Abstract
Background
Two vitamin D pregnancy supplementation trials were recently undertaken in South Carolina: The NICHD (n=346) and Thrasher Research Fund (TRF, n=163) studies. The findings suggest increased dosages of supplemental vitamin D were associated with improved health outcomes of both mother and newborn, including risk of preterm birth (<37 weeks gestation). How that risk was associated with 25(OH)D serum concentration, a better indicator of vitamin D status than dosage, by race/ethnic group and the potential impact in the community was not previously explored. While a recent IOM report suggested a concentration of 20 ng/mL should be targeted, more recent work suggests optimal conversion of 25(OH)D to 1,25(OH)2D takes place at 40 ng/mL in pregnant women.
Objective
Post-hoc analysis of the relationship between 25(OH)D concentration and preterm birth rates in the NICHD and TRF studies with comparison to Charleston County, South Carolina March of Dimes (CC-MOD) published rates of preterm birth to assess potential risk reduction in the community.
Methods
Using the combined cohort datasets (n=509), preterm birth rates both for the overall population and for the subpopulations achieving 25(OH)D concentrations of ≤20 ng/mL, >20 to <40 ng/mL, and ≥40 ng/mL were calculated; subpopulations broken down by race/ethnicity were also examined. Log-binomial regression was used to test if an association between 25(OH)D serum concentration and preterm birth was present when adjusted for covariates; locally weighted regression (LOESS) was used to explore the relationship between 25(OH)D concentration and gestational age (weeks) at delivery in more detail. These rates were compared with 2009-2011 CC-MOD data to assess potential risk reductions in preterm birth.
Results
Women with serum 25(OH)D concentrations ≥40 ng/mL (n=233) had a 57% lower risk of preterm birth compared to those with concentrations ≤20 ng/mL [n=82; RR=0.43, 95% confidence interval (CI)=0.22,0.83]; this lower risk was essentially unchanged after adjusting for covariates (RR=0.41, 95% CI=0.20,0.86). The fitted LOESS curve shows gestation week at birth initially rising steadily with increasing 25(OH)D and then plateauing at ~40 ng/mL. Broken down by race/ethnicity, there was a 79% lower risk of preterm birth among Hispanic women with 25(OH)D concentrations ≥40 ng/mL (n=92) compared to those with 25(OH)D concentrations ≤20 ng/mL (n=29; RR=0.21, 95% CI=0.06,0.69) and a 45% lower risk among Black women (n=52 and n=50; RR=0.55, 95% CI=0.17,1.76). There were too few white women with low 25(OH)D concentrations for assessment (n=3). Differences by race/ethnicity were not statistically significant with 25(OH)D included as a covariate.
Compared to the CC-MOD reference group, women with serum concentrations ≥40 ng/mL in the combined cohort had a 46% lower rate of preterm birth overall (n=233, p=0.004) with a 66% lower rate among Hispanic women (n=92, p=0.01) and a 58% lower rate among black women (n=52, p=0.04).
Conclusions
In this post-hoc analysis, achieving a 25(OH)D serum concentration ≥40 ng/mL significantly decreased the risk of preterm birth compared to ≤20 ng/mL. These findings suggest the importance of raising 25(OH)D levels substantially above 20 ng/mL; reaching 40 ng/mL during pregnancy would reduce the risk of preterm birth and achieve the maximal production of the active hormone.
Introduction
Since its discovery a hundred years ago, vitamin D has emerged as one of the most controversial nutrients and prohormones of the 21st century. Its role in calcium metabolism and bone health is undisputed but its role in immune function and long-term health is still debated. There are clear indicators from in vitro and animal in vivo studies that point to the role of vitamin D in both innate and adaptive immunity (1-3), and an emerging number of observational and cohort studies that support vitamin D's role in pregnancy outcomes (4, 5); however, translation of these findings to clinical practice, including the care of pregnant women, has not yet fully materialized. Until recently, there has been a paucity of data from randomized controlled trials to establish clear-cut beneficial effects of vitamin D supplementation or concentration of circulating 25(OH)D during pregnancy.
The current vitamin D requirements during pregnancy as established by the Institute of Medicine's (IOM) 2010 guidelines are 400 IU/day as the Estimated Average Requirement (EAR) and 600 IU/day as the Recommended Dietary Allowance (RDA) (6). Sufficiency is defined by the IOM as a total circulating 25(OH)D concentration of at least 20 ng/mL (6). While the IOM guidelines focus specifically on bone health, these recommendations are widely interpreted as covering all health conditions. Recent observational studies have shown that there exists a large proportion of women who, despite achieving the EAR intake for vitamin D, have 25(OH)D concentrations below 20 ng/mL, including a disproportionately high number of African American and Hispanic women (7-9).
Two vitamin D pregnancy supplementation trials were recently conducted in South Carolina to determine the optimal vitamin D supplementation regimen necessary to achieve sufficiency in pregnant women [defined a priori in the NICHD and Thrasher Research Fund (TRF) studies (10-12) as a total circulating 25(OH)D level 32 ng/mL or greater; deficiency as less than 20 ng/mL; and insufficiency as 20 to <32 ng/mL]. In those trials, 4000 IU vitamin D/day was found to safely achieve a level of at least 32 ng/mL by early in the second trimester in a diverse group of pregnant women. While 1,25(OH)2D can be normal when 25(OH)D is quite low in non-pregnant individuals, only during pregnancy is 25(OH)D directly related to 1,25(OH)2D. Specifically, in the NICHD trial, it was found that the conversion of 25(OH)D to the active form of the hormone, 1,25(OH)2D, plateaued around 40 ng/mL (10). Further, the impact on pregnancy health, including risk of preterm birth (<37 weeks gestation), was explored with findings suggestive that improved vitamin D status was associated with improved health outcomes of both mother and newborn (12, 13).
While higher vitamin D dose regimens were suggestive of a reduced risk of preterm birth (10, 12, 13), the relationship between preterm birth and 25(OH)D serum concentration was not previously explored in detail. Due to multiple input sources and inter-individual variability in dose response (14), 25(OH)D serum concentration is a better indicator of vitamin D status than dose. The objective of this post-hoc analysis was to examine if an association between 25(OH)D concentration and preterm birth incidence exists within the combined NICHD and TRF cohort both overall and among specific race/ethnic groups, and if so, to compare the rates to the Charleston County, South Carolina March of Dimes (CCMOD) published rates of preterm birth (most recently updated in 2011 at the time of this analysis) to assess potential risk reduction in the community (15).
Methods
Study Design
As previously reported, datasets from the NICHD and TRF vitamin D supplementation trials were combined for this analysis using a common data dictionary (13). Details about both clinical trials and results based on dosage have been published previously (10, 12). Briefly, the studies were conducted concurrently and administered identical questionnaires to produce comparable sociodemographic and clinical characteristics using the same criteria. In the NICHD trial, women with baseline 25(OH)D concentrations ≤40 ng/mL were randomized to one of three treatment groups: control (400 IU vitamin D3/day), 2000 IU/day, or 4000 IU/day. Women with concentrations >40-60 ng/mL (n=22) were randomized to 400 or 2000 IU/day and women with concentrations >60 ng/mL (n=1) were given 400 IU/day. In the TRF trial, women were randomized to either 2000 IU/day or 4000 IU/day without exclusion. Outcome measures for both clinical trials included the following: [1] maternal baseline and delivery 25(OH)D; [2] neonatal 25(OH)D concentration; and [3] gestational age at delivery in weeks. This post-hoc analysis included women who participated in either the NICHD trial or the TRF trial and were followed through delivery and had blood samples available within 6 weeks of delivery (n=509).
Definition of Preterm Birth
Information on gestational age was based on the mother's report of the first day of her last menstrual period generating the obstetrical Expected Date of Confinement or Delivery (EDC or EDD) with ultrasound confirmation at the time of mother's first obstetrical visit. All women were enrolled in either the NICHD trial or the TRF trial <16 weeks of gestation and thus ultrasound confirmation of dating was within the first trimester/early second trimester. Preterm birth was defined as delivery of a liveborn infant at <37 completed weeks of gestation.
Laboratory measurements
Maternal and cord blood/neonatal total circulating 25(OH)D assays—A rapid, direct RIA developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples as previously described (10). The laboratory participated in an independent quality assessment/assurance program (DEQAS) using National Institute of Standards and Technology (NIST) standards in place throughout both clinical trials (16). The inter- and intra-assay coefficient of variation was ≤10%.
Statistical Analyses
Baseline demographic characteristics for the NICHD and TRF cohorts were summarized and compared using chi-square tests for categorical variables (race/ethnicity, insurance status, marital status, education level, and preterm birth), Mann-Whitney tests for maternal age, Poisson regression for count data (parity and gravidity), and t-tests for baseline maternal 25(OH)D. Serum 25(OH)D concentrations were plotted against gestation week at birth and locally weighted regression (LOESS) was used to explore the relationship in more detail.
Two 25(OH)D concentrations were of special interest: 20 ng/mL, from the IOM guidelines, and 40 ng/mL, from the 25(OH)D concentration found to achieve maximal production of the active hormone 1,25(OH)2D (10). Preterm birth rates were calculated both for the overall population and among women achieving 25(OH)D concentrations of ≤20 ng/mL, >20 to <40 ng/mL, and ≥40 ng/mL within 6 weeks of delivery. The incidence of preterm birth across 25(OH)D group was tested for a trend and risk ratios and 95% confidence intervals were calculated.
Univariate log-binomial regression was used to identify covariates for multivariate analysis. Covariates tested included race/ethnicity, maternal age, parity, gravidity, insurance status, marital status, education level, and study. Multivariable log-binomial regression was conducted within the combined cohort to estimate risk ratios and 95% confidence intervals for the association between 25(OH)D serum concentration and the risk of preterm birth, adjusting for the effect of covariates with a univariate association of p<0.20. Participants with a race of “other” were excluded due to small cell sizes. To identify differences by race/ethnic group, preterm birth rates were calculated for each race/ethnic group for those with serum concentrations ≤20 ng/mL vs. ≥40 ng/mL. These rates were then compared to the CC-MOD 2009-2011 data to assess potential risk reduction in the community (15).
Results
The characteristics of the combined NICHD/TRF cohort are found in Table 1. In these diverse groups of women, there were significant differences between the NICHD and TRF cohorts by race/ethnicity, maternal age, parity, gravidity, insurance status, marital status, and education level but not baseline 25(OH)D or proportion of preterm births.
Table 1.
Maternal sociodemographic and clinical characteristics of cohort
| Characteristic | Combined Cohort (N=509 ) | NICHD Cohort (N=346) | TRF Cohort (N=163) | p-value* (comparing NICHD v.TRF) |
|---|---|---|---|---|
| Race/ethnicity (N, %) | <0.0001 | |||
| Hispanic | 207 (41%) | 137 (40%) | 70 (43%) | |
| Black | 169 (33%) | 96 (28%) | 73 (45%) | |
| White | 129 (25%) | 113 (33%) | 16(10%) | |
| Other | 4 (1%) | 0 (0°%) | 4 (3%) | |
| Maternal age, years (median and range) | 26 (16-44) | 26 (16-44) | 24 (16-39) | <0.0001 |
| Parity (median and range) | 1 (0-9) | 2 (0-9) | 1 (0-4) | <0.0001 |
| Gravidity (median and range)** | 2 (1-9) | 2 (1-9) | 2 (1-6) | <0.0001 |
| Insurance status (N,%)** | <0.0001 | |||
| None/Medicaid | 342 (68%) | 214 (63%) | 128 (79%) | |
| Marital status (N,%)** | <0.0001 | |||
| Married | 234 (46%) | 178 (52%) | 56 (34%) | |
| Education level (N,%)** | <0.0001 | |||
| Some college or higher | 267 (54%) | 213 (65%) | 54 (33%) | |
| Baseline Maternal 25(OH)D, ng/mL | 0.47 | |||
| Mean (SD) | 23 (9.7) | 24 (9.7) | 23 (9.6) | |
| Birth Outcome (N,%) | 0.20 | |||
| Preterm (<37 weeks) | 50 (10%) | 30 (9%) | 20 (12%) | |
Statistical comparison of characteristics between NICHD and Thrasher Research Fund (TRF) cohorts. Race, insurance status, marital status, education level, and birth outcome compared using chi-square tests. Maternal age compared using Mann-Whitney test. Parity and gravidity compared using Poisson regression. Baseline maternal 25(OH)D compared using t-test.
24 participants missing gravidity data, 4 missing insurance status data, 2 missing marital status data, and 17 missing education level data.
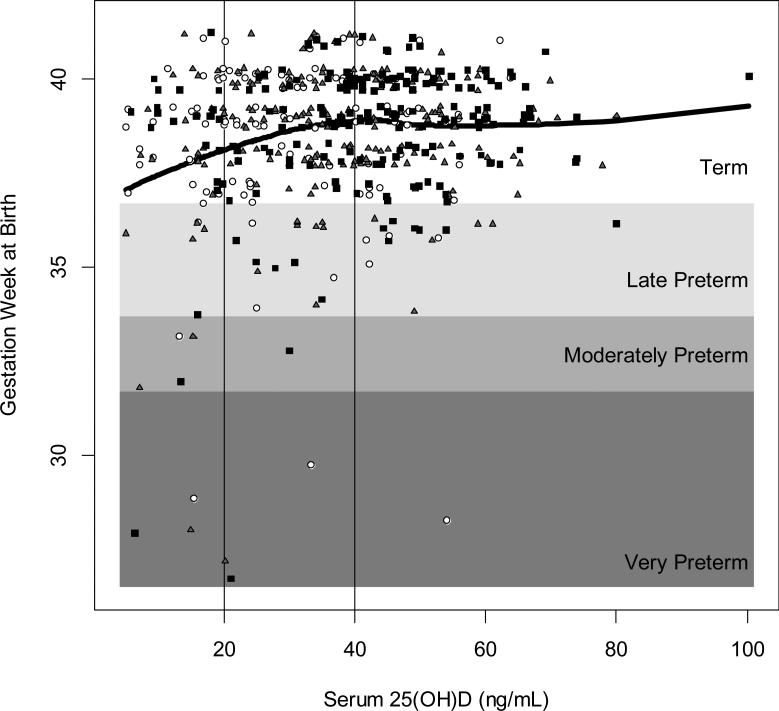
Within the combined cohort, there were 50 preterm births (<37 weeks) (10%), of which 7 were very preterm (<32 weeks), 5 were moderately preterm (32 to <34 weeks), and 38 were late preterm (34 to <37 weeks). A plot of gestation week at birth as a function of 25(OH)D concentration with preterm status and cut points of interest highlighted is shown in Figure 1, with the fitted LOESS curve superimposed.
Figure 1. 25(OH)D concentration within 6 weeks of delivery by gestational age (weeks) at birth (NICHD & TRF, N=509).
Term birth is ≥37 weeks of gestation; late preterm birth is 34 to <37 weeks; moderately preterm is 32 to <34 weeks; and very preterm is <32 weeks. White circles represent women assigned to the control group (400 IU/day); gray triangles represent women assigned to the 2000 IU/day treatment group; and solid black squares represent women assigned to the 4000 IU/day treatment group. Black line represents fitted LOESS curve.
Preterm birth rates were 17% in women with 25(OH)D ≤20 ng/mL (N=82); 10% in women with 25(OH)D >20 to <40 ng/mL (N=194), and 7% in women with 25(OH)D ≥40 ng/mL (N=233). Those with serum 25(OH)D concentrations ≥40 ng/mL within 6 weeks of delivery had a 57% lower risk of preterm birth compared to those with concentrations ≤20 ng/mL (RR=0.43, 95% CI=0.22,0.83), without adjustment for covariates (Table 2). Among women with low baseline 25(OH)D concentrations (≤20 ng/mL) at the beginning of their pregnancy (n=208), those who achieved ≥40 ng/mL within 6 weeks of delivery (n=60) had a 78% lower risk of preterm birth compared to those who did not (RR=0.22, 95% CI=0.05,0.92).
Table 2.
Association between serum 25(OH)D concentration within 6 weeks of delivery and the risk of preterm birth (nichd & trf, n=509)
| Preterm Birth (<37 Weeks) | Term Birth (≥37 Weeks) | p-value (test for trend) | Unadjusted RR (95% CI) | Adjusted† RR (95% CI) | |
|---|---|---|---|---|---|
| ≤20 ng/mL N (%) | 14 (17%) | 68 (83%) | 0.01 | 1.0 | 1.0 |
| >20 to <40 ng/mL N (%) | 19 (10%) | 175 (90%) | 0.57 (0.30, 1.09) | 0.59 (0.31,1.14) | |
| ≥40 ng/mL N (%) | 17 (7%) | 216 (93%) | 0.43 (0.22, 0.83) | 0.41 (0.20, 0.86) |
Bold values signify significance at p<0.05.
Adjusted for race/ethnicity, maternal age, and insurance status.
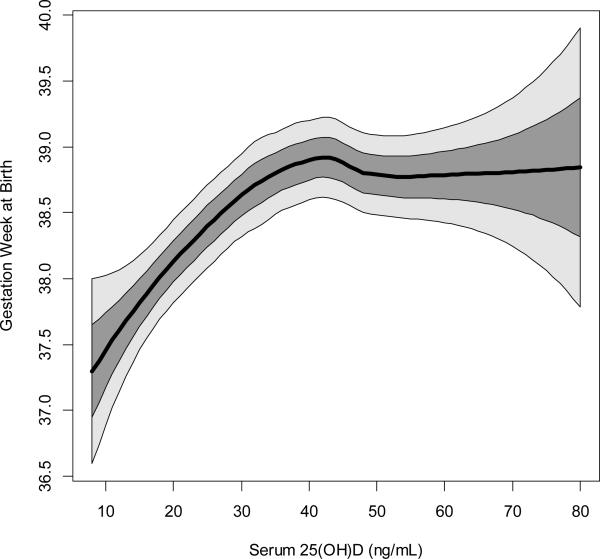
A zoom on the fitted LOESS curve with confidence bounds superimposed to better display the average behavior across its dynamic range shows gestational age at birth initially rising steadily with increasing 25(OH)D concentration and then plateauing at approximately 40 ng/mL (Figure 2). Box plots showing 25(OH)D concentration by birth category are shown in Supplementary Figure 1. The median 25(OH)D within 6 weeks of delivery for those with a preterm birth (<37 weeks) was 31 ng/mL compared to 39 ng/mL for those with a term birth (p=0.01).
Figure 2. Zoom on the fitted LOESS curve of 25(OH)D concentration and gestational age (weeks) at birth to better show the change in average behavior with 1 and 2 SD windows superimposed (NICHD & TRF, N=509).
Black line represents fitted LOESS curve; dark gray area represents 1 standard deviation; and light gray area represents 2 standard deviations. Multivariable log-binomial regression found that 25(OH)D concentrations ≥40 ng/mL reduces the risk of preterm birth by 59% compared to ≤20 ng/mL, adjusted for covariates.
Within the combined cohort, serum 25(OH)D, race/ethnicity, maternal age, and insurance status each had associations of p<0.20 with preterm birth in univariate log-binomial regression analyses and were included in the multivariable log-binomial regression model. Parity, gravidity, marital status, education level, and study had univariate associations of p≥0.20. In multivariable log-binomial regression, the only variable significantly associated with preterm birth was 25(OH)D serum concentration. After adjusting for race/ethnicity, maternal age, and insurance status, participants with 25(OH)D concentrations ≥40 ng/mL had a 59% lower risk of preterm birth than participants with concentrations ≤20 ng/mL (RR=0.41, 95% CI=0.20,0.86) (Table 2). There was a non-significant decrease in risk (41%) between those with concentrations >20 to <40 ng/mL compared to those with concentrations ≤20 ng/mL (RR=0.59, 95% CI=0.31,1.14).
Among race/ethnic groups in the combined cohort, the percentage of preterm birth was 8% among Hispanic women, 13% among black women, and 9% among white women. Supplementary Figure 2 shows the plots of gestational age at birth as a function of 25(OH)D concentration by race/ethnic group, with fitted LOESS curves superimposed. There was a 79% reduction in preterm birth among Hispanic women who attained ≥40 ng/mL compared to those ≤20 ng/mL (RR=0.21, 95% CI=0.06,0.69), and a 45% reduction among black women (RR=0.55, 95% CI=0.17,1.76). In white women, there were not enough women with low 25(OH)D concentrations within 6 weeks of delivery for assessment (n=3). Differences by race/ethnicity were not statistically significant when 25(OH)D concentration was included as a covariate in multivariable regression analysis.
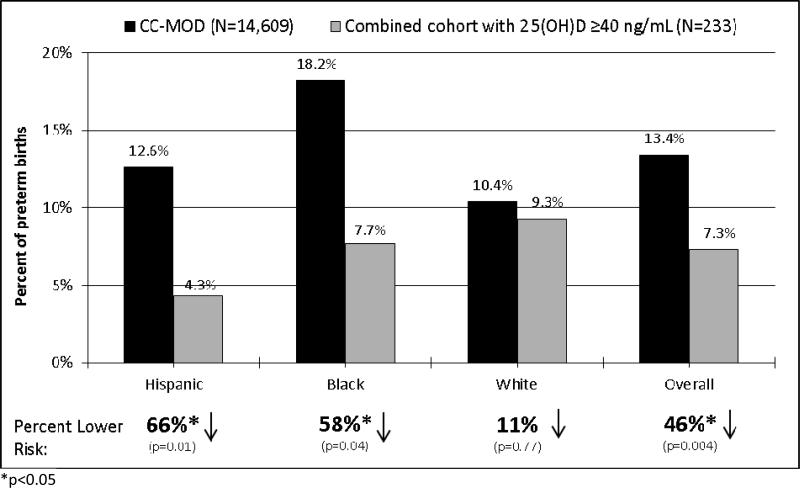
Compared to the CC-MOD reference group, women in the combined cohort with serum 25(OH)D concentrations ≥40 ng/mL had a 46% lower rate of preterm birth (p=0.004) (Figure 3). Among race/ethnic groups, rates were reduced by 66% for Hispanic women (p=0.01), 58% for black women (p=0.04), and 11% for white women (p=0.77). While the 25(OH)D status of the CC-MOD population is not known, as a surrogate measurement for that population, we found that women in the combined cohort control group who received 400 IU/day (the current standard of vitamin D supplementation for pregnant women) and who had a median achieved 25(OH)D concentration of 30 ng/mL had rates of preterm birth that were not significantly different from the CC-MOD population: 9%, 14%, 11%, and 11% for Hispanic, black, white, and all women combined respectively. The data and code used to generate the analyses are available to qualified researchers upon request.
Figure 3. Comparison of preterm birth rates for Charleston County, South Carolina March of Dimes (CC-MOD) vs. NICHD & TRF combined cohort.
CC-MOD rates are the published average percent of preterm births among live births for 2009-2011 in Charleston County, South Carolina; 25(OH)D concentrations unknown (15). Combined cohort includes participants from 2 studies conducted in South Carolina (10, 12). Percent lower risk and p-value between CC-MOD and combined cohort with 25(OH)D ≥40 ng/mL.
Discussion
In this post-hoc analysis of the combined cohort dataset from two pregnancy vitamin D supplementation trials, there was a clear association between 25(OH)D serum concentration within six weeks of delivery and preterm birth. Achieving a 25(OH)D serum concentration of ≥40 ng/mL significantly decreased the risk of preterm birth compared to ≤20 ng/mL. While those women with 25(OH)D concentrations between 20 and 40 ng/mL also had a decreased risk of preterm birth, it was less of a decrease (41% vs. 59% in those women with 25(OH)D >40 ng/mL) and it was not statistically significant. These findings support the premise that 25(OH)D concentrations of 40 ng/mL and above are needed to significantly reduce the risk of preterm birth. In subgroup analyses, the reduction in risk was most notable among those with baseline 25(OH)D ≤20 ng/mL and among Hispanic women. When compared to published 2009-2011 CC-MOD rates of preterm birth (15), there were significant reductions in risk for Hispanic and black women ≥40 ng/mL but there was not a statistically significant reduction for white women. These results suggest the importance of vitamin D status during pregnancy.
A concern that women who deliver prematurely also have lower 25(OH)D concentrations due to less time during gestation for vitamin D supplementation to improve vitamin D status has been raised (17). Women taking 400 IU/day of vitamin D, as well as those on higher doses, reach steady-state by 2 months of supplementation (10). The association in this post-hoc and earlier analyses utilized the 25(OH)D within six weeks of delivery. In a recent publication (4), it was shown that the 25(OH)D concentration closest to the time of delivery, especially in Hispanic women, had a stronger association with gestational age and prematurity than baseline or second trimester 25(OH)D, arguing that the effect is mutable and that even in those women who were deficient at baseline, vitamin D supplementation can affect outcome.
Other studies have found a similar reduction in risk of preterm birth. A recent study by Bodnar et al. (5) found a 1.8-fold (95% CI=1.3,2.6) increase in the risk of preterm birth among those with 25(OH)D <20 ng/mL compared to ≥30 ng/mL and that the risk of preterm birth significantly decreased as 25(OH)D concentration increased to about 36 ng/mL. In NICHD pregnancy vitamin D supplementation trial by Hollis et al., the conversion of 25(OH)D to 1,25(OH)2D, the active hormonal form of vitamin D, was optimized at ≥40 ng/mL (10). There were no reported safety issues at such higher 25(OH)D concentrations. The Institute of Medicine recommends that pregnant women attain a total circulating 25(OH)D concentration of at least 20 ng/mL (6); however, these findings suggest that higher levels can be beneficial for birth outcomes. This post hoc analysis supports this premise.
The lack of a visible risk reduction trend among white participants was likely due to the lack of women with low 25(OH)D levels within 6 weeks of delivery in this race group. Also, white participants in this combined cohort started out with relatively high baseline 25(OH)D concentrations (median = 30 ng/mL), which likely left little opportunity for improvement. Black women had a median baseline 25(OH)D of 16 ng/mL, but only increased to a median of 30 ng/mL, which was likely too low to see a statistically significant change in risk. Comparatively, Hispanic women started with a median baseline 25(OH)D of 24 ng/mL and achieved a median 25(OH)D of 38 ng/mL, a change in nutrient status that better spans the response region seen in Bodnar et al. (5, 18, 19).
Strengths of this analysis include using serum 25(OH)D concentration as the predictor variable, which is statistically more powerful than using treatment group because it accounts for all input sources including cutaneous, food, and supplementation whereas treatment group only accounts for supplemental dose. Also, using 25(OH)D concentration overcomes the inherent bias of compliance and inter-individual variability in response to dose associated with treatment group analyses. Additionally, this randomized trial used supplementation doses ten times higher than the IOM recommendation of 400 IU/day, and therefore a larger spectrum of 25(OH)D concentrations could be assessed in relation to preterm birth risk.
The limitations of this post-hoc analysis are that this was a post-study analysis that was not able to control for some covariates related to preterm birth such as body mass index (BMI) and other sociodemographic factors such as smoking and alcohol use (which was extremely low in this cohort). Observed differences by race for those ≤20 ng/mL vs. ≥40 ng/mL within the combined cohort indicate that socioeconomic factors may play a role and while this analysis adjusted for insurance status as a proxy for socioeconomic status, additional indicators were not available. The second issue is one of generalizability and whether or not the diversity of socioeconomic backgrounds and racial/ethnic groups of the women in the study are representative of the larger population. There is also the limitation of sample size and power, with a larger sample size necessary to investigate 25(OH)D and race/ethnic trends further, particularly for white women. Additionally, the serum concentrations of the CC-MOD comparison population were unknown. Since the rate of preterm birth in the CC-MOD population was not significantly different from the combined cohort control group, it is reasonable to expect that women in the CC-MOD population had a similar average intake of 400 IU vitamin D per day (the standard amount found in prenatal vitamins) and serum 25(OH)D concentration averaging 30 ng/mL, the average 25(OH)D concentration in the control group taking 400 IU vitamin D per day. Furthermore, knowing that some women opt not to take prenatal vitamins, it is likely that the mean baseline 25(OH)D concentration of 23 ng/mL of the combined cohort is even more representative of the CC-MOD population. The similar rates of preterm birth strengthen the finding that improved vitamin D status was associated with lower rates of preterm births.
This post-hoc analysis supports the importance of attaining a 25(OH)D blood concentration substantially above 20 ng/mL during pregnancy for the prevention of preterm birth. Reaching 40 ng/mL would achieve the optimal conversion of 25(OH)D to 1,25(OH)2D (10) and lower preterm birth risk. An intake amount substantially higher than the IOM recommendation of 400 IU/day is needed for most people to attain this level. Less than one third (29%) of participants in the control group receiving 400 IU/day achieved a 25(OH)D concentration of 40 ng/mL compared to 57% in the 4000 IU treatment group, a dose amount with no observed adverse effects. Individuals should aim for a desired 25(OH)D level instead of taking a generic dose amount since 25(OH)D serum concentration is a better indicator of vitamin D status. Serum 25(OH)D testing is recommended to determine the specific intake amount an individual needs to attain a specific 25(OH)D concentration.
The March of Dimes estimates that the annual cost of preterm births in the United States is $12 billion (for 455,918 children) (20). If approximately 50% of preterm births could be prevented in the general population, as this analysis suggests is possible, there could be $6 billion available for other services and, more than 225,000 children and families spared this trauma. In light of this, practice guidelines at the Medical University of South Carolina (MUSC) and other institutions are currently being changed to prospectively target 40 ng/mL for pregnant women with the goal of dramatically lowering preterm birth rates.
Supplementary Material
Highlights.
Women with 25(OH)D ≥40 ng/mL had 59% lower risk of preterm birth than those ≤ 20
Gestation week at birth initially rises steadily with increasing 25(OH)D then plateaus at ~40 ng/mL
46% lower preterm birth rate among women with 25(OH)D ≥40 ng/mL compared to CC-MOD reference group
Findings most robust in Hispanic and Black women
Acknowledgements
We would like to thank the hundreds of women who have participated in our NICHD and Thrasher Research Fund studies; without their willingness to participate, we would not have begun to understand vitamin D's role during pregnancy. We also would like to thank Dr. Becky McNeil for her tireless efforts to provide a pristine dataset and Myla Ebeling for her countless hours in toiling over the NICHD data. Lastly, we would like to thank the study coordinators who made the success of these clinical trials possible.
Supported in part by the Thrasher Research Fund, NICHD R01 HD47511, NIH RR01070, and the South Carolina Clinical and Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, and NIH/NCRR Grant Number UL1 RR029882.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 17th Vitamin D Workshop as a poster presentation on June 20, 2014 in Chicago, IL.
References
- 1.Holick MF. Vitamin D Deficiency. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17(4):348–52. doi: 10.1097/MNH.0b013e3282ff64a3. Epub 2008/07/29. doi: 10.1097/MNH.0b013e3282ff64a3 00041552-200807000-00003 [pii]. PubMed PMID: 18660668. [DOI] [PubMed] [Google Scholar]
- 3.Liu P, Stenger S, Tang D, Modlin R. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 4.Wagner CL, Baggerly C, McDonnell SL, Baggerly L, Hamilton SA, Winkler J, Warner G, Rodriguez C, Shary JR, Smith PG, Hollis BW. Post-hoc comparison of vitamin D status at three timepoints during pregnancy demonstrates lower risk of preterm birth with higher vitamin D closer to delivery. The Journal of steroid biochemistry and molecular biology. 2014 doi: 10.1016/j.jsbmb.2014.11.013. doi: 10.1016/j.jsbmb.2014.11.013. PubMed PMID: 25448734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodnar LM, Platt RW, Simhan HN. Early-Pregnancy Vitamin D Deficiency and Risk of Preterm Birth Subtypes. Obstetrics and gynecology. 2015 doi: 10.1097/AOG.0000000000000621. doi: 10.1097/AOG.0000000000000621. PubMed PMID: 25569002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board . Dietary Reference Intakes for Vitamin D and Calcium. National Academy Press; Washington, D.C.: 2010. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 7.Hamilton SA, McNeil R, Hollis BW, Davis DJ, Winkler J, Cook C, Warner G, Bivens B, McShane P, Wagner CL. Profound Vitamin D Deficiency in a Diverse Group of Women during Pregnancy Living in a Sun-Rich Environment at Latitude 32 degrees N. International journal of endocrinology. 2010;2010:917428. doi: 10.1155/2010/917428. Epub 2011/01/05. doi: 10.1155/2010/917428. PubMed PMID: 21197089; PMCID: 3004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D Deficiency and Insufficiency is Common during Pregnancy. American journal of perinatology. 2011;28(1):7–12. doi: 10.1055/s-0030-1262505. Epub 2010/07/20. doi: 10.1055/s-0030-1262505. PubMed PMID: 20640974. [DOI] [PubMed] [Google Scholar]
- 9.Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized Controlled Trial (RCT) of Vitamin D Supplementation in Pregnancy in a Population With Endemic Vitamin D Deficiency. The Journal of clinical endocrinology and metabolism. 2013;98(6):2337–46. doi: 10.1210/jc.2013-1154. Epub 2013/04/06. doi: 10.1210/jc.2013-1154. PubMed PMID: 23559082. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2341–57. doi: 10.1002/jbmr.463. Epub 2011/06/28. doi: 10.1002/jbmr.463. PubMed PMID: 21706518; PMCID: 3183324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollis BW, Wagner CL. Vitamin d and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcified tissue international. 2013;92(2):128–39. doi: 10.1007/s00223-012-9607-4. Epub 2012/05/25. doi: 10.1007/s00223-012-9607-4. PubMed PMID: 22623177. [DOI] [PubMed] [Google Scholar]
- 12.Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, Bivens B, Davis DJ, Smith PG, Murphy M, Shary JR, Hollis BW. A randomized trial of vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208(2):137, e1–e13. doi: 10.1016/j.ajog.2012.10.888. Epub 2012/11/08. doi: 10.1016/j.ajog.2012.10.888. PubMed PMID: 23131462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW. Health characteristics and outcomes of two randomized vitamin D supplementation trials during pregnancy: a combined analysis. The Journal of steroid biochemistry and molecular biology. 2013;136:313–20. doi: 10.1016/j.jsbmb.2013.01.002. Epub 2013/01/15. doi: 10.1016/j.jsbmb.2013.01.002. PubMed PMID: 23314242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer research. 2011;31(2):607–11. Epub 2011/03/08. PubMed PMID: 21378345. [PubMed] [Google Scholar]
- 15. [August 18, 2014];South Carolina Preterm Birth Rates 2001-2011. [Internet]2014. Available from: http://www.marchofdimes.com/Peristats/ViewSubtopic.aspx?reg=45&top=3&stop=60&lev=1&slev=4&obj=1.
- 16.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12(1):19–28. doi: 10.2174/138945011793591608. Epub 2010/08/28. doi: BSP/CDT/E-Pub/00160 [pii]. PubMed PMID: 20795940. [DOI] [PubMed] [Google Scholar]
- 17.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, Simhan HN. Maternal vitamin D status and spontaneous preterm birth by placental histology in the US Collaborative Perinatal Project. American journal of epidemiology. 2014;179(2):168–76. doi: 10.1093/aje/kwt237. doi: 10.1093/aje/kwt237. PubMed PMID: 24124195; PMCID: 3873106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP. Toward a physiological referent for the vitamin D requirement. Journal of endocrinological investigation. 2014;37(11):1127–30. doi: 10.1007/s40618-014-0190-6. doi: 10.1007/s40618-014-0190-6. PubMed PMID: 25308199. [DOI] [PubMed] [Google Scholar]
- 19.Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014;72(1):48–54. doi: 10.1111/nure.12090. doi: 10.1111/nure.12090. PubMed PMID: 24330136. [DOI] [PubMed] [Google Scholar]
- 20.March of Dimes . Premature Babies Cost Employers $12.7 Billion Annually Whiteplains. March of Dimes; NY: 2014. [August 25, 2014]. Available from: http://www.marchofdimes.org/news/premature-babies-cost-employers-127-billion-annually.aspx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.