Abstract
Objective
To assess the association of reported smoking cessation at various time points during pregnancy with fetal growth restriction (FGR).
Methods
This was a population-based retrospective cohort study of singleton nonanomalous live births using Ohio birth certificates, 2006–2012. Outcomes of women who reported smoking only in the 3 months before conception and women who reported smoking through the first, second, or third trimester were compared to a referent group of nonsmokers. Multivariate logistic regression assessed the association between smoking cessation at various times in pregnancy and FGR less than the 10th and 5th percentiles.
Results
Of 927,424 births analyzed, 75% did not smoke. Of smokers, 24% smoked preconception only, 10% quit after the 1st trimester, 4% quit after the 2nd trimester, and 59% smoked throughout pregnancy. The rate of FGR less than the 10th and 5th percentiles among non-smokers was 8.1% and 3.6%, respectively. Although smoking only in the preconception period did not significantly increase FGR risk, smoking in any trimester did. The adjOR(95%CI) for FGR less than the 10th and 5th percentiles, respectively, of cessation after the 1st trimester was 1.19(1.13,1.24) and 1.25(1.17,1.33), and 1.67(1.57,1.78) and 1.83(1.68,1.99) for cessation after the second trimester. Women who reported smoking throughout pregnancy had the highest risks of FGR, 2.26 (2.22,2.31) and 2.44(2.37,2.51), after accounting for the influence of race, low socioeconomic status, and medical comorbidities.
Conclusions
Smoking of any duration during pregnancy is associated with an increased risk of FGR, with decreasing risk the earlier that cessation occurs. Smoking cessation programs should focus on the benefit of quitting as early in pregnancy as possible.
Introduction
Smoking during pregnancy has been associated with an increased risk of a multitude of adverse outcomes, including fetal growth restriction.1,2 As of 2010, 23% of women nationwide smoked tobacco in the 3 months before pregnancy, and just over half (54%) quit during pregnancy.3 In Ohio, a high-prevalence smoking area, only 24% of women who smoked in the 3 months before pregnancy quit before conception and 16% of pregnant women continued to smoke in the 3rd trimester.3,4
Although several studies have examined the effects of smoking cessation in pregnancy on fetal growth restriction, many have been limited in size or ethnic diversity.5–8 Limited data exist to assess the association between smoking cessation before pregnancy and in each trimester and risk of fetal growth restriction. Additional knowledge about the benefits of quitting in the preconception or early gestational period would be an added incentive to quit earlier for the women who struggle with this addiction. We aimed to perform a large-scale population-based study of recent US births to quantify the influence of smoking before pregnancy or in any trimester on the risk of fetal growth restriction.
MATERIALS AND METHODS
The protocol for this study was approved by the Human Subjects Institutional Review Board of the Ohio Department of Health and a de-identified data set was provided for this analysis. This study was exempt from review by the Institutional Review Board at the University of Cincinnati, Cincinnati, Ohio
We performed a population-based retrospective cohort study of all live births in Ohio over 7 years (2006–2012) using vital statistics birth certificate records from the Ohio Department of Health. Women were divided into 1 of 5 exposure groups based on their reported smoking status: “non-smokers,” “smoked preconception only” (smoked in the 3 months preconception but not in any trimester), “smoked through 1st trimester” (smoked preconception and the 1st trimester but not 2nd or 3rd), “smoked through 2nd trimester” (smoked preconception and the first 2 trimesters but not the 3rd), and “smoked throughout pregnancy” (smoked before and in all 3 trimesters). For each trimester, women who smoked an average of ≥1 cigarette a day were considered smokers. We also performed a sensitivity analysis using a threshold of 5 cigarettes per day. We limited analyses to singleton non-anomalous live births between 20 and 42 weeks gestation with available data on smoking history.
The primary outcome was fetal growth restriction less than the 10th and less than the 5th percentiles, as defined by the 1996 Alexander U.S. growth curves reference.9 We compared baseline demographic, socioeconomic, prenatal and behavioral characteristics among all 5 exposure groups. Additionally, a linear regression was performed to assess the dose-response relationship between cigarettes smoked per trimester and birth weight.
Gestational age was defined by the best obstetric estimate variable in the birth record, which combines last menstrual period and ultrasound parameters. BMI was calculated using maternal preconception height and weight, and gestational weight gain was calculated by subtracting maternal weight at delivery from preconception maternal weight.
Comparisons of dichotomous variables were performed with chi squared tests and continuous variables were compared using ANOVA. Multinomial logistic regression estimated the adjusted odds ratio of FGR less than the 10th and less than the 5th percentiles associated with various smoking behaviors after adjusting for maternal age, race, education level, marital status, pregestational hypertension and diabetes, gestational hypertension and diabetes, and BMI. Covariates were selected based on differences noted in univariate comparisons and those with biologic plausibility. Each smoking group was compared to the referent group of nonsmokers. Additionally, a linear regression was performed to assess the dose-response relationship between cigarettes smoked per trimester and birth weight.
Analyses were performed using STATA 12.1 software (StataCorp, College Station, Texas). Results are reported as rates with associated p values and odds ratios with 95% confidence intervals. Comparisons were considered statistically significant if the p value was <0.01 or the 95% confidence interval did not include the null value 1.0.
RESULTS
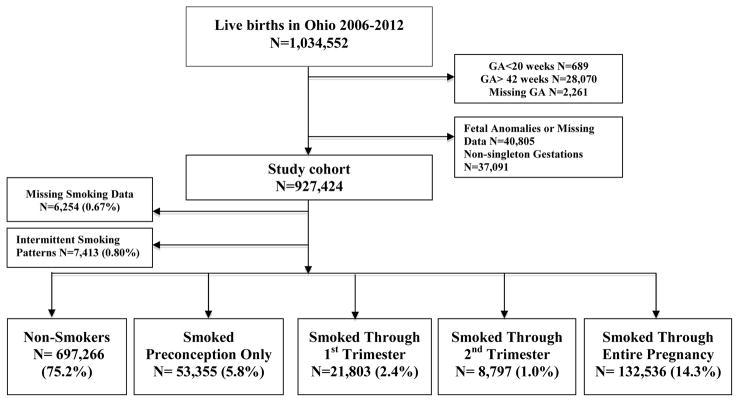
The total number of live births during the study period was 1,034,552. Following exclusions, there were 927,424 live births included in this analysis (figure 1). There was minimal missing data (<2%) for most variables analyzed; however, gestational age at first prenatal visit was missing in 25% and Body Mass Index (BMI) and Medicaid status were missing in 5% and 4%, respectively. There were few cases (N=13,667, 1.5%) with missing smoking data or smoking behaviors that did not fall within any of the 5 exposure groups. The reference group of non-smokers comprised 75.2% of births during the study period. Nearly 1 in 4 women during the study period smoked cigarettes. Of those, 23.8% smoked preconception only, 9.7% smoked through the first trimester, 3.9% smoked through the second trimester, and the majority (59.2%) smoked throughout the entire pregnancy, Figure 1.
Figure 1.
Flow Diagram of the Study Population
Seventy-six percent of the study population comprised births to non-Hispanic white mothers (N=709,297). Non-Hispanic white mothers were more frequently smokers than non-white mothers (N=188,985, 26.6% vs. N=34,628, 16.0%, p<0.01) and more likely to smoke throughout pregnancy (N=114,230, 16.1% vs. N=18,135, 8.4%, p<0.01). Young mothers age <20 years were more likely to be smokers than women ages 20–34 years (N=29,406, 31.5% vs. N=180,099, 24.9%, p<0.01) and more likely to smoke throughout pregnancy (N=16,883,18.1% vs N=106,755,14.8%, p<0.01).
Women who reported quitting smoking in the first trimester were more likely to initiate prenatal care in the first trimester than women who reported smoking throughout pregnancy (N=32,033, 60.0% vs. N=61,947, 46.7%, p<0.01). Other maternal characteristics associated with adverse smoking behaviors included low education level, limited prenatal care, late initiation of prenatal care at ≥20 weeks, unmarried, WIC enrollment, and Medicaid funded delivery, Table I.
Table 1.
Baseline Maternal Characteristics
| Non-Smokers N=697,266 |
Smoked Preconception Only N=53,355 |
Smoked Through 1st Trimester N=21,803 |
Smoked Through 2nd Trimester N=8,797 |
Smoked throughout pregnancy N=132,536 |
|
|---|---|---|---|---|---|
| Demographic Factors | |||||
| Maternal Race | |||||
| Non-Hispanic White | 517,166 (72.9%) | 44,435 (6.3%) | 17,728 (2.5%) | 7,130 (1.0%) | 114,230 (16.1%) |
| Non-Hispanic Black | 120,430 (80.0%) | 6,534 (4.3%) | 3,123 (2.1%) | 1,355 (0.9%) | 14,920 (9.9%) |
| Hispanic | 36,657 (86.1) | 1,858 (4.4%) | 753 (1.8%) | 249 (0.6%) | 2,564 (6.0%) |
| Other | 21,805 (93.4%) | 471(2.0%) | 169 (0.7%) | 48 (0.2%) | 651(2.8%) |
| Parity | 1 (0,2) | 0 (0,1) | 0 (0,1) | 1 (0,2) | 1 (0,2) |
| Maternal Age Mean | 27.7 ± 5.9 | 25.7 ± 5.3 | 24.6 ± 5.2 | 24. 4 ± 5.3 | 25.2 ± 5.4 |
| Maternal Age Median and IQR | 28 (23,32) | 25 (22,29) | 24 (21,28) | 23 (20,27) | 24 (21,29) |
| Maternal Age | 28 (23, 32) | 25 (22, 29) | 24 (20, 28) | 23 (20, 27) | 24 (21, 28) |
| Age Group | |||||
| <20 | 63,110 (67.6%) | 6,163 (6.6%) | 3,580 (3.8%) | 1,524 (1.6%) | 16,883 (18.1%) |
| 20–34 | 538,815 (74.5%) | 43,783(6.1%) | 17,068 (2.4%) | 6,777 (0.9%) | 106,755 (14.8%) |
| ≥35 | 95,341 (86.3%) | 3,409 (3.1%) | 1,155(1.1%) | 496 (0.5%) | 8,898 (8.1%) |
| Socioeconomic Factors | |||||
| Less than High School Diploma | 94,378 (13.5%) | 7,171(13.4%) | 4,243 (19.5%) | 2,192 (24.9%) | 42,280 (31.9%) |
| Unmarried | 238,063 (34.1%) | 29,475 (55.2%) | 15,005 (68.8%) | 6,533 (74.3%) | 94,084 (71.0%) |
| WIC | 239,827 (34.4%) | 26,265 (50.2%) | 13,141 (60.3%) | 5,709 (64.9%) | 90,035 (67.9%) |
| Medicaid | 202,044 (29.0%) | 23,487 (44.0%) | 12,248 (56.2%) | 5,662 (64.4%) | 90,451 (68.3%) |
| Prenatal Care | |||||
| Limited (≤5 Visits) | 49,531 (7.1%) | 3,293 (6.2%) | 1,700 (7.8%) | 976 (11.1%) | 16,423 (12.4%) |
| Early Initiation ≤12 Weeks Gestation | 400,724 (57.5%) | 32,033(60.0%) | 11,185 (51.3%) | 4,054 (46.1%) | 61,947 (46.7%) |
| Late Initiation >20 Weeks Gestation | 41,237 (5.9%) | 2,709 (5.1%) | 1,573 (7.2%) | 788 (9.0%) | 12,073 (9.1%) |
| Maternal Health Indicators | |||||
| Prior C-Section | 90,386 (13.0%) | 5,499 (10.3%) | 2,245 (10.3%) | 1,025 (11.7%) | 18,455 (13.9%) |
| Underweight, Prepregnancy BMI<18.5 | 23,840 (3.4%) | 2,436 (4.6%) | 1,274(5.8%) | 610(6.9%) | 9,836 (7.4%) |
| Obesity, Prepregnancy BMI ≥ 30 | 153,533 (22.0%) | 12,356 (23.2%) | 5,197 (23.8%) | 2,101 (23.9%) | 30,293 (22.9%) |
| Gestational Weight Gain, pounds | 31.2 ± 16.9 | 36.4 ± 18.8 | 36.5 ± 19.2 | 33.6 ± 19.5 | 30.3 ± 18.9 |
| Chronic Hypertension | 13,123 (1.9%) | 980 (1.8%) | 374 (1.7%) | 196 (2.2%) | 2,232 (1.7%) |
| Pre-gestational Diabetes | 5,412 (0.8%) | 402 (0.8%) | 196 (0.9%) | 83 (0.9%) | 1,188 (0.9%) |
| Gestational Hypertension/Pre-eclampsia | 32,252 (4.6%) | 2,754(5.2%) | 1,075 (4.9%) | 373 (4.2%) | 4,407 (3.3%) |
| Gestational Diabetes | 37,789 (5.4%) | 3,076(5.8%) | 1,228 (5.6%) | 483(5.5%) | 6,370 (4.8%) |
All comparisons are statistically significant at p-value ≤0.001 for the X2 statistic corresponding to the 5 IPI group comparison for each maternal characteristic in this table.
Dichotomous variables are presented as percent of n for corresponding smoking group, except for the categorical variables mother’s race and age group, which reflect percentages across rows.
Continuous variables are presented as mean ± standard deviation or median and interquartile range.
Women who reported smoking throughout pregnancy were more likely to be heavy smokers preconception (>20 cigarettes/day) than those who quit smoking early, after the preconception period (N=76,149, 57.5% vs. N=16,595, 31.1%, p<0.01), Table 2. However, women who smoked throughout pregnancy reported a reduction in their average cigarettes smoked per day from 17.1 ± 9.9 preconception to 10.0 ± 7.5 in the third trimester, Table 2. In all groups who reported smoking during pregnancy, women most frequently smoked <10 cigarettes/day.
Table 2.
Smoking Behavior Comparisons
| Non-Smokers N=697,266 |
Smoked Preconception Only N=53,355 |
Smoked Through 1st Trimester N=21,803 |
Smoked Through 2nd Trimester N=8,797 |
Smoked throughout pregnancy N=132,536 |
|
|---|---|---|---|---|---|
| Preconception Smoking Behaviors | |||||
| Average Number of Cigarettes Smoked per Day | 0 | 11.8±9.6 | 14.6±10.4 | 16.1±9.8 | 17.1±9.9 |
| Heavy Smoking (≥20/day) | 0% | 16,595 (31.1%) | 9,617 (44.1%) | 4,644 (52.8%) | 76,149 (57.5%) |
| Smoking Behaviors in Final 3 Months of Smoking | |||||
| Average Number of Cigarettes Smoked | 0 | 11.8 ± 9.7 | 9.0 ± 8.1 | 7.2 ± 7.2 | 10.0 ± 7.5 |
| <10 | 0.0% | 20,897 (39.2%) | 11,806 (54.2%) | 5,832 (66.3%) | 56,815 (42.9%) |
| 10–19 | 0.0% | 15,863 (29.7%) | 5,901 (27.1%) | 1,956 (22.2%) | 49,540 (37.4%) |
| ≥20 | 0.0% | 16,595 (31.1%) | 4,096 (18.8%) | 1,009 (11.5%) | 26,181 (19.8%) |
All comparisons are statistically significant at p-value ≤0.001 for the X2 statistic corresponding to the 5 IPI group comparison for each maternal characteristic in this table.
Dichotomous variables are presented as percent of n for corresponding smoking group.
Continuous variables are presented as mean ± standard deviation.
Birth outcomes associated with smoking throughout pregnancy included preterm birth <37 weeks (N=18,053,13.6% vs. referent N=69,794, 10.0%), low birth weight <2500 g (N=15,052, 11.4% vs. N=39,173, 5.6%), lower average birth weight (3090 g vs. 3340 g), and NICU admission (N=8,298, 6.3% vs. N=34,300, 4.9%), p-values<0.01, Table 3. Absolute rates of other birth outcomes were similar across the various smoking groups, but p values were statistically significant, likely due to the large sample size.
Table 3.
Birth Outcomes
| Non-Smokers N=697,266 |
Smoked Preconception Only N=53,355 |
Smoked Through 1st Trimester N=21,803 |
Smoked Through 2nd Trimester N=8,797 |
Smoked throughout pregnancy N=132,536 |
|
|---|---|---|---|---|---|
| Maternal | |||||
| Vaginal Delivery | 456,531 (65.4%) | 33,739 (63.2%) | 14,007 (64.2%) | 5,536 (62.9%) | 87,168 (65.8%) |
| Operative Vaginal Delivery | 39,144 (5.6%) | 3,392 (6.4%) | 1,367 (6.3%) | 471 (5.4%) | 6,668 (5.0%) |
| Cesarean Delivery | 200,896 (28.8%) | 16,178 (30.3%) | 6,417 (29.4%) | 2,776 (31.6%) | 38,555 (29.1%) |
| Premature Rupture of Membranes | 19,704 (2.8%) | 1,739 (3.3%) | 750 (3.4%) | 468 (5.3%) | 4,296 (3.2%) |
| PTB <37 weeks | 69,794 (10.0%) | 5,096 (9.6%) | 2,477 (11.4%) | 1,590 (18.1%) | 18,053 (13.6%) |
| Meconium | 38,098 (5.5%) | 2,833 (5.3%) | 1,179 (5.4%) | 501 (5.7%) | 7,558 (5.7%) |
| Induction of Labor | 220,696 (31.7%) | 19,660 (36.9%) | 7,977 (36.6%) | 2,971 (33.8%) | 41,281 (31.2%) |
| Fetal Intolerance to Labor | 44,932 (6.4%) | 4,022 (7.5%) | 1,516 (7.0%) | 688 (7.6%) | 7,899 (6.0%) |
| Newborn | |||||
| BW Mean ± SD | 3340.0 ± 557.8 | 3339.0 ± 557.0 | 3280.0 ± 589.8 | 3072.5 ± 762.9 | 3090.1 ± 542.4 |
| LBW <2500 gm | 39,173 (5.6%) | 3,035 (5.7%) | 1,585 (7.3%) | 1,306 (14.9%) | 15,052 (11.4%) |
| ELBW <1500 gm | 7,391 (1.1%) | 535 (1.0%) | 309 (1.4%) | 541 (6.2%) | 1,636 (1.2%) |
| NICU Admission | 34,300 (4.9%) | 2,720 (5.1%) | 1,260 (5.8%) | 930 (10.6%) | 8,298 (6.3%) |
| Infant Transfer | 14,234 (2.0%) | 1,162 (2.2%) | 515 (2.4%) | 368 (4.2%) | 3,869 (2.9%) |
| 5 min. Apgar score <7 | 15,610 (2.2%) | 1,244 (2.3%) | 581 (2.7%) | 401 (4.6%) | 3,008 (2.3%) |
PTB = preterm birth; BW = birth weight; LBW = low birth weight; ELBW = extremely low birth weight; NICU = neonatal intensive care unit
All comparisons are statistically significant at p-value ≤0.001 for the X2 statistic corresponding to the 5 IPI group comparison for each maternal characteristic in this table.
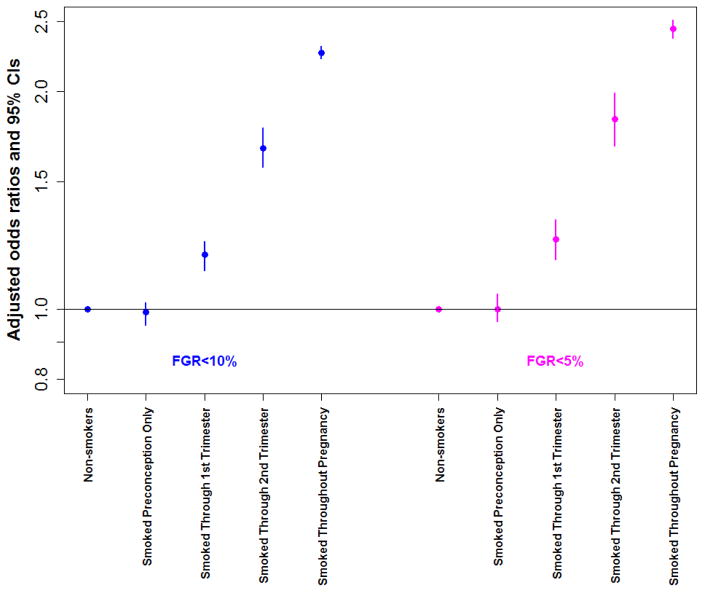
The overall frequency of FGR less than the 10th and 5th percentiles in the study cohort was 9.8% (N=90,613) and 4.6% (N=42,547) respectively. The rate of FGR <10th and <5th percentiles was lowest in non-smokers (8.1%, N=56,666 and 3.6%, N=25,380), followed by those who smoked preconception only (8.4%, N=4,485 and 3.8%, N=2,034). For all groups who smoked in at least 1 trimester of pregnancy, the risk of FGR over non-smokers increased significantly. For women who quit smoking in pregnancy, the adjusted odds ratio (95% CI) of smoking just through the first trimester was 1.19(1.13,1.24) and 1.25(1.17,1.33), and was higher with smoking through the second trimester at 1.67(1.57,1.78) and 1.83(1.68,1.99), respectively for FGR less than the 10th and 5th percentiles, Table 4. Smoking throughout pregnancy carried the highest risk of FGR with over two-fold increase risk for birth weights less than the 10th and 5th percentiles, even after accounting for coexistent influences of maternal age, race, education, marital status, Medicaid funded delivery, pregestational hypertension and diabetes, gestational hypertension and diabetes, and BMI. The attributable risk of smoking throughout pregnancy was 10.2% for FGR less than the 10th percentile and 5.8% for FGR less than the 5th percentile, Table 4.
Table 4.
Rate of Fetal Growth Restriction In Singleton Pregnancies by Timing of Smoking Cessation
| Non-Smokers N=697,266 |
Smoked Preconception Only N=53,355 |
Smoked Through 1st Trimester N=21,803 |
Smoked Through 2nd Trimester N=8,797 |
Smoked throughout pregnancy N=132,536 |
|
|---|---|---|---|---|---|
| FGR <10th Percentile | 56,566 (8.1%) | 4,485 (8.4%) | 2,286 (10.5%) | 1,281 (14.6%) | 24,182 (18.3%) |
| Attributable Risk | 0.3% (0.25,0.35) | 2.4% (2.2,2.6) | 6.5% (5.98, 7.02) | 10.2% (10.04,10.36) | |
| Crude OR | Ref. | 1.04 (1.01, 1.07) | 1.33 (1.27, 1.39) | 1.93 (1.82, 2.05) | 2.53 (2.49, 2.57) |
| * Adjusted OR | Ref. | 0.99 (0.95, 1.02) | 1.19 (1.13, 1.24) | 1.67 (1.57, 1.78) | 2.26 (2.22, 2.31) |
| FGR <5th Percentile | 25,380 (3.6%) | 2,034 (3.8%) | 1,105 (5.1%) | 649 (7.4%) | 12,500 (9.4%) |
| Attributable Risk | 0.2% (0.16,0.24) | 1.5% (1.34,1.66) | 3.8% (3.4,4.2) | 5.8% (5.67,5.93) | |
| Crude OR | Ref. | 1.05 (1.00, 1.10) | 1.41 (1.33, 1.50) | 2.11 (1.95, 2.29) | 2.76 (2.70, 2.82) |
| * Adjusted OR | Ref. | 1.00 (0.96, 1.05) | 1.25 (1.17, 1.33) | 1.83 (1.68, 1.99) | 2.44 (2.37, 2.51) |
Odds ratios were adjusted for maternal age, race, education level, Medicaid, marital status, pregestational hypertension and diabetes, gestational hypertension and diabetes, and Body Mass Index. Births with fetal anomalies and multiple gestations were excluded.
All comparisons are statistically significant at p-value ≤0.001 for the X2 statistic corresponding to the 5 IPI group comparison for each maternal characteristic in this table.
The dose response analysis showed a significant inverse linear correlation between the number of cigarettes smoked per day and birth weight. Each additional cigarette smoked per day in the first, second and third trimesters was associated with a decrease in birth weight of 12.1, 14.8, and 14.5 grams respectively.
The results of a sensitivity analysis performed after redefining smokers as those who reported 5 or more cigarettes per day produced similar results. Using this definition of smokers, the adjusted odds ratio (95% CI) of smoking just through the first trimester was 1.23(1.16,1.30) and 1.28(1.18,1.39), and was higher with smoking through the second trimester at 1.81(1.67,1.97) and 1.96(1.75,2.19) and third trimester at 2.38(2.33,2.43) and 2.57(2.50,2.65) respectively for FGR less than the 10th and 5th percentiles. The risk of FGR was not increased with smoking only before conception [aOR 1.02(0.98, 1.06) and 1.02 (0.97, 1.05) for FGR <10th and 5th percentiles], again similar to the findings of the original analysis displayed in table 4
DISCUSSION
Smoking during any trimester of pregnancy carries an increased risk of fetal growth restriction over non-smokers. Previous studies showed that women who smoke during pregnancy have double the risk of FGR over women who do not smoke1 and that roughly ¼ of all growth restriction cases can be attributed to smoking.10 Fetal growth restriction is a serious pregnancy complication, carrying a 5 to 30-fold increase in infant mortality,11 and an increased risk of preterm birth, necrotizing enterocolitis, and respiratory distress syndrome.12 Recent evidence also suggests that fetal growth restriction contributes to an increased risk of chronic adult diseases, such as Type II Diabetes Mellitus, hypertension, stroke and coronary heart disease.13 In this large population-based cohort study, we found that although preconception smoking was not associated with an increased risk of FGR, smoking during any trimester of pregnancy was associated with FGR risk.
Several studies have concluded that women who quit smoking before the third trimester had similar growth restriction outcomes as those who did not smoke at all during pregnancy.6–8 However, these findings of no significant risk increase in women who smoked but quit earlier in pregnancy may have been limited by smaller sample size (n<11,200) compared to our large cohort of nearly 1 million singleton live births5,6,8, In addition, few similar studies included a population of US women, and the findings of those born in other areas of the world may not be applicable given regional and racial differences in birth weights and smoking patterns across the world.7,8 Finally, one other large U.S. cohort study concluded that quitting smoking in the first trimester reduced risk of FGR compared to a referent group of women who smoked throughout pregnancy.14 This study also drew the conclusion that quitting smoking in the first trimester brought risk of SGA similar to that of non-smokers, but did not use non-smokers as a referent group.14
Fetal growth is influenced by constitutional, environmental, and genetic factors.15 The pharmacogenetics of maternal tobacco use and the effects of smoking on fetal growth are complex. Since fetal growth is continuous during pregnancy, early cessation plausibly results in less effect, if the effect is from genetic interaction or direct toxic effects of nicotine.16 Additionally, the effect of nicotine could theoretically be due to placental-smoking interaction. Decidual invasion occurs during the first trimester. Therefore a critical window of exposure in the first trimester may result in an early pregnancy reversible effect of smoking cessation followed by the remaining gestational period where smoking cessation can no longer salvage normal placentation.17,18 Either or both of these mechanisms could hypothetically explain the greater influence earlier cessation has on normalization of fetal growth as our study suggests. We are unaware of any previously published studies comparing the risk of FGR in women who quit smoking preconception and in each trimester to a referent group of non-smokers.
There are a number of limitations to a study using vital statistics data for research. Some data variables including maternal demographic information and gestational age at birth appear to be very reliably recorded in the birth certificate.19 However, data on pregnancy complications, and medical comorbid conditions are underreported, which may limit the ability to thoroughly adjust for confounding risk factors for FGR.20 Additionally, smoking may be underreported on birth certificate data when compared to anonymous PRAMS surveys, potentially making the effect of smoking even stronger than reported here.21 However, self-reported smoking has been shown to be a valid measure of reproductive smoking behavior, especially when timing of smoking exposure is critical.22 Information regarding alcohol intake, drug use, and second hand smoke exposure was not available in the data set used for this analysis, and thus could not be accounted for as potentially confounding factors.
Our study reinforces prior conclusions that the strongest association between smoking and fetal growth restriction occurs in the third trimester. Additionally, this study provides novel information of an increased risk of FGR with smoking in any trimester of pregnancy, with decreasing risk the earlier smoking cessation occurs. Early initiation of prenatal care may assist women in making the decision to quit smoking early in pregnancy. The only way to achieve the same risk of FGR as non-smoking mothers is by quitting before conception, although quitting at any point is beneficial. Therefore, smoking cessation programs should focus on the benefit of quitting in the preconception period or as early in pregnancy as possible.
Figure 2. Odds of Fetal Growth Restriction in Singleton Pregnancies by Timing of Smoking Cessation.
Odds ratios were adjusted for maternal age, race, education level, Medicaid, marital status, pregestational hypertension and diabetes, gestational hypertension and diabetes, and body mass index. Births with fetal anomalies and multiple gestations were excluded.
Acknowledgments
Kaitlin. Blatt received research funding from an educational grant from the University of Cincinnati Department of Obstetrics and Gynecology Women’s Health Scholars Program. Dr. DeFranco received research funding from the Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio; March of Dimes Grant 22-FY14-470.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presentation information: The abstract for this study has been submitted for consideration of presentation at the ACOG annual meeting May 2–6, 2014 in San Francisco, California, USA.
All of the analysis, interpretations, and conclusions that were derived from the data source and included in this article are those of the authors and not the Ohio Department of Health. Access to de-identified Ohio birth certificate data was provided by the Ohio Department of Health.
References
- 1.Mitchell EA, Thompson JM, Robinson E, et al. Smoking, nicotine and tar and risk of small for gestational age babies. Acta paediatrica. 2002;91(3):323–328. doi: 10.1080/08035250252834003. [DOI] [PubMed] [Google Scholar]
- 2.Raatikainen K, Huurinainen P, Heinonen S. Smoking in early gestation or through pregnancy: a decision crucial to pregnancy outcome. Preventive medicine. 2007 Jan;44(1):59–63. doi: 10.1016/j.ypmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Trends in Smoking Before, During, and After Pregnancy — Pregnancy Risk Assessment Monitoring System, United States, 40 Sites, 2000–2010. MMWR. 2013;62(6):1–19. [PubMed] [Google Scholar]
- 4.Osterman MJ, Martin JA, Curtin SC, Matthews TJ, Wilson EC, Kirmeyer S. Newly released data from the revised U.S. birth certificate, 2011. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013 Dec 10;62(4):1–22. [PubMed] [Google Scholar]
- 5.England LJ, Kendrick JS, Wilson HG, Merritt RK, Gargiullo PM, Zahniser SC. Effects of smoking reduction during pregnancy on the birth weight of term infants. American journal of epidemiology. 2001 Oct 15;154(8):694–701. doi: 10.1093/aje/154.8.694. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman E, Gremy I, Lang JM, Cohen AP. Low birthweight at term and the timing of fetal exposure to maternal smoking. American journal of public health. 1994 Jul;84(7):1127–1131. doi: 10.2105/ajph.84.7.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raisanen S, Sankilampi U, Gissler M, et al. Smoking cessation in the first trimester reduces most obstetric risks, but not the risks of major congenital anomalies and admission to neonatal care: a population-based cohort study of 1,164,953 singleton pregnancies in Finland. Journal of epidemiology and community health. 2014 Feb;68(2):159–164. doi: 10.1136/jech-2013-202991. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K, Sato M, Zheng W, Shinohara R, Yokomichi H, Yamagata Z. Effect of maternal smoking cessation before and during early pregnancy on fetal and childhood growth. Journal of epidemiology / Japan Epidemiological Association. 2014;24(1):60–66. doi: 10.2188/jea.JE20130083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology. 1996 Feb;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 10.Delpisheh A, Kelly Y, Rizwan S, Attia E, Drammond S, Brabin BJ. Population attributable risk for adverse pregnancy outcomes related to smoking in adolescents and adults. Public health. 2007 Nov;121(11):861–868. doi: 10.1016/j.puhe.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Peleg D, Kennedy CM, Hunter SK. Intrauterine growth restriction: identification and management. American family physician. 1998 Aug;58(2):453–460. 466–457. [PubMed] [Google Scholar]
- 12.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American journal of obstetrics and gynecology. 2000 Jan;182(1 Pt 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 13.Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Seminars in perinatology. 2008 Jun;32(3):213–218. doi: 10.1053/j.semperi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polakowski LL, Akinbami LJ, Mendola P. Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstetrics and gynecology. 2009 Aug;114(2 Pt 1):318–325. doi: 10.1097/AOG.0b013e3181ae9e9c. [DOI] [PubMed] [Google Scholar]
- 15.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstetrics and gynecology. 1982 May;59(5):624–632. [PubMed] [Google Scholar]
- 16.Danileviciute A, Grazuleviciene R, Paulauskas A, Nadisauskiene R, Nieuwenhuijsen MJ. Low level maternal smoking and infant birthweight reduction: genetic contributions of GSTT1 and GSTM1 polymorphisms. BMC pregnancy and childbirth. 2012;12:161. doi: 10.1186/1471-2393-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway AC, Salomon A, Soares MJ, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. American journal of physiology Endocrinology and metabolism. 2014 Feb 15;306(4):E443–456. doi: 10.1152/ajpendo.00478.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pringle KG, Kind KL, Sferruzzi-Perri AN, Thompson JG, Roberts CT. Beyond oxygen: complex regulation and activity of hypoxia inducible factors in pregnancy. Human reproduction update. 2010 Jul-Aug;16(4):415–431. doi: 10.1093/humupd/dmp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dietz PM, Bombard JM, Hutchings YL, et al. Validation of obstetric estimate of gestational age on US birth certificates. American journal of obstetrics and gynecology. 2014 Apr;210(4):335, e331–335. doi: 10.1016/j.ajog.2013.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichman NE, Schwartz-Soicher O. Accuracy of birth certificate data by risk factors and outcomes: analysis of data from New Jersey. American journal of obstetrics and gynecology. 2007 Jul;197(1):32, e31–38. doi: 10.1016/j.ajog.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Tong VT, Dietz PM, Farr SL, D’Angelo DV, England LJ. Estimates of smoking before and during pregnancy, and smoking cessation during pregnancy: comparing two population-based data sources. Public health reports. 2013 May-Jun;128(3):179–188. doi: 10.1177/003335491312800308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and perinatal epidemiology. 2005 Sep;19(5):368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]