2 channels.
Early attempts to determine the mechanism for channel-transporter feedback have yielded interesting but not yet definitive results. Co-expression of KCNQ1 with SMIT1 roughly doubled SMIT1 activity versus SMIT1 alone. One might surmise that the increase in SMIT1 activity is a product of the potassium efflux through KCNQ1 channels, thereby increasing the electrical gradient to favor sodium-coupled myo-inositol transport. Consistent with this, we found that when co-expressed with KCNQ1, SMIT1 function was suppressed by XE-991, a pore-binding KCNQ channel-blocker (Abbott et al. 2014). These data provided a basis for targeting transporter activity through a novel drug approach: ion channel manipulation. In addition, they also suggested that K+ flux through KCNQ1 is seemingly important for augmentation of SMIT1 activity. However, we demonstrated the channel-blocking effect was specific to KCNQ1-SMIT1, because when we co-expressed KCNQ4 and SMIT1 (between which we detected neither complex formation nor reciprocal regulation in oocytes), XE-991 had no effect, even though it inhibits KCNQ4, being a pan-KCNQ blocker. This, then, suggests that merely having K+ flux through a channel in the same cell is not sufficient to augment SMIT1 activity – arguing that either the electrical gradient hypothesis is incorrect, or (as mentioned in the discussion of KCNQ1-NIS, above) the effect requires close physical proximity between channel and transporter.
Further suggesting that the chansporter inter-subunit cooperation is more complex than merely electrical gradient enhancement, we found unexpected results when studying the KCNQ1-R231A S4 mutant that locks the voltage sensor of the channel into an open, or “on”, state, forming a constitutively active channel (Panaghie & Abbott 2007). Following the increased driving force hypothesis, this would be theorized to increase Na+-linked myo-inositol influx, yet the addition of KCNQ1-R231A to SMIT1 greatly inhibits transporter function (Abbott et al. 2014). Within the context of the driving force hypothesis these findings are in diametric opposition to each other, and instead suggest the possibility that KCNQ1 voltage sensor conformation, independent of actual conductance, might influence SMIT1 activity. This could also be the reason that KCNE2 inhibits myo-inositol uptake of KCNQ1-SMIT1 complexes, because KCNE2 also holds KCNQ1 open, although KCNE2 also greatly reduces outward current through KCNQ1 despite favoring its open state. Future investigations will be targeted at understanding exactly how KCNQ1 augments SMIT1 and NIS activity and whether there are underlying mechanistic commonalties. Intriguingly, we also found that KCNQ1 and SMIT2 functionally interact, and that SMIT2 inhibits KCNQ1 activity, but we do not yet know the mechanistic basis for these effects either (Abbott et al. 2014).
Physical interaction between MaxiK and GAT3
Very recently, Singh and colleagues uncovered an additional K+ channel-transporter interaction in the brain (Singh et al. 2016). MaxiK channels – voltage- and Ca2+-activated channels named for their large conductance – were demonstrated to physically interact with GABA transporter 3 (GAT3). As both GAT3 and MaxiK channels are localized in glia, the authors propose that the complexes may serve to limit GABA release, although no functional experiments have been published to date on this very recently discovered chansporter complex. Interestingly, GAT3 is encoded by SLC6A11 – adding yet another member of the SLC superfamily to the list of transporters regulated functionally and/or physically by K+ channels.
ION CHANNEL-ATPASE INTERACTIONS
Several examples are known of ion channels functionally interacting with transporters in the transmembrane ATPase family (and to our knowledge just one known case of physical interaction). Transmembrane ATPases are primary active transporters that use ATP hydrolysis to provide the energy to transport ions up their electrochemical gradient. In many cell types, ion channels and pumps are involved in activities such as maintaining ion homeostasis, that require one or more of the proteins to support function of one or more of the others. One broad example is the Na+/K+-ATPase. This ubiquitous protein eliminates 3 Na+ ions while bringing in 2 K+ ions, thus helping to restore the appropriate concentrations of K+ and Na+ ions, and also the negative membrane potential, following action potentials in excitable cells so that the cell is ready for future action potentials. Here, however, we will focus on just two contrasting examples. First we discuss facilitation of gastric H+/K+-ATPase activity by K+ channels. As one of many examples of channels cooperating with pumps to ensure favorable cellular/extracellular ion balance or specific ion secretion but probably without the necessity for direct physical coupling, we focus on this example because it involves KCNQ1, which we also discussed above in the context of its ability to co-assemble with the SLC transporters. Second, we cover physical interaction of the unusual Orai1 Ca2+ channel with the SPCA2 ATPase – we chose this example because it involves formation of one of the thus-far few known examples of a macromolecular channel-transporter complex.
Functional interactions between K+ channels and the gastric H+/K+-ATPase
Under normal conditions, gastric acid secretion is coordinated by a small symphony of cellular events (Figure 4). In response to gastric parietal cell stimulation, H+/K+-ATPase is trafficked to the apical surface of the acid-secreting cells where it can begin the process of H+ efflux in exchange for K+ influx. The excreted H+ becomes the substrate for HCl acid formation, the intracellular K+ causes a hyperpolarization of the parietal cell, preventing further acid secretion (Hersey & Sachs 1995). The gastric acid secretion process would end here, as a one-off terminal event, were it not for the presence of potassium-normalizing K+ channels KCNQ1-KCNE2 (Roepke, Kanda, et al. 2011) and KCNJ1 (ROMK) (Vucic et al. 2015). Together, these channels coordinate the “resetting” of intracellular K+ levels to enable prolonged H+/K+-ATPase function and thus further gastric acid secretion.
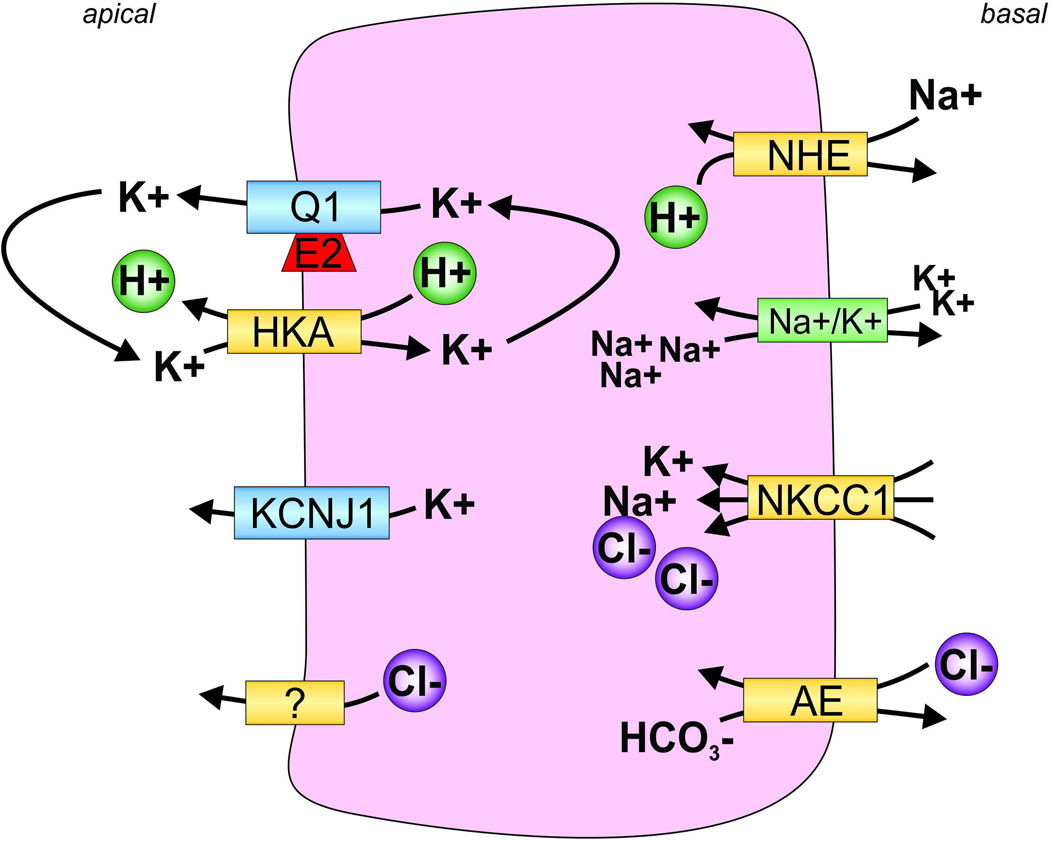
Figure 4. Channel-transporter crosstalk in gastric parietal cells facilitates gastric acid secretion.
Sodium-hydrogen exchanger (NHE) draws Na+ into the cell and expels H+. The sodium-potassium ATPase (Na+/K+) releases Na+ and draws in K+. Na+/K+/Cl− cotransporter 1 (NKCC1) draws Na+, K+, and Cl− into the cell. The anion exchanger (AE) exchanges Cl− and HCO3−. H+/K+-ATPase (HKA) expels H+ into the stomach lumen accompanied by an unknown Cl− channel or transporter to participate in gastric acid formation. KCNQ1-KCNE2 (Q1, E2) and KCNJ channels expel potassium from the cell in order to “reset” the high intracellular K+ accumulated by HKA activity.
A color version of the figure is available online.
Thus, in addition to its roles in the thyroid and choroid plexus, KCNQ1-KCNE2 acts as a “team player” within the parietal cells of the gastric glands of the stomach. Here, the KCNQ1-KCNE2 complex acts to counterbalance and functionally reset the high intracellular K+ levels accumulated by the H+/K+-ATPase (Figure 4) (Roepke et al. 2006; Abbott 2014; Lee et al. 2000). Although KCNQ1 channels are typically inhibited by extracellular protons, the formation of the KCNQ1-KCNE2 complex enables constitutive activation of the channel as well as an augmentation of the channel’s current and function at low extracellular pH (Heitzmann et al. 2004; Heitzmann et al. 2007). This last attribute is critical to maintaining the function of this channel in the very low pH of the stomach. Gastric acid secretion can be strongly inhibited by application of the KCNQ1 blocker chromanol 293B (Grahammer et al. 2001), thereby inhibiting potassium efflux and maintaining a hyperpolarized parietal cell by way of intracellular potassium accumulation. Also crucial is that KCNE2 helps ensure that KCNQ1 is localized at the apical membrane where it can assist the H+/K+-ATPase. Deletion of Kcne2 results in aberrant localization of KCNQ1 to the basolateral membrane of parietal cells, because in this pathologic state KCNE3 is upregulated and hijacks KCNQ1, taking it to the wrong side of the cell and disenabling it from assisting the H+/K+-ATPase. However, even concomitant Kcne3 deletion does not restore gastric acid secretion in Kcne2−/− mice, illustrating the importance of the effects of KCNE2 on KCNQ1 gating and pH sensitivity for the parietal cell function of KCNQ1 (Roepke, King, et al. 2011). It is of no surprise, then, that Kcne2 and Kcnq1 knockout mice each display profound hypochlorhydria and altered gastric morphology (Roepke et al. 2006; Lee et al. 2000).
KCNJ1, also known as Kir1.1, is an inward-rectifier potassium channel comprised of intracellular N- and C-terminals, two transmembrane segments (M1, M2), and one pore-forming region (H5). Like Kv channels, four of the KCNJ1 proteins must tetramerize to form a membrane protein with a functional pore. KCNJ channels are regulated by multiple pathways, including protein kinase A (PKA), protein kinase C (PKC), intracellular pH, and PIP2 (Huang 2001). Well-known for its role in potassium recycling in the kidney, KCNJ1 is also expressed in gastric parietal cells (Vucic et al. 2015; Huang 2001). Like KCNQ1-KCNE2, KCNJ1 channels on the parietal cell apical membrane appear to help facilitate gastric acid secretion by recycling K+ for later participation in ion exchange by the H+/K+-ATPase. Selective blockade of KCNJ1 channels by application of Kir-selective blocker tertiapin-Q (TPNQ), and/or application of KCNQ blocker XE-991, to the gastric lumen suggests the requirement of both KCNQ1-KCNE2 and KCNJ1 channel function for maintenance of normal gastric acid secretory responses. These findings are validated in Kcnj1 knockout mouse studies, which demonstrated reductions in gastric acid secretion independent of any other developmental or morphological changes to the stomach (Vucic et al. 2015). Likewise, we previously showed that although Kcne2−/− mice exhibit abnormal parietal cell morphology in addition to achlorhydria, Kcne2+/− mice exhibit hypochlorhydria in the absence of morphological changes to parietal cells, suggesting KCNQ1-KCNE2 channels are directly involved in supporting H+/K+-ATPase function rather than their disruption causing achlorhydria indirectly via disruption of cell architecture (Roepke et al., 2006).
H+/K+-ATPase is not a member of the SLC gene superfamily, and in fact functions as an antiporter – transporting H+ out of the cell, to participate in gastric acid formation, in exchange for drawing K+ into the cell. This pump operates in stark contrast to the SLC5A proteins which operate as symporters, drawing both sodium and their substrate (MI, glucose, etc.) into the cell. Although crosstalk between K+ channels and the gastric H+/K+-ATPase has been functionally demonstrated, physical interaction of the transporter with KCNQ1-KCNE2 or any of the channels discussed above has not. In the case of KCNQ1-KCNE2, it is considered unlikely that it operates by direct physical interact with H+/K+-ATPase, because while apical membrane expression of the latter is thought to occur only after signaling by secretagogues (which stimulate trafficking of H+/K+-ATPase-containing vesicles to the apical membrane), KCNQ1-KCNE2 is thought to reside in the apical membrane at rest – although this has not yet been fully investigated.
Physical interaction between Orai1 and SPCA2
Previous research has demonstrated Orai1 to be a vital component of store-operated calcium channel activation (Soboloff et al. 2006). Orai1 channels (Figure 1 A), which consist of four-transmembrane segment proteins forming a functional hexamer at the plasma membrane (PM), are activated by the depletion of internal calcium stores, generally referred to as a “store-operated” mechanism (Prakriya et al. 2006; Soboloff et al. 2006). The activity of these channels is greatly increased when co-expressed with STIM proteins – endoplasmic reticulum (ER)-bound Ca2+ store sensors that possess no channel activity on their own (Soboloff et al. 2006; Mercer et al. 2006). Together, the STIM1-Orai1 complex forms a unique Ca2+ sensor-channel pairing between the PM and ER (Soboloff et al. 2006). Perturbations to Orai channel function may cause diverse problems, including immune deficiency (Feske et al. 2006) and myopathy (Nesin et al. 2014).
Secretory pathway Ca2+-ATPase isoform 2 (SPCA2), encoded by (ATP2C2) is a P-type calcium transporter localized to the Golgi (Vanoevelen et al. 2005). The SPCA proteins, spanning 10 transmembrane segments, use ATP to transport Ca2+ and Mn2+ from the cytoplasm and into the Golgi and are critical in their role as Ca2+ signal terminators (Durr et al. 1998; Feng et al. 2010). Although SPCA1 is ubiquitously expressed, SPCA2 expression is restricted to brain and secretory and absorptive epithelial tissues (Cross et al. 2013; Xiang et al. 2005) and is highly upregulated in the mammary epithelium during lactation (Feng et al. 2010). Recently, SPCA2 has been highlighted as a critical component in human breast tissue and tumorigenicity, by way of intracellular Ca2+ level regulation (Feng et al. 2010). In the same study, SPCA2 was demonstrated to form a complex with calcium channel Orai1 (Figure 5 B) (Feng et al. 2010). When physically associated, SPCA2-Orai1 complexes enable Ca2+ signaling independent of Ca2+ stores, STIM sensors, or SPCA2 pump activity, revealing a previously unknown mechanism of Orai1 operation (Feng et al. 2010). These findings provide the first evidence for non-K+ channel participation in chansporter complex crosstalk or formation.
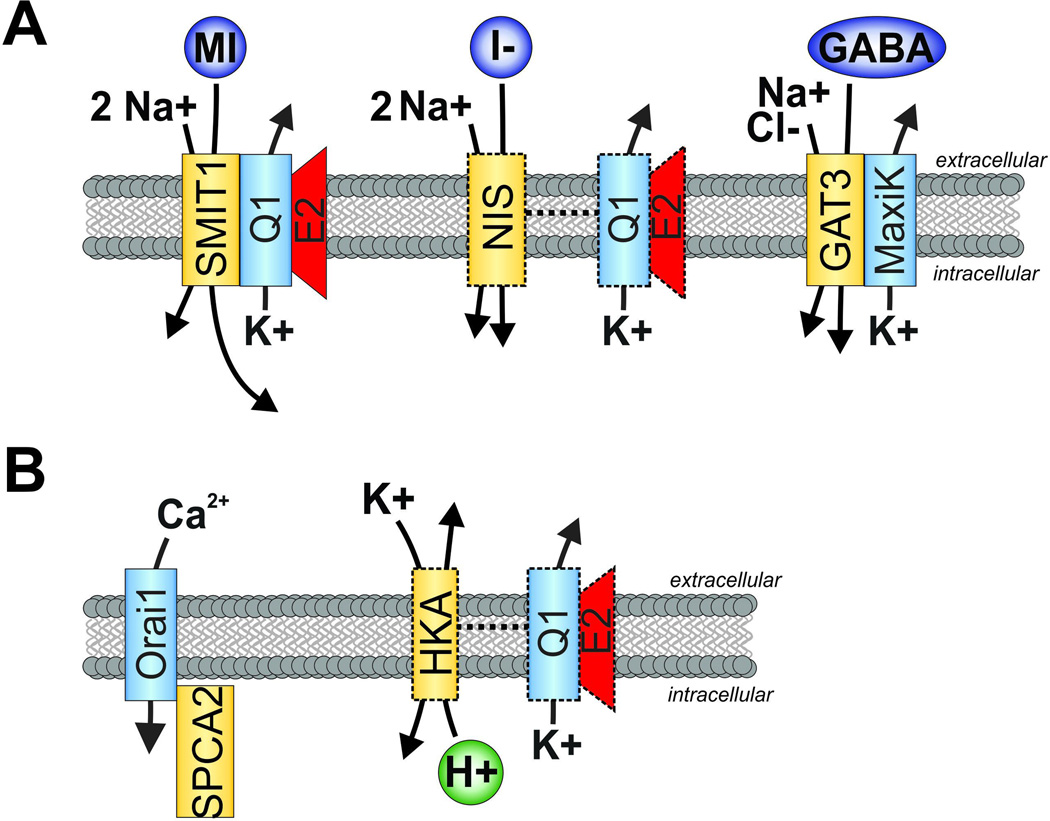
Figure 5. Summary of channel-transporter complex formation and functional crosstalk discussed herein.
A. K+ channel interactions with members of the SLC superfamily. Left, SMIT1 and KCNQ1-KCNE2 form reciprocally regulating, physically interacting complexes in the brain. Center, NIS and KCNQ1-KCNE2 exhibit functional crosstalk in the thyroid, although physical interaction remains to be determined (indicated by dotted lines). Right, GAT3 and MaxiK form physically interacting chansporter complexes.
B. Left, Orai1 and SPCA2 form physically interacting chansporter complexes which enable a novel mode of calcium channel activation, potentially important in tumorigenesis. Right, HKA and KCNQ1-KCNE2 exhibit functional crosstalk in the stomach to enable gastric acid secretion. Physical interaction remains to be determined (indicated by dotted lines).
A color version of the figure is available online.
CONCLUSIONS
It is striking that many of the transporters demonstrated to complex with Kv channels are, to date, from the SLC gene families. In addition to SMIT1, we found that SMIT2 and SGLT1 (SLC5A11 and SLC5A1, respectively) exerted effects when co-expressed with KCNQ1 (Abbott et al. 2014), however in-depth characterization remains to be pursued. Further exploration of SLC members is warranted and may yield additional Kv-SLC complexes.
The potential connections between SMIT1, states of mania, inositol status, and lithium present a tantalizing cluster of biological phenomena. We know that both SMIT1 mRNA levels and brain inositol levels are acutely responsive to lithium treatment, though the latter is of little surprise given the well-established use of lithium to inhibit IMPase in many in vitro experiments. We also know that inositol levels roughly correspond to manic symptom presentation in humans. Together, these data suggest the possibility of altered inositol pathways constituting elements of the etiology of manic disorders, although there have been no pharmaceutical interventions to test or leverage this concept aside from lithium. Given the ability of ion channel-targeted drugs to manipulate co-assembled SMIT1 activity, as described herein, it is interesting to consider the possibility that novel interventions for mania could come in the form of re-purposed, or structurally modified, ion channel drugs. However, it is important to remember that many potassium channels, and KCNQ1 in particular, are widely expressed and fulfil a wide spectrum of physiological roles, therefore targeting even a specific channel isoform with the goal of modulating its associated transporter would present significant challenges with respect to side effects. We have shown that transporter function can be regulated by relatively channel-specific antagonists (Abbott et al. 2014) but can this be therapeutically exploited and/or will it prove to be a gadfly causing unwanted side effects? Increased specificity might potentially be achieved if a drug could target only chansporter complexes rather than non-complexed channels, especially if they could also be tailored for specific transporter-channel-KCNE subunit combinations.
Perhaps most critically, the findings reviewed herein hint at the emergence of a potentially broad paradigm in cell signaling, that of direct channel-transporter crosstalk and co-regulation facilitated by direct physical coupling. There are a number of research directions that should now be pursued to flesh out this idea. First, the scope of chansporter complexes that exist in vivo must be catalogued in order to define the generality of this new principle. Second, mechanistic questions must be addressed, including how channels can augment transporter function, what signaling information is exchanged within chansporter complexes, what is the directionality of this information, and why the necessity for direct interaction – is it merely proximity to limit the distance through which ions must diffuse, or are their intimate interactions between the moving parts of channels and transporters that convey information of a higher order than solely ion movement?
Acknowledgments
DECLARATIONS OF INTEREST
We are grateful for support from the US National Institutes of Health (R01 GM115189, R01 DK41544 to G.W.A.) and American Heart Association (Predoctoral fellowship to D.L.N.).
REFERENCES
- Abbott GW. Biology of the KCNQ1 Potassium Channel. [Accessed June 4, 2014];New Journal of Science. 2014 2014:1–26. Available at: http://www.hindawi.com/journals/njos/2014/237431/ [Google Scholar]
- Abbott GW, et al. KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. [Accessed May 31, 2014];Science signaling. 2014 7(315):ra22. doi: 10.1126/scisignal.2005025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24595108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott GW, et al. MiRP2 Forms Potassium Channels in Skeletal Muscle with Kv3 . 4 and Is Associated with Periodic Paralysis. Channels. 2001;104:217–231. doi: 10.1016/s0092-8674(01)00207-0. [DOI] [PubMed] [Google Scholar]
- Allison JH, Stewart MA. Reduced Brain Inositol in Lithium-treated Rats. Nature New Biology. 1971;233(43):267–268. doi: 10.1038/newbio233267a0. Available at: http://www.nature.com/doifinder/10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- Angelo K, et al. KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophysical journal. 2002;83(4):1997–2006. doi: 10.1016/S0006-3495(02)73961-1. Available at: http://dx.doi.org/10.1016/S0006-3495(02)73961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80. doi: 10.1038/384078a0. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8900282. [DOI] [PubMed] [Google Scholar]
- Bellocq C, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. [Accessed November 27, 2013];Circulation. 2004 109(20):2394–2397. doi: 10.1161/01.CIR.0000130409.72142.FE. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15159330. [DOI] [PubMed] [Google Scholar]
- Berry GT, et al. In vivo brain myo-inositol levels in children with Down syndrome. The Journal of pediatrics. 1999;135:94–97. doi: 10.1016/s0022-3476(99)70334-3. [DOI] [PubMed] [Google Scholar]
- Berry GT, et al. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. The Journal of biological chemistry. 2003;278(20):18297–18302. doi: 10.1074/jbc.M213176200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12582158. [DOI] [PubMed] [Google Scholar]
- Berry GT, et al. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995;25(2):507–513. doi: 10.1016/0888-7543(95)80052-n. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7789985. [DOI] [PubMed] [Google Scholar]
- Boini KM, et al. Enhanced insulin sensitivity of gene-targeted mice lacking functional KCNQ1. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;(1310):22–26. doi: 10.1152/ajpregu.90839.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden La, Caplan MJ. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochemistry International. 1996;29(4):335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Buccafusca R, et al. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels. [Accessed July 6, 2014];Molecular genetics and metabolism. 2008 95(1–2):81–95. doi: 10.1016/j.ymgme.2008.05.008. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18675571. [DOI] [PubMed] [Google Scholar]
- Carrasco N. Iodide transport in the thyroid gland. Biochimica et Biophysica Acta - Reviews on Biomembranes. 1993;1154(1):65–82. doi: 10.1016/0304-4157(93)90017-i. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8507647. [DOI] [PubMed] [Google Scholar]
- Chau JFL, et al. Sodium/myo-inositol cotransporter-1 is essential for the development and function of the peripheral nerves. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2005;19(13):1887–1889. doi: 10.1096/fj.05-4192fje. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16174787. [DOI] [PubMed] [Google Scholar]
- Chen Y-H, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. [Accessed November 10, 2013];Science (New York, N.Y.) 2003 299(5604):251–254. doi: 10.1126/science.1077771. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12522251. [DOI] [PubMed] [Google Scholar]
- Choi E, Abbott GW. A shared mechanism for lipid- and beta-subunit-coordinated stabilization of the activated K+ channel voltage sensor. The FASEB Journal. 2010;24(5):1518–1524. doi: 10.1096/fj.09-145219. Available at: papers3://publication/doi/10.1096/fj.09-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady MJ, et al. Identification of a novel Na+/myo-inositol cotransporter. Journal of Biological Chemistry. 2002;277(38):35219–35224. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]
- Cross BM, et al. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PloS one. 2013;8(6):e67348. doi: 10.1371/journal.pone.0067348. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3696057&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379(6564):458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- Davanzo P, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2001;24(4):359–369. doi: 10.1016/S0893-133X(00)00207-4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11182531. [DOI] [PubMed] [Google Scholar]
- Dohán O, et al. The Sodium/Iodide Symporter (NIS): Characterization, Regulation, and Medical Significance. Endocrine Reviews. 2003;24(1):48–77. doi: 10.1210/er.2001-0029. Available at: http://press.endocrine.org/doi/abs/10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- Durr G, et al. The medial-Golgi Ion Pump Pmr1 Supplies the Yeast Secretory Pathway with Ca2+ and Mn2+ Required for Glycosylation, Sorting, and Endoplasmic Reticulum-Associated Protein Degradation. Molecular Biology of the Cell. 1998;9(5):1149–1162. doi: 10.1091/mbc.9.5.1149. Available at: http://www.molbiolcell.org/cgi/doi/10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faham S, et al. The Crystal Structure of a Sodium Galactose Transporter Reveals Mechanistic Insights into Na+/Sugar Symport. Science. 2008;321(5890):810–814. doi: 10.1126/science.1160406. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, et al. Store-Independent Activation of Orai1 by SPCA2 in Mammary Tumors. Cell. 2010;143(1):84–98. doi: 10.1016/j.cell.2010.08.040. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0092867410010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. Available at: http://www.nature.com/doifinder/10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Frohlich H, et al. Hypothyroidism of gene-targeted mice lacking Kcnq1. Pflugers Arch. 2011;461(1):45–52. doi: 10.1007/s00424-010-0890-5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3644480&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahammer F, et al. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120(6):1363–1371. doi: 10.1053/gast.2001.24053. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0016508501333565. [DOI] [PubMed] [Google Scholar]
- Grunnet M, et al. KCNE4 is an inhibitory subunit to the KCNQ1 channel. The Journal of Physiology. 2002;542(1):119–130. doi: 10.1113/jphysiol.2002.017301. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2290389&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami S, et al. Cell volume and membrane stretch independently control K+ channel activity. The Journal of physiology. 2009;587:2225–2231. doi: 10.1113/jphysiol.2008.163550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harchi AEl, et al. The S140G KCNQ1 atrial fibrillation mutation affects “IKs” profile during both atrial and ventricular action potentials. [Accessed December 4, 2013];J Physiol Pharmacol. 2010 1(6):759–764. Available at: http://www.jpp.krakow.pl/journal/archive/12_10/pdf/759_12_10_article.pdf. [PubMed] [Google Scholar]
- Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. [Accessed March 20, 2014];Molecular psychiatry. 2005 10(1):117–126. doi: 10.1038/sj.mp.4001618. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15558078. [DOI] [PubMed] [Google Scholar]
- Hayashi E, Maeda T, Tomita T. The effect of myo-inositol deficiency on lipid metabolism in rats. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1974;360(2):134–145. doi: 10.1016/0005-2760(74)90163-5. Available at: http://linkinghub.elsevier.com/retrieve/pii/0005276074901635. [DOI] [PubMed] [Google Scholar]
- Hegsted DM, et al. Inositol deficiency: an intestinal lipodystrophy in the gerbil. The Journal of nutrition. 1973;103(2):302–307. doi: 10.1093/jn/103.2.302. Available at: http://www.ncbi.nlm.nih.gov/pubmed/4119310. [DOI] [PubMed] [Google Scholar]
- Heitzmann D, et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. The Journal of physiology. 2004;561(Pt 2):547–557. doi: 10.1113/jphysiol.2004.075168. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1665368&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzmann D, et al. KCNE Beta Subunits Determine pH Sensitivity of KCNQ1 Potassium Channels. Cellular Physiology and Biochemistry. 2007;19(1–4):21–32. doi: 10.1159/000099189. Available at: http://www.karger.com/doi/10.1159/000099189. [DOI] [PubMed] [Google Scholar]
- Hersey, Sachs Gastric acid secretion. Physiological Reviews. 1995;75(1):155–189. doi: 10.1152/physrev.1995.75.1.155. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7831396. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Science’s STKE: signal transduction knowledge environment. 2001;2001(111):re19. doi: 10.1126/stke.2001.111.re19. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11734659. [DOI] [PubMed] [Google Scholar]
- Hitomi K, Tsukagoshi N. cDNA sequence for rkST1, a novel member of the sodium ion-dependent glucose cotransporter family. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1994;1190(2):469–472. doi: 10.1016/0005-2736(94)90110-4. Available at: http://linkinghub.elsevier.com/retrieve/pii/0005273694901104. [DOI] [PubMed] [Google Scholar]
- Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. [Accessed January 8, 2014];Annual review of nutrition. 1986 6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. Available at: http://www.annualreviews.org/doi/pdf/10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- Hou X, et al. Crystal Structure of the Calcium Release-Activated Calcium Channel Orai. Science. 2012;338(6112):1308–1313. doi: 10.1126/science.1228757. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL. Regulation of ROMK trafficking and channel activity. Current opinion in nephrology and hypertension. 2001;10(5):693–698. doi: 10.1097/00041552-200109000-00022. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11496066. [DOI] [PubMed] [Google Scholar]
- Indriati DW, et al. Quantitative Localization of Cav2.1 (P/Q-Type) Voltage-Dependent Calcium Channels in Purkinje Cells: Somatodendritic Gradient and Distinct Somatic Coclustering with Calcium-Activated Potassium Channels. Journal of Neuroscience. 2013;33(8):3668–3678. doi: 10.1523/JNEUROSCI.2921-12.2013. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.2921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nature reviews. Neuroscience. 2000 Oct;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jin X-T, Galvan A, et al. Localization and Function of GABA Transporters GAT-1 and GAT-3 in the Basal Ganglia. Frontiers in Systems Neuroscience. 2011 Jul;5:63. doi: 10.3389/fnsys.2011.00063. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21847373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X-T, Paré JF, Smith Y. Differential localization and function of GABA transporters, GAT-1 and GAT-3, in the rat globus pallidus. European Journal of Neuroscience. 2011;33(8):1504–1518. doi: 10.1111/j.1460-9568.2011.07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HM, et al. Cloning of the cDNA for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. The Journal of biological chemistry. 1992;267(9):6297–6301. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1372904. [PubMed] [Google Scholar]
- Labro AJ, et al. The S4-S5 Linker of KCNQ1 Channels Forms a Structural Scaffold with the S6 Segment Controlling Gate Closure. Journal of Biological Chemistry. 2011;286(1):717–725. doi: 10.1074/jbc.M110.146977. Available at: http://www.jbc.org/cgi/doi/10.1074/jbc.M110.146977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MP, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. Journal of Clinical Investigation. 2000;106(12):1447–1455. doi: 10.1172/JCI10897. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=387258&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB, MacKinnon R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science. 2005;309(5736):897–903. doi: 10.1126/science.1116269. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Loussouarn G. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. The EMBO Journal. 2003;22(20):5412–5421. doi: 10.1093/emboj/cdg526. Available at: http://emboj.embopress.org/cgi/doi/10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussouarn G. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. The EMBO Journal. 2003;22(20):5412–5421. doi: 10.1093/emboj/cdg526. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14532114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubrich B, van Calker D. Inhibition of the high affinity myo-inositol transport system: a common mechanism of action of antibipolar drugs? Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1999;21(4):519–529. doi: 10.1016/S0893-133X(99)00037-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10481836. [DOI] [PubMed] [Google Scholar]
- Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395(6705):900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- Mercer JC, et al. Large Store-operated Calcium Selective Currents Due to Co-expression of Orai1 or Orai2 with the Intracellular Calcium Sensor, Stim1. Journal of Biological Chemistry. 2006;281(34):24979–24990. doi: 10.1074/jbc.M604589200. Available at: http://www.jbc.org/lookup/doi/10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelle J, Goffin E, Devuyst O. Molecular Physiology of Water Balance. The New England journal of medicine. 2015;373(2):196. doi: 10.1056/NEJMc1505505. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26154806. [DOI] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. European journal of endocrinology / European Federation of Endocrine Societies. 2004;151(Suppl):U25–U37. doi: 10.1530/eje.0.151u025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15554884. [DOI] [PubMed] [Google Scholar]
- Nesin V, et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(11):4197–4202. doi: 10.1073/pnas.1312520111. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24591628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou JW, et al. Ca2+- and thromboxane-dependent distribution of MaxiK channels in cultured astrocytes: from microtubules to the plasma membrane. Glia. 2009;57(12):1280–1295. doi: 10.1002/glia.20847. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2713352&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G, Abbott GW. The Role of S4 Charges in Voltage-dependent and Voltage-independent KCNQ1 Potassium Channel Complexes. [Accessed March 21, 2013];The Journal of General Physiology. 2007 129(2):121–133. doi: 10.1085/jgp.200609612. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2154355&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PL, Carafoli E. Ion motive ATPasesIUbiquity, properties, and significance to cell function. Trends in Biochemical Sciences. 1987a;12:146–150. Available at: http://linkinghub.elsevier.com/retrieve/pii/0968000487900715. [Google Scholar]
- Pedersen PL, Carafoli E. Ion motive ATPases. II. Energy coupling and work output. Trends in Biochemical Sciences. 1987b May;12:186–189. Available at: http://linkinghub.elsevier.com/retrieve/pii/0968000487900909. [Google Scholar]
- Porcellati F, et al. Alternate splicing in human Na+-MI cotransporter gene yields differentially regulated transport isoforms. The American journal of physiology. 1999;276:C1325–C1337. doi: 10.1152/ajpcell.1999.276.6.C1325. [DOI] [PubMed] [Google Scholar]
- Porcellati F, et al. Human Na(+)-myo-inositol cotransporter gene: alternate splicing generates diverse transcripts. The American journal of physiology. 1998;274(5 Pt 1):C1215–C1225. doi: 10.1152/ajpcell.1998.274.5.C1215. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9612208. [DOI] [PubMed] [Google Scholar]
- Prakriya M, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443(7108):230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Purtell K, et al. The KCNQ1-KCNE2 K+ channel is required for adequate thyroid I- uptake. The FASEB Journal. 2012;26(8):3252–3259. doi: 10.1096/fj.12-206110. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22549510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, King EC, et al. Genetic dissection reveals unexpected influence of subunits on KCNQ1 K+ channel polarized trafficking in vivo. The FASEB Journal. 2011;25(2):727–736. doi: 10.1096/fj.10-173682. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3023397&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. [Accessed November 12, 2012];Nature medicine. 2009 15(10):1186–1194. doi: 10.1038/nm.2029. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2790327&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, Kanda Va, et al. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. [Accessed November 11, 2012];FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011 25(12):4264–4273. doi: 10.1096/fj.11-187609. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21859894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, et al. Targeted Deletion of Kcne2 Causes Gastritis Cystica Profunda and Gastric Neoplasia X. Guan, ed. PLoS ONE. 2010;5(7):e11451. doi: 10.1371/journal.pone.0011451. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2897890&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TK, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. The Journal of biological chemistry. 2006;281(33):23740–23747. doi: 10.1074/jbc.M604155200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16754665. [DOI] [PubMed] [Google Scholar]
- Rundén-Pran E, et al. BK channel activity determines the extent of cell degeneration after oxygen and glucose deprivation: A study in organotypical hippocampal slice cultures. Neuroscience. 2002;112(2):277–288. doi: 10.1016/s0306-4522(02)00092-1. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, et al. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKS potassium channel. Nature. 1996;384(6604):80–83. doi: 10.1038/384080a0. Available at: http://www.nature.com/doifinder/10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schneider S. Inositol transport proteins. FEBS Letters. 2015;589(10):1049–1058. doi: 10.1016/j.febslet.2015.03.012. Available at: http://linkinghub.elsevier.com/retrieve/pii/S001457931500188X. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403(6766):196–199. doi: 10.1038/35003200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10646604. [DOI] [PubMed] [Google Scholar]
- Sesti F, Goldstein SAN. Single-Channel Characteristics of Wild-Type IKs Channels and Channels formed with Two MinK Mutants that Cause Long QT Syndrome. The Journal of General Physiology. 1998;112(6):651–663. doi: 10.1085/jgp.112.6.651. Available at: http://www.jgp.org/cgi/doi/10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G, et al. Valproate decreases inositol biosynthesis. Biological Psychiatry. 2004;56(11):868–874. doi: 10.1016/j.biopsych.2004.08.027. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15576064. [DOI] [PubMed] [Google Scholar]
- Singh H, et al. MaxiK channel interactome reveals its interaction with GABA transporter 3 and heat shock protein 60 in the mammalian brain. Neuroscience. 2016 Jan;317:76–107. doi: 10.1016/j.neuroscience.2015.12.058. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0306452216000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, et al. Orai1 and STIM reconstitute store-operated calcium channel function. Journal of Biological Chemistry. 2006;281(30):20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, et al. Expression of the sodium iodide symporter in human kidney. Kidney International. 2001;59(3):1013–1023. doi: 10.1046/j.1523-1755.2001.0590031013.x. Available at: http://www.nature.com/ki/journal/v59/n3/pdf/4492116a.pdf. [DOI] [PubMed] [Google Scholar]
- Suh B-C, Hille B. PIP 2 Is a Necessary Cofactor for Ion Channel Function: How and Why? Annual Review of Biophysics. 2008;37(1):175–195. doi: 10.1146/annurev.biophys.37.032807.125859. Available at: http://www.annualreviews.org/doi/abs/10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester DJ, et al. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. [Accessed December 4, 2013];Heart rhythm: the official journal of the Heart Rhythm Society. 2005 2(5):507–517. doi: 10.1016/j.hrthm.2005.01.020. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15840476. [DOI] [PubMed] [Google Scholar]
- Than BLN, et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2014;33(29):3861–3868. doi: 10.1038/onc.2013.350. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3935979&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinel N. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. The EMBO Journal. 2000;19(23):6326–6330. doi: 10.1093/emboj/19.23.6326. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=305874&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L, et al. MaxiK channel and cell signalling. Pflugers Archiv European Journal of Physiology. 2014;466(5):875–886. doi: 10.1007/s00424-013-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Sanguinetti MC. Voltage-dependent inactivation of the human K + channel KvLQT1 is eliminated by association with minimal K + channel (minK) subunits. The Journal of Physiology. 1998;510(1):37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. Available at: http://doi.wiley.com/10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki H, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. [Accessed November 29, 2013];Nature genetics. 2008 40(9):1098–1102. doi: 10.1038/ng.208. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18711366. [DOI] [PubMed] [Google Scholar]
- Vanoevelen J, et al. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. Journal of Biological Chemistry. 2005;280(24):22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- Vayre L, et al. Immunohistochemical analysis of Na+/I- symporter distribution in human extra-thyroidal tissues. European Journal of Endocrinology. 1999;141(4):382–386. doi: 10.1530/eje.0.1410382. [DOI] [PubMed] [Google Scholar]
- Vergara C, et al. Calcium-activated potassium channels. Current Opinion in Neurobiology. 1998;8(3):321–329. doi: 10.1016/s0959-4388(98)80056-1. Available at: http://www.sciencedirect.com/science/article/pii/S0959438898800561. [DOI] [PubMed] [Google Scholar]
- Vucic E, et al. Kir1.1 (ROMK) and Kv7.1 (KCNQ1/KvLQT1) are essential for normal gastric acid secretion: importance of functional Kir1.1. Pflügers Archiv - European Journal of Physiology. 2015;467(7):1457–1468. doi: 10.1007/s00424-014-1593-0. Available at: http://link.springer.com/10.1007/s00424-014-1593-0. [DOI] [PubMed] [Google Scholar]
- Warth R, et al. The role of KCNQ1/KCNE1 K(+) channels in intestine and pancreas: lessons from the KCNE1 knockout mouse. [Accessed December 4, 2013];Pflügers Archiv: European journal of physiology. 2002 443(5–6):822–828. doi: 10.1007/s00424-001-0751-3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11889581. [DOI] [PubMed] [Google Scholar]
- Willmroth F, et al. Sodium-myo-inositol co-transporter (SMIT-1) mRNA is increased in neutrophils of patients with bipolar 1 disorder and down-regulated under treatment with mood stabilizers. [Accessed November 23, 2013];The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2007 10(1):63–71. doi: 10.1017/S1461145705006371. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16420717. [DOI] [PubMed] [Google Scholar]
- Xiang M, Mohamalawari D, Rao R. A novel isoform of the secretory pathway Ca2+,Mn 2+-ATPase, hSPCA2, has unusual properties and is expressed in the brain. Journal of Biological Chemistry. 2005;280(12):11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- Yasuda K, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. [Accessed November 22, 2013];Nature genetics. 2008 40(9):1092–1097. doi: 10.1038/ng.207. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18711367. [DOI] [PubMed] [Google Scholar]
- Zhou J-B, et al. Variants in KCNQ1, AP3S1, MAN2A1, and ALDH7A1 and the risk of type 2 diabetes in the Chinese Northern Han population: a case-control study and meta-analysis. [Accessed December 4, 2013];Medical science monitor: international medical journal of experimental and clinical research. 2010 16(6):BR179–BR183. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20512086. [PubMed] [Google Scholar]