Abstract
Photobiomodulation (PBM) also known as low-level laser (or light) therapy (LLLT), has been known for almost 50 years but still has not gained widespread acceptance, largely due to uncertainty about the molecular, cellular, and tissular mechanisms of action. However, in recent years, much knowledge has been gained in this area, which will be summarized in this review. One of the most important chromophores is cytochrome c oxidase (unit IV in the mitochondrial respiratory chain), which contains both heme and copper centers and absorbs light into the near-infra-red region. The leading hypothesis is that the photons dissociate inhibitory nitric oxide from the enzyme, leading to an increase in electron transport, mitochondrial membrane potential and ATP production. Another hypothesis concerns light-sensitive ion channels that can be activated allowing calcium to enter the cell. After the initial photon absorption events, numerous signaling pathways are activated via reactive oxygen species, cyclic AMP, NO and Ca2+, leading to activation of transcription factors. These transcription factors can lead to increased expression of genes related to protein synthesis, cell migration and proliferation, anti-inflammatory signaling, anti-apoptotic proteins, antioxidant enzymes. Stem cells and progenitor cells appear to be particularly susceptible to LLLT.
Keywords: Low Level Light Therapy, Mechanism, Mitochondria, Cytochrome c oxidase, Photobiomodulation, Light sensitive ion channels
HISTORICAL INTRODUCTION
The first evidence of the action of low-level laser irradiation came from the experiments of Dr. Endre Mester, at the Semmelweis Medical University (Hungary) in 1967. The experiment consisted of shaving the back of mice and implanting a tumor via an incision in the skin. Mester applied light from a ruby laser (694 nm) in an attempt to repeat one of the experiments described by McGuff in Boston [1]. McGuff had used the newly discovered ruby laser to cure malignant tumors both in rats and also tested it in human patients. Unfortunately (or perhaps fortunately for scientific discovery), Mester’s laser had only a small fraction of the power possessed by McGuff’s laser. Therefore Mester failed to cure any tumors, but did observe a faster rate of hair growth in the treated mice compared to the controls [2], calling this effect "laser biostimulation". He later used a HeNe laser (632.8 nm) to stimulate wound healing in animals, as well as in clinical studies [3]. For several decades, the profession believed that coherent laser light was necessary, but as of today, non-coherent light sources such as light emitting diodes (LED) have proved to be just as efficient as lasers in promoting photobiomodulation (PBM) [4].
Low-level light therapy (LLLT) or PBM consists of the application of light with the purpose of promoting tissue repair, decreasing inflammation, and producing analgesia, usually using a low-power light source (laser or LED) [5]. Because of the low power, (usually below 500 mW depending on the target tissue) the treatment causes no evident temperature rise in the treated tissue and, therefore, no significant change in the gross tissue structure [6]. PBM/LLLT differs from other light-based treatments because it does not ablate and is not based on heating. It also differs from photodynamic therapy (PDT), which is based on the effect of light to excite exogenously delivered chromophores to produce toxic reactive oxygen species (ROS) [7].
With the advantage of being non-invasive, the applications of PBM are broad, going from pain relief to promoting the recovery of tendinopathies, nerve injuries, osteoarthritis and wound healing. The complete mechanism of action is still elusive, but the knowledge that has been gained so far is the subject of the present review. The importance of parameters in PBM will be discussed, together with the possible chromophores or photoacceptors, signaling molecules produced after photon absorption, transcription factors that may be activated to account for the lasting effects of a brief light exposure, downstream effector molecules that follow on, and specific mechanisms that may be applicable to the different cells and tissues being treated with PBM.
PARAMETERS OF PBM
The light parameters and the doses applied are fundamental in PBM. The most important parameters regarding the light source and the light doses are described on the following tables (table 1 and table 2, respectively):
Table 1.
Description of the irradiation parameters.
| IRRADIATION PARAMETERS | ||
|---|---|---|
|
Irradiation Parameter |
Measurement unit | Description |
| Wavelength | nm | Light is an electromagnetic form of
energy with a wave-like behavior. Its wavelength is measured in nanometers (nm), and it is visible within the 400–700 nm range. |
| Irradiance | W cm−2 | It can also be called Power Density
or Intensity, and corresponds to the power (in W) divided by the area (in cm−2). |
|
Pulse Structure |
Peak Power (W) Pulse frequency (Hz) Pulse width (s) Duty cycle (%) |
If the beam is pulsed, the Power should
be called Average Power, which is calculated as follows: Average Power (W) = Peak Power (W) x pulse width (s) x pulse frequency (Hz) |
| Coherence | Coherence length depends on spectral bandwidth |
Coherent light produces laser speckle,
which is believed to play an important role on photobiomodulation interaction with cells and organelles. |
| Polarization | Linear polarized or circular polarized |
Polarized light is known to lose its polarity
in highly scattering media such as biological tissues, therefore this property is not considered very often on the effects of PBM. |
Table 2.
Description of the light dose parameters.
| LIGHT DOSE PARAMETERS | ||
|---|---|---|
|
Irradiation Parameter |
Measurement unit |
Description |
| Energy | Joules (J) | It cannot be mistook as dose, as it
assumes reciprocity (the inverse relationship between power and time). It is calculated as: Energy (J) = Power (W) x Time (s) |
| Energy Density | J cm−2 | This is n important descriptor of dose, but
it could be unreliable when we consider that it assumes a reciprocity relationship between irradiance and time. |
| Irradiation Time | s | Possibly the best way to prescribe and to
record PBM would be to define the four parameters of table 1 and then define the irradiation time as the real "dose". |
|
Treatment Interval |
Hours, days or weeks |
Different time intervals may result in
different outcomes, but more data need to be gathered in order to define the extent of the differences between them. |
Low level light therapy refers to the use of light in the red or near-infrared region, with wavelengths usually in the range of 600 to 700nm and 780 to 1100 nm, and the laser or LEDs typically having an irradiance or power density between 5 mW cm−2 to 5 W cm−2. This type of irradiation can be a continuous wave or a pulsed light consisting of a relatively low-density beam (0.04 to 50 J cm−2), but the output power can vary widely from 1 mW up to 500 mW in order not to allow thermal effects [8]. The wavelength range between 700 and 780 nm has been found to be rather ineffective as it coincides with a trough in the absorption spectrum of cytochrome c oxidase (see later). Moreover red/NIR light is chosen because its penetration through tissue is maximal in this wavelength range, due to lower scattering and absorption by tissue chromophores. Although for many years it was thought that the monochromatic nature and coherence of laser light provided some sort of added benefit over non-coherent LED light, this view is no longer widely held. Continuous or pulsed light sources have both been used. The studies performed for PBM on acute pain and pre-operative analgesia show that a single treatment (usually only 30–60 seconds) is enough to cause analgesia, while for chronic pain and some degenerative conditions, more sessions are required [5].
It is known that if the incorrect parameters are applied, the treatment is likely to be ineffective. There is a biphasic dose response curve (or the phenomenon known as hormesis) in which when too low or too high doses (fluence (J/cm2), irradiance (mW/cm2), delivery time, or number of repetitions) can lead to no significant effect or, sometimes, excessive light delivery can lead to unwanted inhibitory effects [8], [9]. This biphasic response follows the "Arndt-Schulz Law" (which states that weak stimuli slightly accelerate vital activity, stronger stimuli raise it further until a peak is reached, whereas even stronger stimuli suppress it until a negative response is achieved), and has been demonstrated several times in low level light works [10]–[16].
For instance, Bolton irradiated macrophages with the same energy density (in J cm−2) but with different irradiances (W cm−2), and observed different results between the two conditions [17]; Karu and Kolyakov, in 2005, found that the stimulation of DNA synthesis rate is dependent on light intensity at a constant energy density of 0.1 J cm−2 with a clear maximum at 0.8 mW cm−2 [18]; Orion and co-workers worked with a constant energy density and different irradiances on an infarct model in rats after induced heart attack, and found that the beneficial effects were obtained at 5 mW cm−2, while with irradiances as low as 2.5 mW cm−2 or as high as 25 mW cm−2 there were significantly less effects [11]; finally, Lanzafame and collaborators used a fixed energy density of 5 J cm−2 and variable irradiances, ranging from 0.7 to 40 mW cm−2, observing that only with 8 mW cm−2 there were improvements on pressure ulcers in the treated mice [10].
There were some studies with constant irradiance and varying fluences. al-Watban and Andres, for instance, observed the effects of He-Ne laser on the proliferation of Chinese hamster ovary and human fibroblast. The light was delivered at a constant irradiance of 1.25 mW cm−2, and a biphasic dose response was found with a peak at 0.18 J cm−2 [19]. Zhang and collaborators also found a biphasic dose response when they observed a maximum increase in human fibroblast cells after irradiation of light at 628 nm with fluence of 0.88 J cm−2, while there was a marked reduction in the proliferation rate at 9 J cm−2 [20].
Regarding the time interval between treatments, Brondon and colleagues found that the best results for human HEP-2 and murine L-929 cells proliferation rates were achieved with two treatments per day, in comparison with one or four treatments per day. They used an LED with light at 670 nm and irradiance fixed at 10 mW cm−2, and each treatment consisted on the delivery of 5 J cm−2 (the course was stopped after 50 J cm−2 had been delivered) [21].
There are also some systematic reviews and meta analyses of randomized, double-blind, placebo-controlled, clinical trials (RCTs) available in the literature. We can give as an example the review from Bjordal, who identified 14 RCTs of suitable methodological quality. 4 of them failed to report significant effects because the irradiance was either too low or too high, or because there was an insufficient delivery of energy [22]. Another review was performed by Tumilty with 25 RCTs of tendinopathies, 55% of which failed to produce positive outcomes because of an excessive irradiance delivery in comparison with the guidelines set by the World Association for Laser Therapy [23].
As we have seen, at low doses (up to 2 J cm−2), PBM stimulates proliferation, whereas at higher doses (16 J cm−2 or higher) PBM is suppressive, pointing to the dose dependence of biological responses after light exposure [24]. Other authors, however, have observed stimulating effects outside the cited range [25], [26]. A number of different laser light sources, including helium-neon, ruby, and galliumaluminum-arsenide, have been used to deliver PBM in different treatments and on different schedules.
Many researchers fail to consider the importance of selecting the optimum parameters, or they do not have the necessary instrumentation or trained personnel to measure them accurately, resulting in treatment failures. Another cause of failure occurs whenever the terms are misused or wrongly reported. For instance, energy (J) or energy density (J cm−2) are both usually referred to as "dose", but they are, in fact, different calculations, as demonstrated in table 2 [27].
MOLECULAR MECHANISMS OF PBM
Chromophores
Cytochrome c oxidase
Cytochrome C oxidase (Cox) is the terminal enzyme of the electron transport chain, mediating the electron transfer from cytochrome c to molecular oxygen. Several lines of evidence show that Cox acts as a photoacceptor and transducer of photosignals in the red and near-infrared regions of the light spectrum [28]. It seems that PBM increases the availability of electrons for the reduction of molecular oxygen in the catalytic center of Cox, increasing the mitochondrial membrane potential (MMP) and the levels of ATP, cAMP and ROS as well [29].
PBM increases the activity of complexes I, II, III, IV and succinate dehydrogenase in the electron transfer chain. Cox is known as complex IV and, as mentioned before, appears to be the primary photoacceptor. This assumption is supported by the increased oxygen consumption during low-level light irradiation (the majority of the oxygen consumption of a cell occurs at complex IV in the mitochondria), and by the fact that sodium azide, a Cox inhibitor, prevents the beneficial effect of PBM. Besides ATP and cAMP, nitric oxide (NO) level is increased, either by release from metal complexes in Cox (Cox has two heme and two copper centers) or by up-regulation of Cox activity as a nitrite reductase [30].
In fact, it was proposed that PBM might work through the photodissociation of NO from Cox, thereby reversing the mitochondrial inhibition of cellular respiration due to excessive NO binding [31]. NO is photodissociated from its binding sites on the heme iron and copper centers from Cox, where it competes with oxygen and reduces the necessary enzymatic activity. This allows an immediate influx of oxygen and, thus, the resumption of respiration and generation of reactive oxygen species. NO can also be photo-released from other intracellular sites, such as nitrosylated hemoglobin and myoglobin [32].
Retrograde mitochondrial signaling
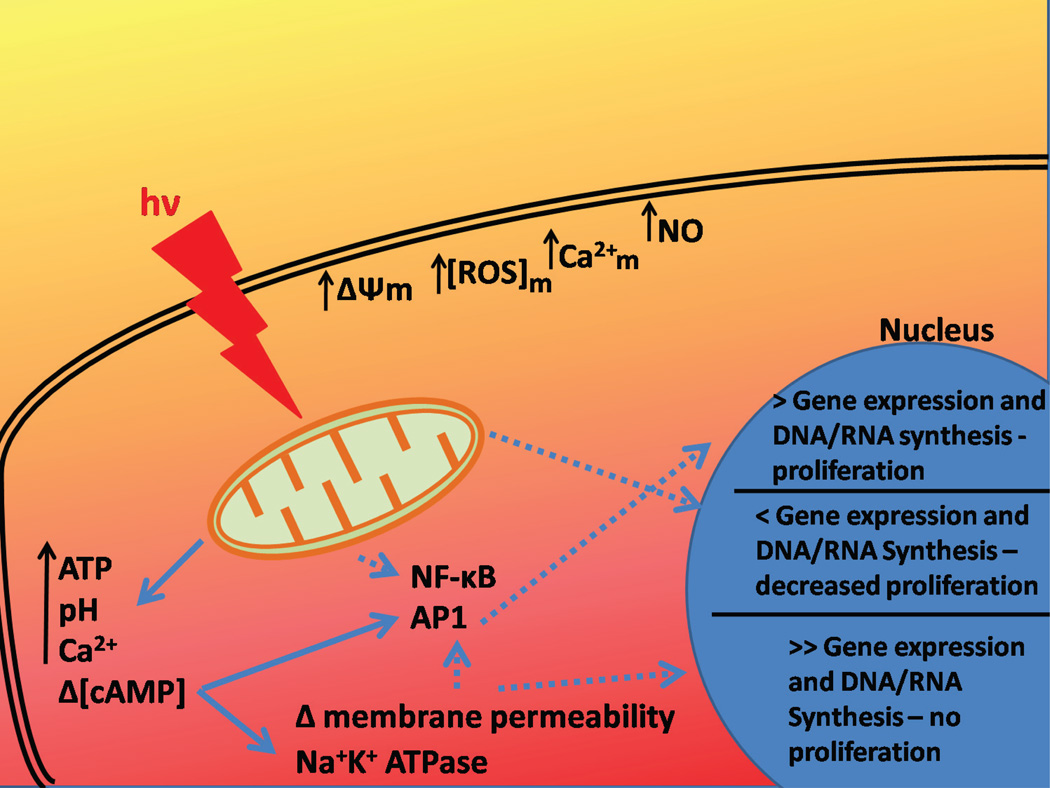
One of the most accepted mechanisms for light-cell interaction was proposed by Karu[33], referring to the retrograde mitochondrial signaling that occurs with light activation in the visible and infrared range (Figure 1). According to Karu, the first step is the absorption of a photon with energy hv by the chromophore Cox. This interaction increases mitochondrial membrane potential (Δψm), causing an increase in the synthesis of ATP and changes in the concentrations of reactive oxygen species (ROS), Ca2+ and NO. Furthermore, there is a communication between mitochondria and the nucleus, driven by changes in the mitochondria ultrastructure, i.e. changes in the fission-fusion homeostasis in a dynamic mitochondrial network. The alteration in the mitochondrial ultrastructure induces changes in ATP synthesis, in the intracellular redox potential, in the pH and in cyclic adenosine monophosphate (cAMP) levels. Activator protein-1 (AP1) and NF-κB have their activities altered by changes in membrane permeability and ion flux at the cell membrane. Some complementary routes were also suggested by Karu, such as the direct up-regulation of some genes [34].
Figure 1.
Scheme of mitochondrial retrograde signaling pathways as proposed by Karu. The main pathway is represented by continuous arrows, and the complementary ones are represented by segmented arrows.
Light sensitive ion channels
The most well-known ion channels that can be directly gated by light are the channelrhodopsins (ChRs), which are seven-transmembrane-domain proteins that can be naturally found in algae providing them with light perception. Once activated by light, these cation channels open and depolarize the membrane. They are currently being applied in neuroscientific research in the new discipline of optogenetics [35].
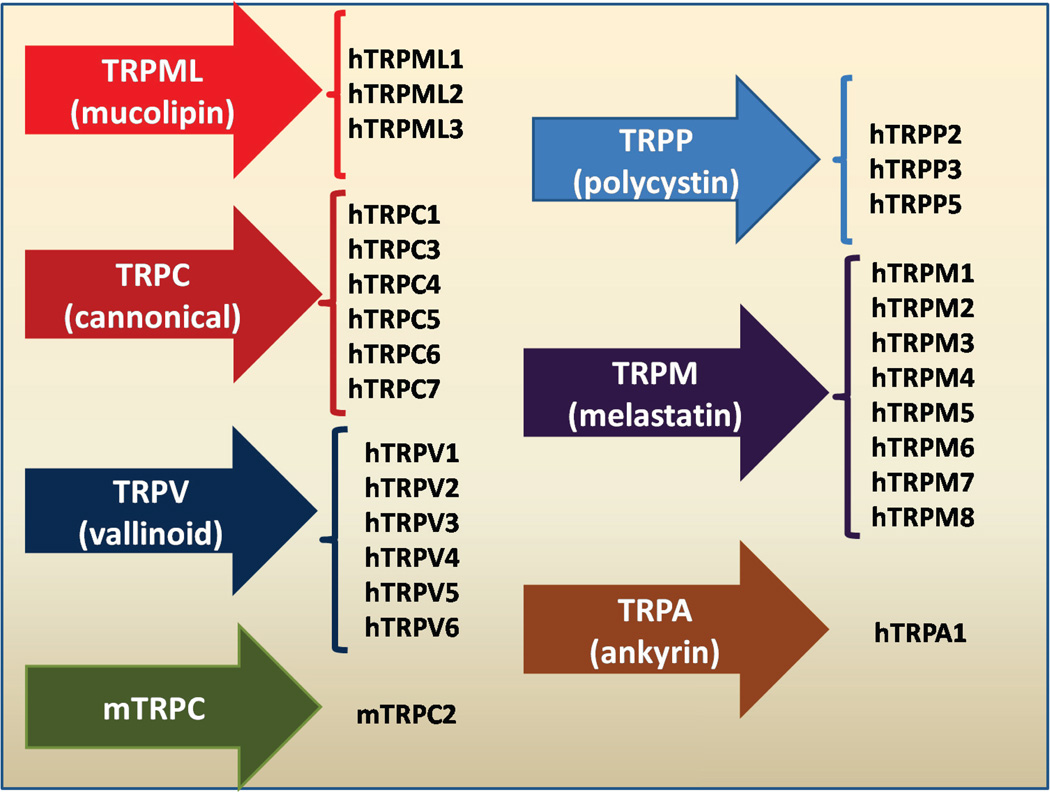
However, members of another broad group of ion-channels are now known to be light sensitive [36]. These channels are called "transient receptor potential" (TRP) channels as they were first discovered in a Drosophila mutant [36] and are responsible for vision in insects. There are now at least 50 different known TRP isoforms distributed amongst seven subfamilies [37], namely the TRPC (‘Canonical’) subfamily, the TRPV (‘Vanilloid’), the TRPM (‘Melastatin’), the TRPP (‘Polycystin’), the TRPML (‘Mucolipin’), the TRPA (‘Ankyrin’) and the TRPN (‘NOMPC’) subfamilies (see Figure 2). A wide range of stimuli modulate the activity of different TRP such as light, heat, cold, sound, noxious chemicals, mechanical forces, hormones, neurotransmitters, spices, and voltage. TRP are calcium channels modulated by phosphoinositides [38].
Figure 2.
All the seven subfamilies of Transient Receptor Potential Channels (TRP).
The evidence that light mediated activation of TRP is responsible for some of the mechanisms of action of PBM is somewhat sparse at present, but is slowly mounting. Mast cells are known to accumulate at the site of skin wounds, and there is some degree of evidence suggesting that these cells play a role in the biological effects of laser irradiation on promoting wound healing. Yang and co-workers demonstrated that after laser irradiation (532 nm), the intracellular [Ca2+] was increased and, as a consequence, there was a release of histamine. If the TRPV4 inhibitor, ruthenium red, was used, the histamine release was blocked, indicating the central role of these channels in promoting histamine-dependent wound healing after laser irradiation [39].
It seems that TRPV1 ion channels are involved in the degranulation of mast cells and laser-induced mast cell activation. It was demonstrated that capsaicin, temperatures above 42°C and acidic pH could induce the expression of TRPV1 in oocytes, and these ion channels can be activated by green light (532 nm) in a power-dependent manner, although blue and red light were not able to activate them [40]. Infrared light (2,780 nm) attenuates TRVP1 activation by capsaicin in cultured neurons, decreasing the generation of pain stimuli. TRPV4 is also attenuated by laser light, but the antinociceptive effect was less intense, therefore the antinociception in this model is mainly dependent on TRPV1 inhibition [41] The stimulation of neurons with pulsed infrared light (1,875 nm) is able to generate laser-evoked neuronal voltage variations and, in this case, TRPV4 channels were demonstrated to be the primary effectors of the chain reaction activated by the laser [42]. However, these effects after exposure to light above 1,500 nm might occur due to thermal effects, since water is the main absorber in this region of infrared spectrum. If it turns out that green light is primarily needed to activate ion channels then clinical applications may be limited due to lack of penetration into tissue.
Direct cell-free light-mediated effects on molecules
There have been some scattered reports that light can exert effects on some important molecules in cell free systems (in addition to the established effect on Cox). The latent form of transforming growth factor beta has been reported to be activated by light exposure [43]. Copper/Zinc Superoxide dismutase (Cu-Zn-SOD) from bovine erythrocytes that had been inactivated by exposure to pH 5.9 was reactivated by exposure to He-Ne laser light (632.7 nm) [44]. The same treatment also reactivated the heme-containing catalase. Amat et al. showed that irradiation of ATP in solution by 655 nm or 830 nm light appeared to produce changes in its enzyme reactivity, fluorescence and Mg2+ binding capacity [45]. However other workers were unable to repeat this somewhat surprising result [46].
Signaling Molecules
Adenosine triphosphate (ATP)
An increase in intracellular ATP is one of the most frequent and significant findings after PBM both in vitro and in vivo [47]. The stimulated synthesis of ATP is caused by an increased activity of Cox when activated by light. According to Ferraresi et al. [48], increased Cox activity is the mechanism of enhanced muscle performance when PBM is carried out before various types of exercises, for example. The authors found an increased ATP synthesis after LED (850±20 nm and 630±10 nm) therapy in different muscles (one with a predominantly aerobic metabolism, and other with mixed aerobic and glycolytic metabolism), just like previous data from Ferraresi et al. [49].
Extracellular ATP participates in a wide array of signaling pathways, known as purinergic signaling [50]. Originally discovered by Burnstock [51] as a non-adrenergic, non-cholinergic neurotransmitter, ATP purinergic signaling is mediated by P2Y G-protein-coupled receptors, and P2X ligand-gated ion channels [52]. ATP can be hydrolyzed to adenosine that carries out signals via the P1 G-protein-coupled receptor [53]. Up to the present date we are not aware of any studies that specifically show that extracellular (as opposed to intracellular) ATP or adenosine can be stimulated by PBM.
Cyclic AMP (cAMP)
Several workers have shown an increase in adenosine-3’,5’-cyclic-monophosphate (cAMP) after PBM [54], [55]. Although it is tempting to suppose that this increase in cAMP is a direct consequence of the rise in ATP caused by light, firm evidence for this connection is lacking. It has been reported that cAMP-elevating agents, i.e. prostaglandin E2, inhibit the synthesis of TNF and, therefore, down-regulate the inflammatory process. Lima and co-authors investigated the signaling pathways responsible for the anti-inflammatory action of PBM (660 nm, 4.5 J cm−2) in lung and airways. They found reduced TNF levels in the treated tissue, probably because of an increase in cAMP levels. Furthermore, the authors demonstrated that the inflammation caused by LPS or by TNF in mice lungs was inhibited by cAMP-elevating agents. Rolipram, a cAMP-elevating agent, acts through inhibition of the enzyme phosphodiesterase, but it does not share this mechanism with low level light [54].
cAMP exerts its cellular effects via activation of three different kinds of sensors: cAMP-dependent protein kinase A (PKA) which phosphorylates and activates cAMP response element-binding protein (CREB), which then binds to CRE domain on DNA and in turn activates genes [56];cyclic nucleotide-gated channels (CNGC) [57] and exchange proteins directly activated by cAMP (Epac) [58].
Reactive oxygen species (ROS)
It was shown that PBM can produce mitochondrial ROS leading to activation of the transcription factor nuclear factor kappa B (NF-κB), which can act as a redox-sensor. The fact that the addition of antioxidants inhibits the activation of NF-κB by 810 nm light reinforces this assumption [59].
ROS are one of the classic “Janus face” mediators; beneficial in low concentrations and harmful at high concentrations; beneficial at brief exposures and harmful at chronic long-term exposures [60]. ROS are produced at a low level by normal mitochondrial metabolism [61]. The concept of mitohormesis was introduced to describe the beneficial of low controlled amounts of oxidative stress in the mitochondria [62]. However when the mitochondrial membrane potential is altered either upwards or downwards, the amount of ROS is increased. In normal cells, absorption of light by Cox leads to an increase in mitochondrial membrane potential and a short burst of ROS is produced. However when the mitochondrial membrane potential is low because of pre-existing oxidative stress [63], excitotoxicity [64], or inhibition of electron transport [63], light absorption leads to an increase in mitochondrial membrane potential towards normal levels and the production of ROS is lowered.
There are many different cellular systems that are designed by evolution to detect excessive levels of ROS and activate transcription factors to produce extra levels of antioxidant defenses [65]. Hydrogen peroxide and lipid hydroperoxides [66] are thought to be the ROS most likely to carry out beneficial redox signaling by reversible oxidation of cysteine thiols in the sensor protein.
Calcium (Ca2+)
Changes in the mitochondrial ultrastructure may lead to alterations in Ca2+ concentration. The increment might be a result of Ca2+ influx from the extracellular environment and gated by the Ca2+ channel TRPV. There is evidence that cytosolic alkalinization can facilitate the opening of TRPV channels and, since laser irradiation can induce cellular alkalinization, PBM could induce TRPV opening and a consequent Ca2+ influx. In mast cells, this Ca2+ influx can mediate histamine release [67]. However it is also possible that light can directly activate TRPV channels as discussed above. It should be noted that PBM usually leads to an increase in intracellular Ca2+ as shown by fluorescent probes [68]. However when intracellular Ca2+ levels have been artificially raised (for instance by causing excitotoxicity with excess glutamate), then PBM can produce a drop in intracellular calcium and protect the neurons from dying [64]. The increase in calcium seen after PBM could also be a result of the release of Ca2+ from intracellular stores [69].
Calcium-sensitive signaling pathways are too numerous to cover in detail here, but include calcium sensitive enzymes like protein kinase C (PKC), calcium-calmodulin dependent kinase II (CamKII) and calcineurin (CaCN) [70], the extracellular calcium–sensing receptor (CaSR) [71], mitochondrial calcium signaling [72], calcium-sensitive adenylyl cyclase [73], and many others.
Nitric oxide (NO)
As mentioned above, NO is often found to be produced after PBM [74]. NO is a well-known vasodilator acting via stimulation of soluble guanylate cyclase to form cyclic-GMP (cGMP). cGMP activates protein kinase G, which causes reuptake of Ca2+ and opening of calcium-activated potassium channels. The fall in concentration of Ca2+ prevents myosin light-chain kinase (MLCK) from phosphorylating the myosin molecule, leading to relaxation of the smooth muscle cells in the lining of blood vessels and lymphatic vessels [75]. There are several other mechanisms by which NO could carry out signaling pathways, including activation of iron-regulatory factor in macrophages [76], modulation of proteins such as ribonucleotide reductase [77] and aconitase [78], stimulating ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase [79], and protein sulfhydryl group nitrosylation [80].
Activation of transcription factors
Nuclear factor kappa B (NFkB)
NF-κB is a transcription factor that regulates the expression of various genes related to many cellular functions, i.e. inflammatory and stress-induced responses and survival. Its activity is regulated by a negative feedback mediated by an inhibitor called IκB, which binds to NF-κB to inactivate it, or can undergo ubiquitination and go to proteasomal degradation in order to release NF-κB. The transcription factor, then, can be translocated to the nucleus and promote gene transcription. Several lines of evidence reveal that NF-κB is redox-sensitive, since ROS can directly activate it, or alternatively ROS could be involved in indirect activation of NF-κB via TNF, interleukin-1 (IL-1) and phorbol esters. PBM can boost ROS generation, and it was shown that light irradiation can induce NF-κB activation [59].
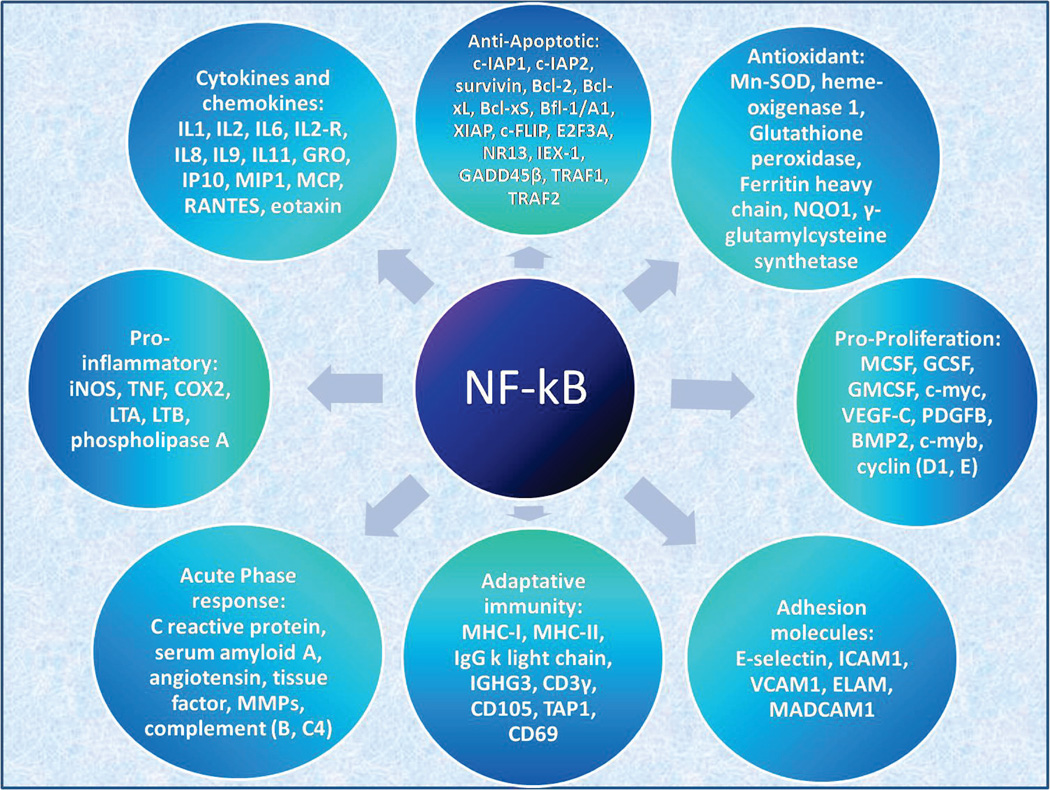
The increased NF-κB production after PBM stimuli leads to enhanced gene transcription that leads to reduced cell death, to cell proliferation, to cell migration [81] and enhanced neurological function. Figure 3 shows an overview of the different groups of genes that have NF-kB response elements.
Figure 3.
Overview of the different groups of genes and molecules that have NF-kB response elements. In principle these could be activated by NK-kB signaling pathway triggered by the ROS produced during LLLT
If the total energy density delivered is too high, however, the injury paradoxically tends to be exacerbated by increased oxidative stress, and an over-abundant activation of NF-κB. The biphasic dose effects of PBM are thought to occur due to an excessive generation of ROS, excessive production of NO, to the activation of some cytotoxic pathways, and to excessive NF-κB activation [82]. In addition, if the tissue is stressed or ischemic, mitochondria can synthesize NO that can displace oxygen from binding to Cox, but this leads to a reduced ATP synthesis and to an increased oxidative stress that can lead to inflammation when NF-κB is activated [83].
Classical mitochondrial inhibitors such as rotenone are known to decrease mitochondrial ATP levels, produce ROS and activate NF-κB. Low-level light still produces ROS and activates NF-κB, but in this case increases ATP levels. Antioxidants do not inhibit this ATP increase, suggesting that light augments the electron transport and potentially causes electron leakage (in the absence of antioxidants) and superoxide production [59].
RANKL
Receptor activator of nuclear factor kappa-B ligand (RANKL) is a transmembrane protein member of the TNF superfamily, involved in bone regeneration and remodeling (acting on osteoclast differentiation and activation). It is also a ligand for osteoprotegerin (OPG). The RANKL/OPG ratio determines whether bone is removed or formed during the remodeling process. The remodeling cycle consists in the increase in the expression of RANKL by osteoblasts, and subsequent binding to RANK receptor, which is highly expressed on osteoclastic membrane. This causes an expansion of the osteoclast progenitor pool, differentiation into mononucleated progenitor cells, increased survival, fusion into multinucleated osteoclasts and, finally, their activation. Osteoblasts can modulate this process by expressing OPG, which is a secretory soluble receptor and inhibitor of RANK receptor.
Parenti et al. investigated the RANKL/OPG ratio in osteoblast-like cells that were irradiated with GaAlAs laser (915 nm) using doses ranging from 1 to 50 J cm−2. Although the differences were not statistically significant, there was a trend for a rapid and transitory increase in the RANKL/OPG ratio for all the tested doses. It seems that this ratio after PBM depends on the tissue and on the parameters used, since there is evidence of an increase in RANKL/OPG ratio in human alveolar bone-derived cells irradiated with 780 nm light, while in rat calvarial cells irradiated with 650 nm light the results were the opposite [84].
Hypoxia inducible factor (HIF-1α)
HIF-1α is a protein involved in cellular adaptation to hypoxia. It is stabilized at low oxygen tensions, but in the presence of higher oxygen concentrations it is rapidly degraded by prolyl hydroxylase enzymes, which are oxygen-dependent. HIF-1α activates genes that are important to the cellular response to hypoxic conditions, such as vascular endothelial growth factor (VEGF), VEGF-receptor, glucose carrier (GLUT-1) and phosphoglycerate kinase (PGK) genes. Since there is no significant changes in gross tissue oxygen concentration during PBM, HIF-1α activation may be mediated by the mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway, by growth factors or cytokines [85]. Another possible explanation is that the sudden boost in cellular respiration caused by light activation of Cox depletes the low amount of oxygen that is present in hypoxic tissues but which is not being rapidly consumed because of inhibited electron transport. This sudden oxygen depletion then rapidly activates HIF-1α.
Cury demonstrated the pro-angiogenic effect of PBM using 660 nm and 780 nm light on skin flaps in rats. He observed that angiogenesis was induced by an increase in HIF-1α and VEGF expression, as well as by a decrease in matrix metalloproteinase 2 (MMP-2) activity [85]. Cury observed that only 660 nm light was able to increase HIF-1α expression, and although VEGF induction occurred in all light doses used, only 40 J cm−2 was able to induce angiogenesis, as well as an increase MMP-2 activity.
Akt/GSK3β/β-catenin pathway
Low-level light may exert a prosurvival effect on cells via the activation of AKT/GSK3β/β-catenin pathway. Basically, protein kinase B (also known as AKT) can be activated by LLL irradiation, and then interact with glycogen synthase kinase 3β (GSK3β), inhibiting its activity. GSK3B is a serine-threoninekinase which mediates various cellular signaling pathways, exerts metabolic control, influences embryogenesis, and is involved in cell death and in oncogenesis. There is evidence that this kinase is involved in the pathogenesis of Alzheimer’s disease, since it promotes hyperphosphorylation of tau protein and causes the formation of neurofibrillary tangles (NTFs), both classic hallmarks of this disease.
The decreased activity of GSK3β is due to the fact that PBM-activated AKT increases the phosphorylation level of its Ser9 residue, which allows the N-terminus of GSK3β to bind with its own binding site. This leads to an accumulation of β-catenin and its translocation into the nucleus, where it can exert its prosurvival action. β-catenin is an important component of Wnt signalling pathway, responsible for the inhibition of axin-mediated β-catenin phosphorylation by GSK3β. This helps to stabilize the under-phosphorylated form of β-catenin, and ensure that it is no longer marked for proteasome degradation, so it can accumulate and travel to the nucleus. Once there, the prosurvival action of β-catenin relies on the increased TCF/LEF-dependent transcriptional activity. This prosurvival effect can be useful in the treatment of neurodegenerative diseases, such as Alzheimer’s [86].
One of the most important regulators of apoptosis is Bax, a member of Bcl-2 family. It is translocated from the cytosol to the mitochondria when a pro-apoptotic stimulus is present, and this translocation is inhibited by PBM, according to Zhang et al. The authors hypothesized that GSK3β is the mediator between Akt and Bax during the PBM anti-apoptotic process. The authors found that GSK3β interacts with Bax and activates it, promoting its translocation directly, but PBM activates Akt which inhibits the activation of GSK3β, thus inhibiting Bax translocation. Using inhibitor compounds such as wortmannin and lithium chloride, there was a significant inhibition of the anti-apoptotic effect observed after PBM, suggesting that PI3K/Akt pathway (inhibited by wortmannin) and GSK3β translocation (inhibited by lithium chloride) play a key role in the protection against apoptosis caused by low level light. LiCl, however, was not able to reduce Bax translocation and apoptosis like PBM, so there must be other upstream regulators of Bax translocation during apoptosis. In conclusion, PBM exerted a pro-survival action through selectively activating the PI3K/Akt pathway and suppressing GSK3β/Bax pathway [87].
Akt/mTOR/CyclinD1 pathway
PBM has been demonstrated to be useful for stimulating proliferation of normal cells, but for dysplastic and malignant cells it could be dangerous. Sperandio et al. provided an example of this situation, observing that oral dysplastic cells, considered pre-malignant, had their viability increased after PBM (660 or 780 nm, 2 to 6 J cm−2). Moreover, these workers showed higher expression of proteins related to cancer progression and invasion, i.e. Akt, HSP90, pS6ser240/244, and Cyclin D1. The data suggest that Akt/mTOR/Cyclin D1 pathway was important for this phenotype differentiation, since the tested oral cancer cells showed higher levels of the signaling mediators that are part of this pathway [88].
ERK/FOXM1
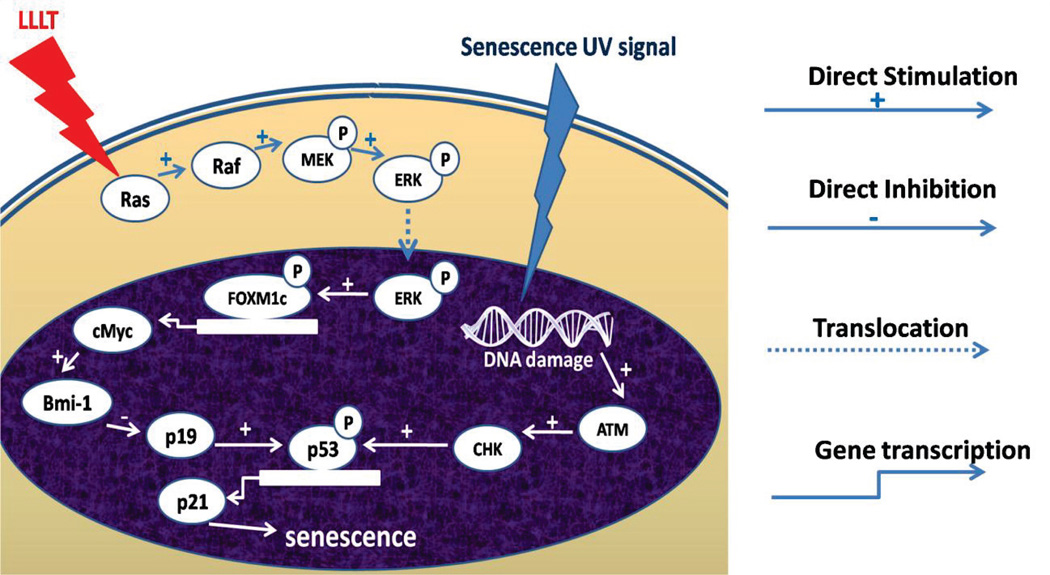
Forkhead box protein M1 (FOXM1) is a protein involved in the regulation of the transition from G1 to S phase of the cell cycle and progression to mitotic division. Ling et al. investigated the protective effect of PBM using red light at 632.8 nm against senescence caused by UV light, and reported an activation of the ERK/FOXM1 pathway that caused a reduction in the expression of p21 protein and G1 phase arrest. Senescence was attenuated by over-expression of FOXM1c with or without PBM, and if FOXM1 was inhibited by shRNA, the effect of PBM in reducing cell senescence was abrogated. PBM promoted the nuclear translocation of extracellular signal-regulated kinase (ERK), increasing FOXM1 accumulation in the nucleus and the transactivation of c-Myc and p21 expression.
Inhibition of the mitogen-activated kinase (MEK)/ERK pathway with an MEK inhibitor PD98059 prevented the nuclear translocation of FOXM1 after PBM, suggesting that Raf/MEK/MAPK/ERK signaling is crucial for the anti-cell senescence effect of PBM mediated by FOXM1 [89]. Figure 4 summarizes these findings.
Figure 4.
A model of the signaling pathways for LLLT protecting cell from UVB-induced senescence.
PPARy
Peroxisome proliferator-activated receptors (PPAR) are mostly present in airway epithelial cells, but also in smooth muscle cells, myofibroblasts, endothelial cells of the pulmonary vasculature and in inflammatory cells such as alveolar macrophages, neutrophils, eosinophils, lymphocytes and mast cells. They are nuclear receptors with transcription factors that regulate gene expression. PPAR-y is involved in the generation of heat shock protein 70 (HSP-70), which is anti-inflammatory, while PPAR-c expression occurs due to an inflammatory response and are associated with massive lung injury and neutrophil infiltration in lungs of mice subjected to endotoxic shock [90]. Lima and co-authors reported a study in which rats were irradiated with 660-nm light (5.4 J) on the skin over the bronchus (chest). They observed a marked rise in the expression of PPAR mRNA after PBM, as well as increased PPAR-y activity in bronchoalveolar lavage (BALF) cells from animals subjected to laser treatment. In conclusion, Lima proposed that PBM can work as a homeostatic facilitator, increasing the expression of a transcription factor that is signaling the synthesis of HSP70 and other anti-inflammatory proteins[90].
RUNX2
Runt-related transcription factor 2 (RUNX-2) is related to osteoblastic differentiation and skeletal morphogenesis, acting as a scaffold for nucleic acids and regulatory factors that are involved in the expression of skeletal-related genes. It regulates the expression of genes related to extracellular matrix components during bone cell proliferation. PBM can increase the expression of RUNX-2, contributing to a better tissue organization, even in diabetic animals as seen by Patrocínio-Silva [91].
Effector molecules
Transforming growth factor (TGF-β)
TGF-β is a strong stimulator of collagen production, inducing the expression of extracellular matrix components and inhibiting its degradation by inhibiting matrix metalloproteinases (MMPs). TGF-β expression is elevated during the initial phase of inflammation after an injury, and stimulates cellular migration, proliferation and interactions within the repair zone [92].
Dang and co-workers suggested that TGF-β/SMAD signaling pathway might play a role in PBM used for non ablative rejuvenation [93]. They found that 800 nm diode laser irradiation was able to induce collagen synthesis through the activation of TGF-β/SMAD pathway in a light dose-dependent manner. 40 J cm−2 was the most effective light dose in enhancing the gene expression of procollagen type I and IV, compared to 20 and 60 J cm−2. The dermal thickness followed the results for the synthesis of collagen, demonstrating that this process was indeed dose-dependent [93].
Aliodoust et al. treated rats with 632.8 nm light and observed increased expression of TGF-β1 (one of the three isoforms of TGF-β) mRNA. TGF-β1 is responsible for the initial scar tissue formed at the wound site. It enhances tendon repair during the fibrosis period via the stimulation of cell proliferation and migration, as well as the synthesis of collagen and proteoglycans [92].
Oxidative stress
The inflammatory process involves an increase in ROS and RNS production, accompanied by a reduction in the activity of antioxidant defenses. This oxidative stress situation can activate NF-κB, as mentioned before, leading to modifications in the expression of genes for pro-inflammatory cytokines, growth factors, chemokines and adhesion molecules.
Assis et al. investigated the effects of PBM on muscle injury using 808 nm light (1.4 J), and observed reduced lipid peroxidation accompanied by a decreased COX-2 mRNA expression and an increased SOD mRNA expression after irradiation. There was a reduced formation of nitrotyrosine, indicating that iNOS activity was lower and, consequently, NO and peroxynitrite production was decreased. In conclusion, the inhibition of oxidative and nitrosative stress contributed to a decrease in the deleterious effects observed after muscle injury [94].
Pro- and anti-inflammatory cytokines
Many cytokines and inflammatory mediators have their levels altered by low-level light irradiation, regardless if they have pro- or anti-inflammatory actions, i.e. TNF, various interleukins, histamine, TGF-β, prostaglandins and eicosanoids. It seems that when inflammation is present, PBM exerts an anti-inflammatory action, but in the absence of inflammation, PBM provide pro-inflammatory mediators that could help in tissue remodeling and to mediate cell function. Wu and co-workers investigated the photoacceptor role of Cox and found that the excitation of Cox initiates a photoreaction that results in histamine release in vitro. The induced signals from mitochondria to cytosol cause alkalinization of the cytosol, which leads to the opening of TRPV channels. This results in an increment of [Ca2+] and, consequently, in an enhanced histamine release [67]. Chen demonstrated in 2014 that an increased calcium influx occurred in mast cells after laser irradiation, and this caused histamine release that could help promoting wound healing. Furthermore, he found that during short-term muscle remodeling after cryoinjury, cytokines expression is also modulated by PBM, leading to a decreased expression of TNF and TGF-β[95].
Although NF-κB activation is known to be pro-inflammatory, PBM has a pronounced anti-inflammatory activity even with NF-κB activation. In fact, the anti-inflammatory effects of PBM could be abrogated if a NF-κB inhibitor is used. This probably occurs because the initial response to cell stress typical of NF-κB activation triggers another response to lower NF-κB activation after PBM had its therapeutic effect. Another possibility is that the initial pro-inflammatory response induced by PBM leads to the expression of eicosanoids that are able to decrease and to end inflammation [95].
Brain-derived neurotrophic factor (BDNF)
BDNF is part of the family of neurotrophins, molecules that exert actions on nerve cells. BDNF, specifically, seems to modulate dendritic structure and to potentiate synaptic transmission in the central nervous system. In order to investigate the effects of low-level light on BDNF levels, Meng et al. treated nerve cells with 632.8 nm light (doses from 0.5 to 4 J cm−2). There was a regulatory role of PBM in neuroprotection and dendritic morphogenesis. PBM attenuated the decrease of BDNF, apparently by the ERK/CREB pathway, and this could be useful in the treatment of neurodegenerative disorders [96].
Vascular endothelial growth factor (VEGF)
Angiogenesis is a complex mechanism, requiring several cell types, mediators and signaling pathways. It is initiated by cell migration and invasion of endothelial cells, subsequent lumen formation and connection of the new vascular segments with preexisting ones, and finally, remodeling of extracellular matrix. This remodeling is dependent on an adequate matrix metalloproteinases (MMPs) activity. VEGF and HIF-1α are critical to the angiogenic process.
PBM has been reported to induce angiogenesis in several experimental models. For example, Cury et al. observed a marked increase in the number of vessels in the skin flap of animals treated with 660 and 780 nm PBM, alongside with a marked increase in VEGF mRNA expression [85].
Hepatocyte growth factor (HGF)
HGF is a cytokine that regulates cell proliferation, motility, morphogenesis and exerts anti-apoptotic and anti-inflammatory activity during hepatic regeneration. The activation of its transmembrane tyrosine kinase receptor, called Met receptor, leads to autophosphorylation of tyrosine residues and phosphorylation of downstream signaling molecules, such as PI3K and MAPK pathway proteins. Araújo and co-workers observed that, after 632.8 nm PBM, hepatectomized animals showed an increase in the expression of HGF followed by increased phosphorylation of Met and its downstream signaling molecules Akt and ERK. This indicates that PBM could enhance liver regeneration after hepatectomy [97].
Basic fibroblast growth factor (bFGF) and keratinocyte growth factor (KGF)
Growth factors play a key role in the wound healing process, mediating the transfer of signals between the epithelium and the connective tissue, especially bFGF and KGF. bFGF is known to be a potent mitogen and chemoattractant for endothelial cells and fibroblasts, as well as accelerating the formation of granulation tissue and to induce re-epithelization. KGF is produced by fibroblasts and exerts a paracrine action on keratinocytes, therefore, it is responsible for the proliferation and migration of epithelial cells, as well as for the maintenance of the epithelium normal structure.
When gingival fibroblasts from a primary culture were irradiated twice with 660 or 780 nm low-level light in a study from Damante et al., production of KGF and bFGF was increased. Red light was more effective in stimulating KGF production, but no significant change in bFGF production was seen with red light. Near-infrared light, however, was capable of inducing bFGF release [98]. These results could explain how PBM can help the wound healing process.
Heat Shock Proteins (HSP)
Heat shock protein 27 (HSP27) is an important member of the small HSP family, with an ATP-independent chaperone activity that is produced in response to oxidative stress in order to modulate inflammation and to regulate the dynamics of the actin cytoskeleton. When HSP27 is activated, it facilitates the phosphorylation of IκB, causing it to be degraded in the proteasome and increasing NF-κB activity. It also contributes to the regulation of NO and ROS production, iNOS expression and TNF secretion. However HSP27 plays a negative role in TNF-mediated IκB kinase (IKK) activation. The results of a study performed by Lim and co-workers with HSP27-silenced cells showed that 635 nm light irradiation was not able to decrease ROS generation if HSP27 was not present, indicating that this chaperone plays an important role in ROS decreasing during inflammation and PBM [99].
HSP70 is part of the normal wound healing process, alongside IL-6 and TGF-β1. Visible (532 nm) and NIR (815 nm) light have been demonstrated to induce HSP70 expression in treated skin cells, and this is important for skin rejuvenation interventions, since there is a consequent effect consisting on the assistance of the correct folding and transport of newly synthesized collagen [93].
HSP90 is another chaperone, which assists the maturation of Akt enabling it to perform its downstream actions. Increased activity of chaperones is certainly not desired in cancer, but it could be useful in healing processes. Sperandio et al. found higher levels of HSP90 in laser-treated cells, and an isoform of this chaperone, HSP90N, which has an oncogenic potential, was found in the experimental groups. This isoform is commonly overexpressed in tumor tissues and is secreted by advantage stages of melanoma [88].
Cellular mechanisms
Inflammation
Lim and co-workers found that 635 nm light irradiation at low power can lead to an anti-inflammatory effect by inhibiting prostaglandin E2 (PGE2) production and cyclooxygenase 1 and 2 (COX-1 and COX-2) mRNA expression. The light irradiation was able to decrease intracellular ROS, which mediate the expression of calcium-dependent phospholipase A2 (cPLA2), secretory phospholipase A2 (sPLA2), and COX-2, and also inhibit the release of PGE2 [99].
PGE2 synthesis is dependent on NF-κB modulation of the cellular signaling mechanism. NF-κB is found in the cytosol in its dimeric form of NF-κB/IκB (the latter is an inhibitory protein). Pro-inflammatory stimuli, such as LPS, are able to activate the NF-κB upstream signaling regulator IκB kinase (IKK), responsible for the phosphorylation and degradation of IκB. The free NF-κB is translocated to the nucleus and induces the expression of pro-inflammatory genes [8]. Lim demonstrated that 635 nm light irradiation suppressed the release of PGE2, possibly through a mechanism related to the inhibition of NF-κB pathway. It did not affect the phosphorylation of IκB, IKK and NF-κB in HSP27-silenced human gingival fibroblasts (hGFs), suggesting that NF-κB modulation by 635 nm light through HSP27 is required for the down-regulation of pro-inflammatory gene expression in these fibroblasts [99].
Macrophages are important antigen-presenting cells, and are involved in the induction of primary immunologic response. Interferon gamma (IFN-γ) polarization (either via classical or M1 activation) programs monocytes for increased phagocytic activity, as well as for anti-tumor activity and allergy suppression. Recently, Chen reported that 660 nm PBM could promote M1 polarization of monocytes, and influence the expression of cytokines and chemokines at the level of mRNA and protein expression. The effect was dose-dependent, since the optimal light dose found was that of 1 J cm−2, compared to 2 and 3 J cm−2. Furthermore, the author could also clarify the mechanisms of epigenetic regulation by PBM in immune cells. Modifications on histones, usually carried out by histone acetyl- or methyltransferases, could be induced by PBM: histone H3 and H4 acetylation and H3K4 trimethylation in the TNF gene promoter area, and histone H3 acetylation in the IP-10 gene promoter region. M1-related immunoregulation is important for antiviral immunity, antitumor immunity, and for the pathogenesis of infammation in autoimmune conditions, therefore PBM could help promoting anti-viral and anti-tumor immunity, but could enhance autoimmune and rheumatoid diseases [95].
Cytoprotection
Studies have shown that PBM in vitro protects cells at risk from dying due to treatment with various different toxins. Methanol, for instance, generates a toxic metabolite (formic acid) that inhibits cytochrome c oxidase. Since PBM enhances mitochondrial activity via stimulation of cytochrome c oxidase, it also promotes cell survival during formic acid toxicity. This was demonstrated by Eells, who used red light (670 nm) in a rodent model of methanol toxicity and found that the light irradiation induced a significant recovery of cone- and rod-mediated function in the retina of rats after methanol intoxication, as well as a protection against histological damage resulting from formic acid [100].
Cyanide is another toxic compound that can have its effects attenuated by PBM. Potassium cyanide-induced apoptosis of neurons was decreased with a pretreatment with 670 nm light. This is explained by the fact that PBM decreased the expression of caspase-3 (commonly increased by cyanide) and reversed the cyanide-induced increased expression of Bax, while decreasing the expression of Bcl-2 and inhibiting ROS generation [101]. Wong-Riley and co-workers show that LED pretreatment was not able to restore enzymatic activity in cells to control levels after cyanide toxicity, but it successfully reversed the toxic effect of tetrodotoxin, especially with 670 and 830 nm light. These wavelengths correspond to the peaks in the absorption spectrum of cytochrome c oxidase, suggesting that this photobiomodulation is dependent of the up-regulation of Cytochrome c oxidase [102].
PBM can be useful in the treatment of Alzheimer’s disease, since low-power laser irradiation promotes Yes-associated protein (YAP) cytoplasmic translocation and amyloid-β-peptide (Aβ) inhibition. Aβ deposition is a known hallmark of Alzheimer’s disease, while YAP translocation is involved in the regulation of Aβ-induced apoptosis. Zhang published a study demonstrating that 832.8 nm light irradiation is able to reduce Aβ-induced toxicity by inhibiting apoptosis through the activation of Akt/YAP/p73 signaling pathway [103].
Proliferation
Several cell types can have their proliferation levels increased by PBM. Keratinocytes, for example, showed an enhanced proliferation after 660 nm light irradiation, accompanied by an increased expression of Cyclin D1 and a faster maturation of keratinocytes in migration to the wound sites, via the expression of proteins involved in the epithelial proliferation process, namely p63, CK10 and CK14. This is useful for the improvement of epithelial healing [104]. Furthermore, fibroblasts irradiated with 632.8 nm light had their proliferation stimulated and their cell viability increased, demonstrating the stimulatory effect of PBM and the usefulness of this therapy in the wound healing process [105].
Vascular endothelial cells exposed to 635 nm irradiation proliferate faster than non-irradiated cells, showing a decreased VEGF concentration. This suggests that laser-induced cell proliferation is related to a decrease in VEGF concentration. 830 nm irradiation decreased TGF-β secretion by the endothelial cells [106].
Amid et al. published a review about the influence of PBM on the proliferation of osteoblasts. According to the studies reviewed by the authors, wavelengths between 600 nm and 1000 nm have been used, and resulted in positive effects on dentistry, on anti-inflammatory process and on osteoblastic proliferation [107].
Fibroblasts irradiated with 632.8 nm light had their proliferation stimulated and their cell viability increased, demonstrating the stimulatory effect of PBM and the usefulness of this therapy in the wound healing process.
Migration
Tendon healing requires migration of tenocytes to the injured area, with consequent proliferation and synthesis of extracellular matrix. Tsai and co-workers evaluated the effect of 660 nm light on rat Achilles tendon-derived tenocytes, and found that dynamin-2 expression was enhanced and the migration was stimulated in vitro. Inhibiting dynamin-2 with dynasore suppressed this stimulatory effect of PBM, leading to the conclusion that tenocyte migration stimulated by low-level light was mediated by the up-regulation of dynamin-2 [108].
Other cell types are also influenced by light irradiation. Melanocytes, for instance, showed an enhanced viability and proliferation after blue and red light irradiation. Melanocytes migration was enhanced by UV, blue and red light in lower doses, but a non-stimulatory effect was observed for higher light doses. Blue light seemed to be more effective compared to UV and red lasers [109]. Human epidermal stem cell migration and proliferation were increased alongside an increased phosphorylation of autocrine extracellular signal-regulated kinase (ERK), which contributed to accelerated wound re-epithelialization [110]. Finally, 780 nm irradiation seemed to be able to accelerate fiber sprouting and neuronal cell migration, at least in embryonic rat brain cultures. Large-size neurons with a dense branched interconnected network of neuronal fibers were also observed after laser irradiation. These results can be seen in Rochkind’s work, and may contribute for future treatment modalities for neuronal injuries or diseases [111].
Protein synthesis
As mentioned before, PBM was able to increase the expression of proteins related to the proliferation and maturation of epithelial cells: p63, CK10 and CK14 [104]. In fact, low level light can increase the expression of several other proteins. A good example is the enhanced collagen I expression by fibroblasts 2 days after 810 nm light irradiation, as demonstrated by Frozanfar and co-workers in 2013 [112]. Moreover, osteoblasts irradiated with 830 nm light increased the expression of proteins and proteoglycans such as osteoglycin and mimecan. Osteoglycin is a leucine-rich proteoglycan, once called osteoinductive factor, easily found in bone matrix, cartilage cells and connective tissues. They play a regulatory role in cell proliferation, differentiation and adhesion of osteoblastic cells, therefore PBM applied on the early proliferation stage of osteoblasts are important for the stimulation of bone formation, in concert with some growth factors and matrix proteins [113].
Stem cells
It appears that stem cells are particularly sensitive to light. PBM induces stem cell activity shown by increased cell migration, differentiation, proliferation and viability, as well as by activating protein expression [114]. Mesenchymal stem cells, usually derived from bone marrow, dental pulp, periodontal ligament and from adipose tissue, proliferate more after light irradiation (usually with wavelengths ranging from 600 to 700 nm). Since stem cells in their undifferentiated form show a lower rate of proliferation, this may be a limiting factor for the clinical effectiveness of stem cell therapies, PBM offers a viable alternative to promote the translation of stem cell research into the clinical arena [115].
Min and co-workers reported that the cell viability of adipose-derived stem cells was found to be increased after irradiation with 830 nm light. Their in vivo results also revealed elevated numbers of stem cells compared to the control group [116]. Epidermal stem cells can also be influenced by light, as demonstrated by Liao et al. The authors reveal that 632.8 nm light has photobiological effects on cultured human epidermal stem cells, such as an increase in proliferation and migration in vitro [110]. Soares observed a similar effect on human periodontal ligament stem cells irradiated with a 660 nm diode laser [117].
Tissue mechanisms
Muscles
We already mentioned the positive results for PBM in muscle recovery, reported by Ferraresi et al. The authors demonstrated the usefulness of PBM in muscle recovery after injury. The authors concluded that it takes between 3 and 6 hours for the PBM to exert maximum effect on the muscle physiology, consisting of increased matrix metalloproteinase activity and ATP synthesis. This effect could still be observed 24 hours after the laser irradiation [49].
Rochkind and co-workers have also worked with PBM applied to muscles, investigating the influence of low power laser irradiation on creatine kinase (CK) and the amount of acetylcholine receptors (AChRs) present in intact gastrocnemius muscle in vivo, as well as the synthesis of DNA and of CK in muscle cells in vitro. The authors found that PBM significantly increased CK activity and AChR level in one and two months, when compared to control animals. The biochemical changes on muscle cells might be due to a trophic signal for increased activity of CK, which leads to a preservation of a reservoir of high-energy phosphate that is available for rapid ATP synthesis [118].
Brain
Regarding the neurological field, PBM can lead to cognitive benefits and memory enhancement in case of brain damage caused by controlled cortical impact (CCI). Khuman and co-workers found that a 500 mW cm−2 laser irradiation (60 J cm−2) for two minutes improved spatial learning and memory of mice with CCI, and this was not observed in sham-injured mice. The authors observed a brief increase in the temperature of brain, but it returned to baseline before 5 minutes of irradiation. They also observed reduction of microgliosis at 48 h. Low level light can be useful in traumatic brain injury (TBI) treatment, since suboptimal light doses demonstrated to affect spatial memory, as assessed by visible platform trials, even in the absence of non-spatial procedural learning, which is hippocampus-independent [82].
Near-infrared (NIR) light exerts a protective effect on neurons, but the mechanisms are not fully understood. However, two mechanisms may be involved, and the first that will be discussed is the direct action of NIR light on the cells, improving mitochondrial function, reducing inflammation, and helping the brain to repair itself. Xuan et al reported that transcranial NIR light could stimulate the process of neurogenesis in the hippocampus and subventricular zone (SVZ) in mice with CCI TBI [119]. These newly formed neuroprogenitor cells could travel to the injured region of the cortex to help in the repair of the damaged region. In another study the same group showed that BDNF was increased in the hippocampus and SVZ at one week post TBI, and that at 4 weeks post TBI there as an increase in synaptogenesis in the cortex showing that new connections between existing brain cells could be stimulated by light [120].
The second mechanism is based on the hypothesis that NIR can trigger a systemic response, this time not so directly, suggesting the involvement of one or more circulating molecules or cell types. This assumption is based on studies reporting remote effects on tissues after irradiation of NIR light on specific sites, such as skin wounds. Another study reported brain protection in mice after remote irradiation with NIR light to the dorsum of the animals, without any direct irradiation on the head. One possibility to explain these remote effects is the stimulation of mast cells and macrophages, which could help to protect cells in the brain, as well as the modulation of inflammatory mediators, like the down-regulation of pro-inflammatory cytokines and up-regulation of anti-inflammatory cytokines. Another possibility is the involvement of bone-marrow derived stem cells, since NIR light can increase the proliferation of c-kit-positive cells located in the bone marrow of the skull, which are then recruited to damaged tissues, especially the myocardial infarct site. These progenitor cells can, alongside with immune cells, secrete trophic and pro-survival factors such as nerve growth factor (NGF) and VEGF. Finally, mitochondria itself could be secreting an unidentified extracellular signal, called by Durieux et al. a “mitokine”, which is then transmitted to remotely located cells[82].
Nerves (repair and pain)
Some clinical studies have demonstrated the efficacy of laser-induced analgesia [121], [122], usually with a low power red or near-infrared laser, and it seems that the pain reduction is due to a conduction block of central and peripheral nerve fibers and to the release of endorphins. In this field, for instance, Chan and co-workers used a Nd:YAG pulsed laser (1064 nm) with average power 1.2 W and power density 0.3–0.45 J cm−2 in a randomized, double-blind clinical trial, and demonstrated the efficacy of this treatment on pulpal analgesia of premolar teeth[123].
Analgesia mediated by low level light therapy is due to various effects, such as light absorption by mitochondrial chromophores (mainly Cox) biomodulation, vasodilation, stimulation of cell division, release of NO, increase in cortisol levels and protein synthesis, increase in intracellular calcium concentration and increased activity of the antioxidant enzyme superoxide dismutase. Serra and Ashmawi investigated recently if serotonin played a role in PBM-induced analgesia, but their results indicated that this effect is mediated by peripheral opioid receptors, but not by peripheral serotoninergic receptors [124].
Low-level light therapy can be used for inhibition of pain and for pathological conditions associated with the nervous system. In 2011, Yan et al. postulated that PBM could suppress afferent fiber signaling as well as modulate synaptic transmission to dorsal horn neurons, including inhibition of substance P, and this can lead to long-term pain depression [125]. PBM exerts potent anti-inflammatory effects in the peripheral nervous system, can reduce myocardial infarction, promotes functional recovery and regeneration of peripheral nerves after injury, and can improve neurological deficits after stroke and TBI [82].
Light with irradiance higher than 300 mW cm−2, when absorbed by nociceptors, can inhibit Aδ and C pain fibers, slowing of conduction velocity, reducing of the compound action potential amplitude, and suppression of neurogenicin inflammation. In case of PBM, the light can block anterograde transport of ATP-rich mitochondria in dorsal root ganglion neurons. This inhibition is completely reversible within 48 hours, and leads to the formation of varicosities, which are usually associated with the disruption of microtubules (interruption of fast axonal flow can reduce ATP availability, which is necessary for the polymerization of microtubules and for the maintenance of the resting potential) [83].
Healing (bones, tendons, wounds)
Regarding bones, low power laser irradiation is not believed to affect osteosynthesis, but it is likely that it creates environmental conditions that accelerate bone healing. PBM stimulates proliferation and differentiation of osteoblasts in vivo and in vitro, leading to an increased bone formation, accompanied by an increase in the activity of alkaline phosphatase (ALP) and in osteocalcin expression. This indicates that laser irradiation can directly stimulate bone formation and, according to Fujimoto et al., this effect can be attributed to an increased expression of insulin-like growth factor (IGF), although other differentiation factors might be involved as well, such as BMPs. BMPs-2, −4, −6 and −7 are members of the TGF-β superfamily, and potent promoters of osteoblastic differentiation and of bone formation (promoting the change of mesenchymal cells into chondroblasts and osteoblasts) [126].
According to Fujimoto, BMP-2 might be most involved in the effects of PBM on bone. PBM stimulated mineralization in vitro via increased gene and protein expression of BMPs and Runx-2, as well as differentiation of osteoblasts into MC3T3-E1cells. Since BMPs are one of the most important and potent bone-inductive mediators and are expressed in skeletal tissues, it is possible that the bone nodules formed after PBM are mediated in part by BMP-2 expression [126].
The balance between oxidants and antioxidants is directly related to the time and quality of the wound healing process[127]. This process can be divided in four overlapping phases: hemostasis, inflammation, proliferation and remodeling or resolution. Hemostasis is initiated as soon as the blood vessels are damaged, and consists on the adherence of platelets to the extracellular matrix and further releasing of growth factors (mostly platelet-derived growth factor, PDGF and TGF-β), culminating in the production of thrombin which acts on fibrinogen to produce a fibrin clot. Thrombin also acts as a chemotactic agent and proliferating agent on monocytes, keratinocytes, fibroblasts and endothelial cells, therefore a defective thrombin activity can lead to a delay in the wound healing process. Hoffman reported that PBM could be beneficial in promoting healing when there is a defect in the hemostasis process [128].
Hair
Different mechanisms have been proposed to explain the reason for the first light-mediated effect observed by Mester in 1968 (hypertrichosis in mice [2]) but now widely used clinically to restore hair growth in adult humans [129]. Some researchers have hypothesized that this effect was due to polycystic ovarian syndrome present in 5 out of 49 female patients under laser treatment for facial hirsutism, others suggested that even if the heat generated by PBM was not able to ablate cells from the hair follicle, the small amount of heat supposedly produced could induce follicular stem cells to proliferate and differentiate, due to the increased level of heat shock proteins. Another possibility relies on the release of certain factors that could affect the cell cycle and induce angiogenesis [129]. The exact mechanism still needs clarifying, but the effects of PBM on hair growth are already well described.
Hair growth is divided basically in three phases: anagen, catagen and telogen. The anagen is the growth phase and can last from 2 to 6 years. Catagen phase lasts from 1 to 2 weeks and consists of club hair transitions upwards toward the skin pore, while the dermal papilla separates from the follicle. In the telogen phase, the dermal papillae fully separate from the hair follicle. It lasts from 5 to 6 weeks, until the papillae move upward to meet the hair follicles again and the hair matrix begins to form new hair, returning to the anagen phase. It has been observed that PBM is able to stimulate telogen hair follicles to enter the anagen phase, as well as to prolong the duration of the anagen phase itself. PBM is also capable of increasing the rate of proliferation of anagen hair follicles and to prevent premature catagen phase entry. This could be due to induced protein synthesis by the transcription factors activated by PBM, followed by cell migration and proliferation, alteration in cytokines levels, growth factors and inflammatory mediators. NO is also augmented in LLL treated tissues, usually dissociated from Cox, and since it is a well known vasodilator, it is likely that there is a vasodilation effect on hair follicles after PBM that could help hair growth. Some inflammatory mediators also have their expression inhibited by PBM (such as IFN-γ, IL-1a, IL-1b, TNF and Fas-antigen) and, considering that inflammation is highly disruptive for hair follicles, the anti-inflammatory effect of PBM could be useful in the treatment of hair conditions such as alopecia areata [129].
High Fluence Low Power Laser Irradiation (HF-LPLI)
Fluence, according to the International System of Units, is the energy density integrated over the unit surface of a sphere. Just like PBM using low fluences of light, high-fluence low-power laser irradiation (HF-LPLI) stimulates mitochondrial chromophores, but this time it overstimulates them, which in turn activates the mitochondrial apoptosis pathway, altering the cell cycle, inhibiting cell proliferation and even causing cell death. HF-LPLI (usually fluences above 80 J cm−2) induces apoptosis by activating caspase-3, and mitochondrial permeability transition after HF-LPLI is the main mechanism of mitochondrial injury. In 2010, Sun et al. reported that signal transducer and activator of transcription 3 (Stat3) was involved in HF-LPLI-induced apoptosis in vitro, and this effect is time- and dose-dependent. Steroid receptor coactivator (Src) seems to be the main upstream kinase of Stat3 activation, and the increased ROS generation plays a key role in this process [130].
Recently, Wu et al. found that HF-LPLI, using light at 633 nm and 120 J cm−2, could ablate tumors via activation of mitochondrial apoptotic pathway after ROS generation. The evidence is based on the inactivation of caspase-8, activation of caspase-9 and by the release of cytochrome C. When this high dose is used, light inactivates Cox (instead of activating Cox), inducing a superoxide burst in the electron transport chain and, finally, produces oxidative damage against cancer cells [29]. Chu and co-workers already observed that PBM could induce a mitochondrial permeability pore transition when higher levels of ROS are produced. As a consequence, the decrease of mitochondrial transmembrane potential causes the permeabilization of the mitochondrial outer membrane and, subsequently, the release of cytochrome c and caspase cascade reaction [131].
Cho also observed the interference that a protein, called survivin, could affect the outcomes of HF-LPLI. Light treatment can activate survivin by inducing an increase in its phosphorylation levels. The activated survivin is able to inhibit the permeabilization of the mitochondrial outer membrane, and therefore prevents the release of cytochrome c, the activation of Bax and caspase-9. Cho then concluded that survivin mediates self protection of tumor cells against HF-LPLI-induced apoptosis, through ROS/cdc25c/CDK1 signaling pathway [131].
Conclusions
Low levels of red/NIR light can interact with cells, leading to changes at the molecular, cellular and tissue levels. Each tissue, however, can respond to this light-interaction differently, although it is well known that the photons, especially in the red or NIR, are predominantly absorbed in the mitochondria [132]. Therefore, it is likely that even the diverse results observed with PBM share the basic mechanism of action. What happens after the photon absorption is yet to be fully described, since many signaling pathways seem to be activated. It seems that the effects of PBM are due to an increase in the oxidative metabolism in the mitochondria [133]. Different outcomes can occur depending on the cell type, i.e. cancer cells that tend to proliferate when PBM is delivered [88]. In this review we have not discussed the response of cells and tissues to wavelengths longer than NIR, namely far IR radiation (FIR) (3 µm to 50 µm). At these wavelengths water molecules are the only credible chromophores, and the concept of structured water layers that build up on biological lipid bilayer membranes has been introduced to explain the selective absorption [134]. Nevertheless FIR therapy has significant medical benefits that are somewhat similar to those of PBM [135], and it is possible that activation of light/heat sensitive ion channels could be the missing connection between the two approaches.
As we have shown, PBM can regulate many biological processes, such as cell viability, cell proliferation and apoptosis, and these processes are dependent on molecules like protein kinase c (PKC), protein kinase B (Akt/PKB), Src tyrosine kinases and interleukin-8/1a (IL-8/1a). The effects of light on cell proliferation can be stimulatory at low fluences (which is useful in wound healing, for instance), but could be inhibitory at higher light doses (which could be useful in certain types of scar formation such as hypertrophic scars and keloids) [131].
The applications of PBM are broad. Four clinical targets, however, are the most common: shining light on injured sites to promote healing, remodeling and/or to reduce inflammation; on nerves to induce analgesia; on lymph nodes in order to reduce edema and inflammation; and on trigger points (a single one of as many as 15 points) to promote muscle relaxation and to reduce tenderness. Since it is non invasive, PBM is very useful for patients who are needle phobic or for those who cannot tolerate therapies with non-steroidal anti-inflammatory drugs [83].
The positive outcomes depend on the parameters used on the treatment. The anti-inflammatory effect of light in low intensity was reported on patients with arthritis, acrodermatitis continua, sensitive and erythematous skin, for instance [136]. With the same basic mechanism of action, which is the light absorption by mitochondrial chromophores, mainly Cox, the consequences of PBM are various, depending on the parameters used, on the signaling pathways that are activated and on the treated tissue. In order to apply PBM in clinical procedures, the clinicians should be aware of the correct parameters and the consequences for each tissue to be treated. More studies have to be performed in order to fill the gaps that still linger in the basic mechanisms underlying LLLT and PBM.
Figure 5.
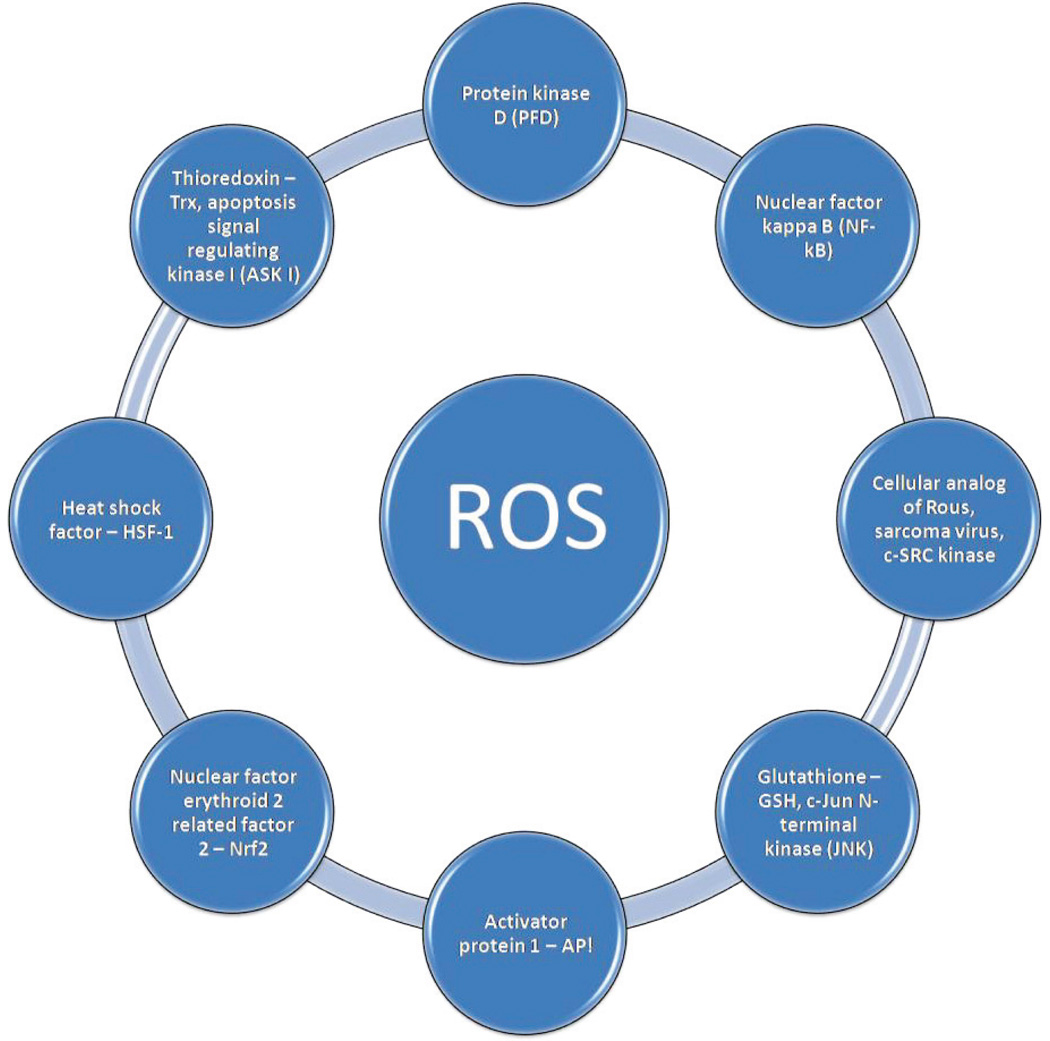
Reactive Oxygen Species sensors and signaling
Acknowledgments
MRH was supported by US NIH grant R01AI050875.
Lucas Freitas de Freitas was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP.
Biography

Michael R Hamblin Ph.D. is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital, an Associate Professor of Dermatology at Harvard Medical School and is a member of the affiliated faculty of the Harvard-MIT Division of Health Science and Technology. He was trained as a synthetic organic chemist and received his PhD from Trent University in England. His research interests lie in the areas of photodynamic therapy (PDT) for infections, cancer, and stimulation of the immune system, and in low-level light therapy (LLLT) for wound healing, arthritis, traumatic brain injury, neurodegenerative diseases and psychiatric disorders. He directs a laboratory of around a dozen post-doctoral fellows, visiting scientists and graduate students. His research program is supported by NIH, CDMRP, USAFOSR and CIMIT among other funding agencies. He has published over 320 peer-reviewed articles, over 150 conference proceedings, book chapters and International abstracts and holds 10 patents. He is Associate Editor for 7 journals, on the editorial board of a further 30 journals and serves on NIH Study Sections. For the past 11 years Dr Hamblin has chaired an annual conference at SPIE Photonics West entitled "Mechanisms for low level light therapy" and he has edited the 11 proceedings volumes together with four other major textbooks on PDT and photomedicine. He has several other book projects in progress at various stages of completion. In 2011 Dr Hamblin was honored by election as a Fellow of SPIE. He is a Visiting Professor at universities in China, South Africa and Northern Ireland.
REFERENCES
- 1.McGuff PE, Deterling RA, Gottlieb LS. Tumoricidal effect of laser energy on experimental and human malignant tumors. N. Engl. J. Med. 1965;273(9):490–492. doi: 10.1056/NEJM196508262730906. [DOI] [PubMed] [Google Scholar]
- 2.Mester E, Szende B, Gärtner P. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother. (Berl) 1968;9(5):621–626. [PubMed] [Google Scholar]
- 3.Kovács IB, Mester E, Görög P. Stimulation of wound healing with laser beam in the rat. Experientia. 1974;30(11):1275–1276. doi: 10.1007/BF01945182. [DOI] [PubMed] [Google Scholar]
- 4.Em M, Chaves A, Piancastelli CC. Effects of low-power light therapy on wound healing. An. Bras. Dermatol. 2014;89(4):616–623. doi: 10.1590/abd1806-4841.20142519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung H, Dai T, Sharma S, Huang Y, Carroll J, Hamblin M. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso-Davis MK, Guillot TS, Podichetty VK, Mashtalir N, Dhurandhar NV, Dubuisson O, Yu Y, Greenway FL. Efficacy of low-level laser therapy for body contouring and spot fat reduction. Obes. Surg. 2011;21(6):722–729. doi: 10.1007/s11695-010-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agostinis P, Berg K, a Cengel K, Foster TH, Girotti aW, Gollnick SO, Hahn SM, Hamblin MR, a Juzeniene, Kessel D, Koberlik M, Moan J, Mroz P, Nowis D, Piette J, Wilson B, Golab J. Photodynamic Therapy of Cancer: an Update. 2012;61(4):250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y-Y, Chen AC-H, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose. Response. 2009;7(4):358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y-Y, Sharma SK, Carroll J, Hamblin MR. Biphasic Dose Response in Low Level Light Therapy - An Update. Dose-Response. 2011;9(4):602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanzafame RJ, Stadler I, Kurtz AF, Connelly R, Peter TA, Timothy PA, Brondon P, Olson D. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg. Med. 2007 Jul;39(6):534–542. doi: 10.1002/lsm.20519. [DOI] [PubMed] [Google Scholar]
- 11.Oron U, Yaakobi T, Oron A, Hayam G, Gepstein L, Rubin O, Wolf T, Ben Haim S. Attenuation of infarct size in rats and dogs after myocardial infarction by low-energy laser irradiation. Lasers Surg. Med. 2001;28(3):204–211. doi: 10.1002/lsm.1039. [DOI] [PubMed] [Google Scholar]
- 12.Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006 Sep;124(1–2):201–210. doi: 10.1016/j.pain.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins D, Abrahamse H. Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed. Laser Surg. 2006 Dec;24(6):705–714. doi: 10.1089/pho.2006.24.705. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins DH, Abrahamse H. The role of laser fluence in cell viability, proliferation, and membrane integrity of wounded human skin fibroblasts following helium-neon laser irradiation. Lasers Surg. Med. 2006 Jan;38(1):74–83. doi: 10.1002/lsm.20271. [DOI] [PubMed] [Google Scholar]
- 15.Lubart R, Lavi R, Friedmann H, Rochkind S. Photochemistry and photobiology of light absorption by living cells. Photomed. Laser Surg. 2006 Apr;24(2):179–185. doi: 10.1089/pho.2006.24.179. [DOI] [PubMed] [Google Scholar]
- 16.Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J. Clin. Laser Med. Surg. 2001 Feb;19(1):29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 17.Bolton P, Young S, Dyson M. Macrophage responsiveness to light therapy with varying power and energy densities. Laser Ther. 1991;3(3):105–111. [Google Scholar]
- 18.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed. Laser Surg. 2005 Aug;23(4):355–361. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 19.al-Watban FA, Andres BL. The effect of He-Ne laser (632.8 nm) and Solcoseryl in vitro. Lasers Med. Sci. 2001;16(4):267–275. doi: 10.1007/pl00011363. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Song S, Fong C-C, Tsang C-H, Yang Z, Yang M. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Invest. Dermatol. 2003 May;1205:849–857. doi: 10.1046/j.1523-1747.2003.12133.x. [DOI] [PubMed] [Google Scholar]
- 21.Brondon P, Stadler I, Lanzafame RJ. A study of the effects of phototherapy dose interval on photobiomodulation of cell cultures. Lasers Surg. Med. 2005 Jun;36(5):409–413. doi: 10.1002/lsm.20183. [DOI] [PubMed] [Google Scholar]
- 22.Bjordal JM, Couppé C, Chow RT, Tunér J, Ljunggren EA. A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust. J. Physiother. 2003;49(2):107–116. doi: 10.1016/s0004-9514(14)60127-6. [DOI] [PubMed] [Google Scholar]
- 23.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed. Laser Surg. 2010 Feb;28(1):3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]
- 24.Bolton P, Young S, Dyson M. THE DIRECT EFFECT OF 860 nm LIGHT ON CELL PROLIFERATION AND ON SUCCINIC DEHYDROGENASE ACTIVITY OF HUMAN FIBROBLASTS INVITRO. LASER Ther. 1995;72:55–60. [Google Scholar]
- 25.Byrnes KR, Wu X, Waynant RW, Ilev IK, Anders JJ. Low power laser irradiation alters gene expression of olfactory ensheathing cells in vitro. Lasers Surg. Med. 2005 Aug;37(2):161–171. doi: 10.1002/lsm.20202. [DOI] [PubMed] [Google Scholar]
- 26.Kushibiki T, Awazu K. Blue laser irradiation enhances extracellular calcification of primary mesenchymal stem cells. Photomed. Laser Surg. 2009 Jun;27(3):493–498. doi: 10.1089/pho.2008.2343. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins Pa, Carroll JD. How to Report Low-Level Laser Therapy (LLLT)/Photomedicine Dose and Beam Parameters in Clinical and Laboratory Studies. Photomed. Laser Surg. 2011;29(12):785–787. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 28.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62(8):607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid. Redox Signal. 2014;20(5):733–746. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov. Med. 2011;11(57):154–159. [PubMed] [Google Scholar]
- 31.Lane N. Cell biology: power games. Nature. 2006 Oct;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 32.Shiva S, Gladwin MT. Shining a light on tissue NO stores: near infrared release of NO from nitrite and nitrosylated hemes. J. Mol. Cell. Cardiol. 2009 Jan;46(1):1–3. doi: 10.1016/j.yjmcc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008;84(5):1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 34.Magrini TD. Low-level laser therapy on MCF-7 cells: a micro-Fourier transform infrared spectroscopy study. J. Biomed. Opt. 2012;17(10):101516. doi: 10.1117/1.JBO.17.10.101516. [DOI] [PubMed] [Google Scholar]
- 35.Lin JY. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp. Physiol. 2011;96(1):19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardie RC. Photosensitive TRPs. 2014:795–826. doi: 10.1007/978-3-319-05161-1_4. [DOI] [PubMed] [Google Scholar]
- 37.Nilius B, Voets T. TRP channels: A TR(I)P through a world of multifunctional cation channels. Pflugers Arch. Eur. J. Physiol. 2005;451(1):1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- 38.Rohacs T. Phosphoinositide Regulation of TRP Channels. 2014:1143–1176. doi: 10.1007/978-3-319-05161-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W-Z, Chen J-Y, Yu J-T, Zhou L-W. Effects of low power laser irradiation on intracellular calcium and histamine release in RBL-2H3 mast cells. Photochem. Photobiol. 2007;83(4):979–984. doi: 10.1111/j.1751-1097.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 40.Gu Q, Wang L, Huang F, Schwarz W. Stimulation of TRPV1 by green laser light. Evidence-based Complement. Altern. Med. 2012;2012 doi: 10.1155/2012/857123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryu J-J, Yoo S, Kim KY, Park J-S, Bang S, Lee SH, Yang T-J, Cho H, Hwang SW. Laser modulation of heat and capsaicin receptor TRPV1 leads to thermal antinociception. J. Dent. Res. 2010;89(12):1455–1460. doi: 10.1177/0022034510381394. [DOI] [PubMed] [Google Scholar]
- 42.Albert ES, Bec JM, Desmadryl G, Chekroud K, Travo C, Gaboyard S, Bardin F, Marc I, Dumas M, Lenaers G, Hamel C, Muller a, Chabbert C. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J. Neurophysiol. 2012;107(12):3227–3234. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- 43.Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC-H, Padwa BL, Hamblin MR, Barcellos-Hoff MH, Kulkarni AB, Mooney DJ. Photoactivation of Endogenous Latent Transforming Growth Factor-1 Directs Dental Stem Cell Differentiation for Regeneration. Sci. Transl. Med. 2014 May;6(238):238ra69–238ra69. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vladimirov YA, Gorbatenkova EA, Paramonov NV, Azizova OA. Photoreactivation of superoxide dismutase by intensive red (laser) light. Free Radic. Biol. Med. 1988;5(5–6):281–286. doi: 10.1016/0891-5849(88)90098-6. [DOI] [PubMed] [Google Scholar]
- 45.Amat A, Rigau J, Waynant RW, Ilev IK, Tomas J, Anders JJ. Modification of the intrinsic fluorescence and the biochemical behavior of ATP after irradiation with visible and near-infrared laser light. J. Photochem. Photobiol. B Biol. 2005;81(1):26–32. doi: 10.1016/j.jphotobiol.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Heger M, Heemskerk AMa, van der der Zwan G. Absence of 633-nm laser irradiation-induced effects on glucose phosphorylation by hexokinase. J. Photochem. Photobiol. B Biol. 2010;98(3):216–222. doi: 10.1016/j.jphotobiol.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J. lasers Med. Sci. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 48.Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: Performance, fatigue and repair benefited by the power of light. Photonics and Lasers in Medicine. 2012;1(4):267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferraresi C, Kaippert B, Avci P, Huang Y-Y, de Sousa MVP, Bagnato VS, Parizotto Na, Hamblin MR. Low-level Laser (Light) Therapy Increases Mitochondrial Membrane Potential and ATP Synthesis in C2C12 Myotubes with a Peak Response at 3–6 h. Photochem. Photobiol. 2015;91(2):411–416. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubyak GR. Signal transduction by P2-purinergic receptors for extracellular ATP. American journal of respiratory cell and molecular biology. 1991;4(4):295–300. doi: 10.1165/ajrcmb/4.4.295. [DOI] [PubMed] [Google Scholar]
- 51.Burnstock G. Purinergic nerves. Pharmacol. Rev. 1972;24(3):509–581. [PubMed] [Google Scholar]
- 52.Burnstock G, Verkhratsky a. Evolutionary origins of the purinergic signalling system. Acta Physiol. 2009;195(4):415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 53.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J. Mol. Med. 2013 Feb;91(2):173–181. doi: 10.1007/s00109-013-0997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Lima FM, Moreira LM, Villaverde aB, Albertini R, Castro-Faria-Neto HC, Aimbire F. Low-level laser therapy (LLLT) acts as cAMP-elevating agent in acute respiratory distress syndrome. Lasers Med. Sci. 2011;26(3):389–400. doi: 10.1007/s10103-010-0874-x. [DOI] [PubMed] [Google Scholar]
- 55.Wu JY, Chen CH, Wang CZ, Ho ML, Yeh ML, Wang YH. Low-Power Laser Irradiation Suppresses Inflammatory Response of Human Adipose-Derived Stem Cells by Modulating Intracellular Cyclic AMP Level and NF-κB Activity. PLoS One. 2013;8(1):1–9. doi: 10.1371/journal.pone.0054067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taskén K, Aandahl EM. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 2004;84(1):137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- 57.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 58.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 2003;4(9):733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 59.Chen AC-H, Arany PR, Huang Y-Y, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-Level Laser Therapy Activates NF-kB via Generation of Reactive Oxygen Species in Mouse Embryonic Fibroblasts. PLoS One. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Popa-wagner A, Mitran S, Sivanesan S, Chang E, Buga A. ROS and Brain Diseases : The Good, the Bad, and the Ugly. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/963520. Article ID 963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011;51(2):327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Yun J, Finkel T. Mitohormesis. Cell Metabolism. 2014;19(5):757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang Y-Y, Nagata K, Tedford CE, McCarthy T, Hamblin MR. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics. 2013;6(10):829–838. doi: 10.1002/jbio.201200157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang YY, Nagata K, Tedford CE, Hamblin MR. Low-level laser therapy (810 nm) protects primary cortical neurons against excitotoxicity in vitro. J. Biophotonics. 2014;7(8):656–664. doi: 10.1002/jbio.201300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bindoli A, Rigobello MP. Principles in redox signaling: from chemistry to functional significance. Antioxid. Redox Signal. 2013;18(13):1557–1593. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 66.Forman HJ, Ursini F, Maiorino M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014;73:2–9. doi: 10.1016/j.yjmcc.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu ZH, Zhou Y, Chen JY, Zhou LW. Mitochondrial signaling for histamine releases in laser-irradiated RBL-2H3 mast cells. Lasers Surg. Med. 2010;42(6):503–509. doi: 10.1002/lsm.20924. [DOI] [PubMed] [Google Scholar]
- 68.Sharma SK, Kharkwal GB, Sajo M, Huang Y, De Taboada L, Mccarthy T, Hamblin MR. Dose Response Effects of 810 nm Laser Light on Mouse Primary Cortical Neurons. NIH Public Access. 2012;43(8):851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santulli G, Marks AR. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr. Mol. Pharmacol. 2015 doi: 10.2174/1874467208666150507105105. [DOI] [PubMed] [Google Scholar]
- 70.Kühl M. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front. Biosci. 2004;9:967–974. doi: 10.2741/1307. [DOI] [PubMed] [Google Scholar]
- 71.Ray K. Calcium-Sensing Receptor: Trafficking, Endocytosis, Recycling, and Importance of Interacting Proteins. Prog. Mol. Biol. Transl. Sci. 2015;132:127–150. doi: 10.1016/bs.pmbts.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Finkel T, Menazza S, Holmstrom KM, Parks RJ, Liu J, Sun J, Liu J, Pan X, Murphy E. The Ins and Outs of Mitochondrial Calcium. Circ. Res. 2015;116(11):1810–1819. doi: 10.1161/CIRCRESAHA.116.305484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krishnan V, Graham A, Mazei-Robison MS, Lagace DC, Kim KS, Birnbaum S, Eisch AJ, Han PL, Storm DR, Zachariou V, Nestler EJ. Calcium-Sensitive Adenylyl Cyclases in Depression and Anxiety: Behavioral and Biochemical Consequences of Isoform Targeting. Biol. Psychiatry. 2008;64(4):336–343. doi: 10.1016/j.biopsych.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Karu TI, Pyatibrat LV, Afanasyeva NI. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005;36(4):307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- 75.Murad F. Discovery of some of the biological effects of nitric oxide and its role in cell signaling. Biosci. Rep. 2005;24(4–5):453–474. doi: 10.1007/s10540-005-2741-8. [DOI] [PubMed] [Google Scholar]
- 76.Drapier JC, Hirling H, Wietzerbin J, Kaldy P, Kühn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lepoivre M, Fieschi F, Coves J, Thelander L, Fontecave M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem. Biophys. Res. Commun. 1991;179(1):442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- 78.Drapier JC, Hibbs JB. Aconitases: a class of metalloproteins highly sensitive to nitric oxide synthesis. Methods Enzymol. 1996;269:26–36. doi: 10.1016/s0076-6879(96)69006-5. [DOI] [PubMed] [Google Scholar]
- 79.Dimmeler S, Lottspeich F, Brune B. Nitric oxide causes ADP-ribosylation and inhibition of glyceraldehyde-3-phosphate dehydrogenase. J. Biol. Chem. 1992;267(24):16771–16774. [PubMed] [Google Scholar]
- 80.Stamler JS, Simon DI, a Osborne J, Mullins ME, Jaraki O, Michel T, Singel DJ, Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. U. S. A. 1992;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avci P, Nyame TT, Gupta GK, Sadasivam M, Hamblin MR. Low-level laser therapy for fat layer reduction: A comprehensive review. Lasers Surg. Med. 2013;45(6):349–357. doi: 10.1002/lsm.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khuman J, Zhang J, Park J, Carroll JD, Donahue C, Whalen MJ. Low-Level Laser Light Therapy Improves Cognitive Deficits and Inhibits Microglial Activation after Controlled Cortical Impact in Mice. J. Neurotrauma. 2012;29(2):408–417. doi: 10.1089/neu.2010.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carroll JD, Milward MR, Cooper PR, Hadis M, Palin WM. Developments in low level light therapy (LLLT) for dentistry. Dent. Mater. 2014;30(5):465–475. doi: 10.1016/j.dental.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 84.Incerti Parenti S, Checchi L, Fini M, Tschon M. Different doses of low-level laser irradiation modulate the in vitro response of osteoblast-like cells. J. Biomed. Opt. 2014 Oct;19(10):108002. doi: 10.1117/1.JBO.19.10.108002. [DOI] [PubMed] [Google Scholar]
- 85.Cury V, Moretti AIS, Assis L, Bossini P, De Souza Crusca J, Neto CB, Fangel R, De Souza HP, Hamblin MR, Parizotto NA. Low level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1α and MMP-2. J. Photochem. Photobiol. B Biol. 2013;125:164–170. doi: 10.1016/j.jphotobiol.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang J, Liu L, Xing D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radic. Biol. Med. 2012;53(7):1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, Zhang Y, Xing Da. LPLI inhibits apoptosis upstream of bax translocation via a GSK-3β-inactivation mechanism. J. Cell. Physiol. 2010;224(1):218–228. doi: 10.1002/jcp.22123. [DOI] [PubMed] [Google Scholar]
- 88.Sperandio FF, Giudice FS, Corrêa L, Pinto DS, Hamblin MR, de Sousa SCOM. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J. Biophotonics. 2013 Apr;29(6):n/a–n/a. doi: 10.1002/jbio.201300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling Q, Meng C, Chen Q, Xing D. Activated ERK/FOXM1 pathway by low-power laser irradiation inhibits UVB-induced senescence through down-regulating p21 expression. J. Cell. Physiol. 2013 Jun;:n/a–n/a. doi: 10.1002/jcp.24425. [DOI] [PubMed] [Google Scholar]
- 90.de Lima FM, Albertini R, Dantas Y, Maia-Filho AL, de Loura Santana C, Castro-Faria-Neto HC, França C, Villaverde AB, Aimbire F. Low-Level Laser Therapy Restores the Oxidative Stress Balance in Acute Lung Injury Induced by Gut Ischemia and Reperfusion. Photochem. Photobiol. 2013 Jan;89(1):179–188. doi: 10.1111/j.1751-1097.2012.01214.x. [DOI] [PubMed] [Google Scholar]
- 91.Patrocínio-Silva TL, de Souza AMF, Goulart RL, Pegorari CF, Oliveira JR, Fernandes K, Magri A, Pereira RMR, Araki DR, Nagaoka MR, Parizotto NA, Rennó ACM. The effects of low-level laser irradiation on bone tissue in diabetic rats. Lasers Med. Sci. 2013 Aug; doi: 10.1007/s10103-013-1418-y. [DOI] [PubMed] [Google Scholar]
- 92.Aliodoust M, Bayat M, Jalili MR, Sharifian Z, Dadpay M, Akbari M, Bayat M, Khoshvaghti A, Bayat H. Evaluating the effect of low-level laser therapy on healing of tentomized Achilles tendon in streptozotocin-induced diabetic rats by light microscopical and gene expression examinations. Lasers Med. Sci. 2014 Jul;29(4):1495–1503. doi: 10.1007/s10103-014-1561-0. [DOI] [PubMed] [Google Scholar]
- 93.Dang Y, Liu B, Liu L, Ye X, Bi X, Zhang Y, Gu J. The 800-nm diode laser irradiation induces skin collagen synthesis by stimulating TGF-β/Smad signaling pathway. Lasers Med. Sci. 2011;26(6):837–843. doi: 10.1007/s10103-011-0985-z. [DOI] [PubMed] [Google Scholar]
- 94.Assis L, Moretti AIS, Abrahão TB, Cury V, Souza HP, Hamblin MR, a Parizotto N, Carlos S. NIH Public Access. 2013;44(9):726–735. doi: 10.1002/lsm.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, Wang C, Wang Y, Liao W, Chen Y, Kuo C, Kuo H, Hung C. Effects of Low-Level Laser Therapy on M1-Related Cytokine Expression in Monocytes via Histone Modification. Mediators Inflamm. 2014:1–13. doi: 10.1155/2014/625048. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J. Neurosci. 2013;33(33):13505–13517. doi: 10.1523/JNEUROSCI.0918-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Araújo TG, de Oliveira AG, Tobar N, Saad MJA, Moreira LR, Reis ER, Nicola EMD, de Jorge GL, dos Tártaro RR, Boin IFSF, Teixeira ARF. Liver regeneration following partial hepatectomy is improved by enhancing the HGF/Met axis and Akt and Erk pathways after low-power laser irradiation in rats. Lasers Med. Sci. 2013 Nov;28(6):1511–1517. doi: 10.1007/s10103-013-1264-y. [DOI] [PubMed] [Google Scholar]
- 98.Damante CA, De Micheli G, Miyagi SPH, Feist IS, Marques MM. Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts. Lasers Med. Sci. 2009 Nov;24(6):885–891. doi: 10.1007/s10103-008-0582-y. [DOI] [PubMed] [Google Scholar]
- 99.Lim W, Kim J, Kim S, Karna S, Won J, Jeon SM, Kim SY, Choi Y, Choi H, Kim O. Modulation of lipopolysaccharide-induced NF-κB signaling pathway by 635 nm irradiation via heat shock protein 27 in human gingival fibroblast cells. Photochem. Photobiol. 2013;89(1):199–207. doi: 10.1111/j.1751-1097.2012.01225.x. [DOI] [PubMed] [Google Scholar]
- 100.Eells JT, Henry MM, Summerfelt P, Wong-Riley MTT, V Buchmann E, Kane M, Whelan NT, Whelan HT. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc. Natl. Acad. Sci. U. S. A. 2003;100(6):3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang HL, Whelan HT, Eells JT, Meng H, Buchmann E, Lerch-Gaggl a, Wong-Riley M. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006;139(2):639–649. doi: 10.1016/j.neuroscience.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 102.Wong-Riley MTT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J. Biol. Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Wu S, Xing D. Inhibition of Aβ 25–35-induced cell apoptosis by Low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell. Signal. 2012;24(1):224–232. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 104.Sperandio FF, Simões A, Corrêa L, Aranha ACC, Giudice FS, Hamblin MR, Sousa SCOM. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics. 2014;9999(9999) doi: 10.1002/jbio.201400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esmaeelinejad M, Bayat M, Darbandi H, Bayat M, Mosaffa N. The effects of low-level laser irradiation on cellular viability and proliferation of human skin fibroblasts cultured in high glucose mediums. Lasers Med. Sci. 2014;29(1):121–129. doi: 10.1007/s10103-013-1289-2. [DOI] [PubMed] [Google Scholar]
- 106.Szymanska J, Goralczyk K, Klawe JJ, Lukowicz M, Michalska M, Goralczyk B, Zalewski P, Newton JL, Gryko L, Zajac a, Rosc D. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J. Physiol. Pharmacol. 2013;64(3):387–391. [PubMed] [Google Scholar]
- 107.Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A. Effect of Low Level Laser Therapy on Proliferation and Differentiation of the Cells Contributing in Bone Regeneration. 2014;5(4):163–170. [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai WC, Hsu CC, Pang JHS, Lin MS, Chen YH, Liang FC. Low-level laser irradiation stimulates tenocyte migration with up-regulation of dynamin II expression. PLoS One. 2012;7(5):1–7. doi: 10.1371/journal.pone.0038235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.AlGhamdi KM, Kumar A, Ashour AE, AlGhamdi Aa. A comparative study of the effects of different low-level lasers on the proliferation, viability, and migration of human melanocytes in vitro. Lasers Med. Sci. 2015;30(5):1541–1551. doi: 10.1007/s10103-015-1758-x. [DOI] [PubMed] [Google Scholar]
- 110.Liao X, Xie G-H, Liu H-W, Cheng B, Li S-H, Xie S, Xiao L-L, Fu X-B. Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: proposed mechanism for enhanced wound re-epithelialization. Photomed. Laser Surg. 2014;32(4):219–225. doi: 10.1089/pho.2013.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rochklnd S, El-Ani D, Nevo Z, Shahar A. Increase of neuronal sprouting and migration using 780 nm laser phototherapy as procedure for cell therapy. Lasers Surg. Med. 2009;41(4):277–281. doi: 10.1002/lsm.20757. [DOI] [PubMed] [Google Scholar]
- 112.Frozanfar A, Ramezani M, Rahpeyma A, Khajehahmadi S, Arbab HR. The effects of low level laser therapy on the expression of collagen type I gene and proliferation of human gingival fibroblasts (HGF3-PI 53): In vitro study. Iran. J. Basic Med. Sci. 2013;16(10):1071–1074. [PMC free article] [PubMed] [Google Scholar]
- 113.Hamajima S, Hiratsuka K, Kiyama-Kishikawa M, Tagawa T, Kawahara M, Ohta M, Sasahara H, Abiko Y. Effect of low-level laser irradiation on osteoglycin gene expression in osteoblasts. Lasers Med. Sci. 2003;18(2):78–82. doi: 10.1007/s10103-003-0255-9. [DOI] [PubMed] [Google Scholar]
- 114.Abrahamse H. Regenerative Medicine, Stem Cells, and Low-Level Laser Therapy: Future Directives. Photomed. Laser Surg. 2012;30(12):681–682. doi: 10.1089/pho.2012.9881. [DOI] [PubMed] [Google Scholar]
- 115.Ginani F, Soares DM, Barreto MPEV, Barboza CAG. Effect of low-level laser therapy on mesenchymal stem cell proliferation: a systematic review. Lasers Med. Sci. 2015 doi: 10.1007/s10103-015-1730-9. [DOI] [PubMed] [Google Scholar]
- 116.Min KH, Byun JH, Heo CY, Kim EH, Choi HY, Pak CS. Effect of Low-Level Laser Therapy on Human Adipose-Derived Stem Cells: In Vitro and In Vivo Studies. Aesthetic Plast. Surg. 2015 doi: 10.1007/s00266-015-0524-6. [DOI] [PubMed] [Google Scholar]
- 117.Soares DM, Ginani F, Henriques ÁG, Barboza CAG. Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med. Sci. 2015 Apr;30(3):1171–1174. doi: 10.1007/s10103-013-1436-9. [DOI] [PubMed] [Google Scholar]
- 118.Rochkind S, Geuna S, Shainberg A. Phototherapy and Nerve Injury. (1st) 2013;109 doi: 10.1016/B978-0-12-420045-6.00004-3. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- 119.Bouvet-Gerbettaz S, Merigo E, Rocca J-P, Carle GF, Rochet N. Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg. Med. 2009;41(4):291–297. doi: 10.1002/lsm.20759. [DOI] [PubMed] [Google Scholar]
- 120.Xuan W, Agrawal T, Huang L, Gupta GK, Hamblin MR. Low-level laser therapy for traumatic brain injury in mice increases brain derived neurotrophic factor (BDNF) and synaptogenesis. J. Biophotonics. 2015;8(6):502–511. doi: 10.1002/jbio.201400069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Marković AB, Todorović L. Postoperative analgesia after lower third molar surgery: contribution of the use of long-acting local anesthetics, low-power laser, and diclofenac. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology. 2006;102(5) doi: 10.1016/j.tripleo.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 122.Tanboga I, Eren F, Altinok B, Peker S, Ertugral F. The effect of low level laser therapy on pain during dental tooth-cavity preparation in children. Eur. Arch. Paediatr. Dent. 2011;12(2):93–95. doi: 10.1007/BF03262786. [DOI] [PubMed] [Google Scholar]
- 123.Chan a, Armati P, Moorthy aP. Pulsed Nd: YAG Laser Induces Pulpal Analgesia: A Randomized Clinical Trial. J. Dent. Res. 2012;91(7 Suppl):S79–S84. doi: 10.1177/0022034512447947. [DOI] [PubMed] [Google Scholar]
- 124.Peres e Serra A, a Ashmawi H. Influence of naloxone and methysergide on the analgesic effects of low-level laser in an experimental pain model. Rev. Bras. Anestesiol. 2010;60(3):302–310. doi: 10.1016/S0034-7094(10)70037-4. [DOI] [PubMed] [Google Scholar]
- 125.Yan W, Chow R, Armati PJ. Inhibitory effects of visible 650-nm and infrared 808-nm laser irradiation on somatosensory and compound muscle action potentials in rat sciatic nerve: Implications for laser-induced analgesia. J. Peripher. Nerv. Syst. 2011;16(2):130–135. doi: 10.1111/j.1529-8027.2011.00337.x. [DOI] [PubMed] [Google Scholar]
- 126.Fujimoto K, Kiyosaki T, Mitsui N, Mayahara K, Omasa S, Suzuki N, Shimizu N. Low-intensity laser irradiation stimulates mineralization via increased BMPs in MC3T3-E1 cells. Lasers Surg. Med. 2010;42(6):519–526. doi: 10.1002/lsm.20880. [DOI] [PubMed] [Google Scholar]
- 127.Gonçalves RV, Novaes RD, Do Carmo Cupertino M, Moraes B, Leite JPV, Do Carmo Gouveia Peluzio M, De Mello Pinto MV, Da Matta SLP. Time-dependent effects of low-level laser therapy on the morphology and oxidative response in the skin wound healing in rats. Lasers Med. Sci. 2013;28(2):383–390. doi: 10.1007/s10103-012-1066-7. [DOI] [PubMed] [Google Scholar]
- 128.Hoffman M, Monroe DM. Low intensity laser therapy speeds wound healing in hemophilia by enhancing platelet procoagulant activity. Wound Repair Regen. 2012;20(5):770–777. doi: 10.1111/j.1524-475X.2012.00828.x. [DOI] [PubMed] [Google Scholar]
- 129.Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014;46(2):144–151. doi: 10.1002/lsm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun X, Wu S, Xing D. The reactive oxygen species-Src-Stat3 pathway provokes negative feedback inhibition of apoptosis induced by high-fluence low-power laser irradiation. FEBS J. 2010;277(22):4789–4802. doi: 10.1111/j.1742-4658.2010.07884.x. [DOI] [PubMed] [Google Scholar]
- 131.Chu J, Wu S, Xing D. Survivin mediates self-protection through ROS/cdc25c/CDK1 signaling pathway during tumor cell apoptosis induced by high fluence low-power laser irradiation. Cancer Lett. 2010;297(2):207–219. doi: 10.1016/j.canlet.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 132.Gavish L, Rubinstein C, Berlatzky Y, Gavish LY, Beeri R, Gilon D, Bulut a, Harlev M, Reissman P, Gertz SD. Low level laser arrests abdominal aortic aneurysm by collagen matrix reinforcement in apolipoprotein E-deficient mice. Lasers Surg Med. 2012;44(8):664–674. doi: 10.1002/lsm.22068. [DOI] [PubMed] [Google Scholar]
- 133.Kilík R, Lakyová L, Sabo J, Kruzliak P, Lacjaková K, Vasilenko T, Vidová M, Longauer F, Radoňak J. Effect of equal daily doses achieved by different power densities of low-level laser therapy at 635 nm on open skin wound healing in normal and diabetic rats. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/269253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Binder H. Water near lipid membranes as seen by infrared spectroscopy. Eur. Biophys. J. 2007;36(4–5):265–279. doi: 10.1007/s00249-006-0110-6. [DOI] [PubMed] [Google Scholar]
- 135.Vatansever F, Hamblin MR. Far infrared radiation (FIR): its biological effects and medical applications\Ferne Infrarotstrahlung: Biologische Effekte und medizinische Anwendungen. Photonics and Lasers in Medicine. 2012:1–12. doi: 10.1515/plm-2012-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Choi M, Kim JE, Cho KH, Lee JH. In vivo and in vitro analysis of low level light therapy: A useful therapeutic approach for sensitive skin. Lasers Med. Sci. 2013;28(6):1573–1579. doi: 10.1007/s10103-013-1281-x. [DOI] [PubMed] [Google Scholar]