Abstract
Inflammation is a major contributor to neuronal injury and is associated with poor outcome after acute brain injury such as stroke. The pro-inflammatory cytokine interleukin (IL)-1 is a critical regulator of cerebrovascular inflammation after ischemic injury, mainly through action of both of its isoforms, IL-1α and IL-1β, at the brain endothelium. In contrast, the differential action of these ligands on endothelial activation and post-stroke angiogenesis is largely unknown. Here we demonstrate that IL-1α is chronically elevated in the brain after experimental stroke suggesting that it is present during post-stroke angiogenic periods. Furthermore, we demonstrate that IL-1α is a potent mediator of endothelial activation and inducer of angiogenic markers in endothelial cells in vitro. Using brain endothelial cell lines, we found that IL-1α was significantly more potent than IL-1β at inducing endothelial cell activation, as measured by expression of the pro-angiogenic chemokine CXCL-1. IL-1α also induced strong expression of the angiogenic mediator IL-6 in a concentration-dependent manner. Furthermore IL-1α induced significant proliferation and migration of endothelial cells, and promoted formation of tube-like structures that are established key hallmarks of angiogenesis in vitro. Finally, all of those responses were blocked by the IL-1 receptor antagonist (IL-1RA). In conclusion, our data highlights a potential new role for IL-1 in brain repair mechanisms and identifies IL-1α as a potential new therapy to promote post-stroke angiogenesis.
Keywords: inflammation, cerebral ischemia, interleukin-1, endothelia, angiogenesis
INTRODUCTION
Inflammation is a key host defense response to infection and injury, the aim of which is host protection against pathogens, regeneration of injured tissues and re-establishment of tissue homeostasis (Nathan, 2002). It is now recognized that inflammation is also a key contributor to neuronal ischemic injury that occurs after stroke, a leading cause of death and morbidity worldwide with limited therapeutic options (Paul et al. 2007). This inflammatory response after cerebral ischemia has long been considered detrimental and due to its slowly evolving nature, has been identified as a potential therapeutic target in acute ischemic stroke (Denes et al. 2011). Inflammation after cerebral ischemia is characterized by central expression of inflammatory mediators, activation of the local innate immune cells of the brain, activation of the brain cerebrovasculature and subsequent opening of the blood-brain barrier (BBB) that allows for edema and circulating immune factors into the brain parenchyma, contributing to secondary brain damage (Wang et al. 2007). A key mediator of inflammation after cerebral ischemia is the pro-inflammatory cytokine interleukin-1 (IL-1) (Denes et al. 2011). Microglia, astrocytes and endothelial cells express both of the IL-1 isoforms, IL-1α and IL-1β, after cerebral ischemia (Basu et al. 2004), and both cytokines contribute to neuronal injury by acting primarily on endothelial cells and astrocytes (Allen et al. 2012). IL-1 action at the cerebrovasculature is considered a primary trigger of post stroke inflammation (Luheshi et al. 2011), and may represent a therapeutic target (Pradillo et al. 2012). Although IL-1-driven inflammation is detrimental during the acute phase of ischemic injury, inflammation also exerts beneficial effects by promoting brain repair mechanisms and functional recovery; Indeed, IL-1 induces generation of LG3 in brain cell cultures, a potentially neuroprotective and pro-angiogenic protein fragment of the extracellular-matrix component perlecan (Saini et al. 2011; Saini et al. 2012). Furthermore, IL-1 induces central expression of the acute phase protein pentraxin-3, a key regulator of repair mechanisms after cerebral ischemia, including glial scar formation, BBB integrity and resolution of edema (Rodriguez-Grande et al. 2014), as well as neurogenesis and angiogenesis (Rodriguez-Grande et al. 2015). Although IL-1-driven repair mechanisms at the cerebrovasculature are gradually being unravelled, the potential role of IL-1 as a direct regulator of angiogenesis after ischemic injury is completely unknown. Importantly, we have demonstrated a selective role for IL-1α (but not IL-1β) in activating endothelial cells to produce LG3 (Saini et al. 2011), and although IL-1α has been reported to exert pro-angiogenic effects during peripheral inflammation (Salven et al. 2002; Matsuo et al. 2009), the potential role of IL-1α on brain angiogenesis has never been explored. Using cultured brain endothelial cells, we demonstrate for the first time that IL-1α induces several stages of angiogenesis as well as expression of key pro-angiogenic markers in brain endothelial cells in vitro, highlighting the important role of angiogenesis driven by IL-1α during the repair phase after acute CNS inflammatory conditions.
MATERIALS AND METHODS
Tandem Ipsilateral Common Carotid and Middle Cerebral Artery Occlusion Stroke Model
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Kentucky and experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health as well as the ARRIVE guidelines. Male (3 months old) C57/Bl6 mice were subjected to transient tandem ipsilateral common carotid artery (CCA)/middle cerebral artery (MCA) occlusion (MCAo) for 60 min (Lee et al. 2011), followed by reperfusion of both arteries for up to 7 days. A small burr hole was made in the skull to expose the MCA and a metal wire with a diameter of 0.005 inch was placed under the artery. Slight elevation of the metal wire causes visible occlusion of the MCA. The CCA was then isolated and occluded using an aneurysm clip. Diminished blood flow was confirmed with Laser Doppler Perfusion Monitor (Perimed, USA) and only those animals with a diminished blood flow of at least 80% and re-establishment of at least 75% of baseline levels were included in subsequent experimentation.
Preparation of brain lysates
Mice were transcardially perfused with 0.9% NaCl, and brains were extracted and homogenised in buffer (50mmol/L Tris-HCl pH 7.4, 150mmol/L NaCl, 5mmol/L CaCl2, 0.02% NaN3, 1% Triton-X) containing protease inhibitors. After centrifugation (17,000 g for 30 min), supernatants were collected and protein concentrations calculated by bicinchoninic acid protein assay (Thermo-Fisher Scientific, UK). Protein concentration was adjusted to 1 mg/mL for all samples before quantification of IL-1α levels by enzyme-linked immunosorbent assay (ELISA).
Endothelial cell cultures
Brain microvascular endothelial cells (BECs) from C57BL/6 mice maintained as cells lines (Clarke et al. 2012; Sapatino et al. 1993), and the mouse bEnd.5 cell line (which closely resemble primary brain endothelial cells), were used in this study. C57BL/6 BECs were cultured on porcine gelatin-coated tissue culture plates (unless stated otherwise) in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% fetal bovine serum (FBS), 1 U/mL penicillin, 100 mg/mL streptomycin and 1% L-glutamine, and were kept at 37°C and 5% CO2. bEnd.5 were cultured in Dulbecco’s modified Eagle medium (DMEM) (high glucose, 4.5 g/L; Invitrogen, Paisley, UK), supplemented with 10% FBS, 1% nonessential amino acid, 2 mM glutamine, 1 U/mL penicillin, and 100 mg/mL streptomycin, and were kept at 37°C and 5% CO2.
Cell treatments
Mouse recombinant IL-1α and IL-1β (R&D Systems, UK) were diluted in sterile phosphate buffered saline (PBS) containing 0.1% low endotoxin bovine serum albumin (BSA) (also used as vehicle control). Cultures were treated with vehicle, IL-1α or IL-1β (0.1 – 100 ng/mL) (based on the known biological activity of IL-1α (Saini et al. 2011) in the presence or absence of IL-1RA (Kineret: Lot# 26458 IF) (10 μg/mL) for 5 h (CXCL-1 release), 6 h (for chemotaxis chamber and Tube-like Structure Morphogenesis Assays) or 24 h (for IL-6, MTS, Cell Count, and Scratch Assays).
Measurement of CXCL1, IL-1α, and IL-6 release by enzyme-linked immunosorbent assay (ELISA)
Levels of CXCL1, and IL-6 levels in the culture media or IL-1α in brain lysates were quantified using DuoSet® mouse CXCL1/CINC-1, mouse IL-1α, or mouse IL-6 ELISAs, respectively, according to the manufacturer’s instructions (R&D Systems, UK). The detection limits of the assay were 47 pg/mL for CXCL1, 23 pg/mL for IL-6, and 16 pg/mL for IL-1α. Levels for all cytokines analyzed were expressed as pg/mL.
Cellular proliferation assay assessed by MTS
BECs were seeded on uncoated tissue culture plates at a density of 10,000 cells/cm2 in 96-well plates in IMDM containing 10% FBS. After 24 h, the media was changed to IMDM containing 1% FBS and cytokine treatment conditions. After 24 h, MTS reagent (Cell titer96; Promega, Madison, WI, USA) was added to each well and the absorbance was read at 1, 1.5 and 2-h time points at 490 nm. Data were reported as a percentage of the control conditions.
Cellular proliferation assay measured by cell count
24-well plates were coated with porcine gelatin for 20 min prior to cell seeding. BECs were seeded at 5,000 cells/cm2 in IMDM containing 10% FBS. After 24 h, the media was changed to IMDM containing 1% FBS. After treatments with cytokines for 24 h, the conditioned media was collected and 250 μL trypsin was added to each well and allowed to incubate for 5 min. 500 μL 1% FBS-containing IMDM was then added to each well to inactivate the trypsin. The detached cells were then transferred to 1.5 mL microcentrifuge tubes and spun at 1,000 rpm for 5 min at room temperature. The media was removed and the pellet was then re-suspended in 50 μL of 1% FBS-containing media. The cells were then counted on a hemocytometer using a 1:2 dilution with Trypan Blue. Data were expressed as cells counted on hemocytometer and reported as percentage of the control conditions.
Scratch migration assay
BECs were seeded at a density of 10,500 cells/cm2 and grown for 48 h in 10 % FBS-containing IMDM, after which the cell layer was scratched mechanically with a 200 μL pipet tip. The culture medium was then removed and new media containing 1 % FBS was added. After treatments with cytokines for 24 h, bright field microscopic pictures of the scratches were taken, and the area of the remaining, unfiled scratch was measured using ImageJ Software. Results were expressed as a percent of the scratch filled/repaired by the cell monolayer. Data were expressed as percentage of scratch area filled.
Chemotactic migration assay
Poycarbonate membranes (Neuro Probe, Inc., USA) with 8 µm pores were coated with 100 μg/mL Collagen Type I (Corning; USA) in 0.02N acetic acid overnight at 4°C. The bottom wells of a Chemotaxis Chamber (Neuro Probe, USA) contained chemotactic controls (0 and 1% serum media) and treatment conditions (IL-1α ± IL-1RA). After assembly of the chamber as per the manufacturer’s instructions, the upper wells were seeded at 15,000 cells/well in 0% serum media or 10 μg/mL IL-1RA conditions and the chamber was incubated at 37°C for 6 h. The cells on the underside of the membrane were fixed in methanol and stained with crystal violet (0.5%). The membrane was then cut into sections, mounted on a slide, and visualized. Upon visualization, migrated cells were counted and recorded. Results were expressed as mean number of cells that migrated to the bottom of the membrane as a percentage of the serum starved (control) condition.
Matrigel tube-like morphology assays
Slow thawed growth factor reduced Matrigel (Corning, USA) was coated on the wells of a 24-well plates (35μL per well) for 1 h 37°C for 1 h. Cells were passaged into their diluted concentrations in IMDM supplemented with 1% serum, as well as cytokine treatment conditions and were incubated at 37°C for 20 min. Cells were seeded at 15,000 cells/cm2and allowed on the Matrigel for 6 h at 37°C before being fixed with 4% paraformaldehyde at 4°C overnight. Pictures were taken using a Nikon Eclipse Ti Microsoft camera using Nikon software. Using the ImageJ software, hyper-reflective borders of capillary-like structures were quantified using a consistent threshold. Results were expressed as mean percentage of hyper-reflective (tube) pixels per high-powered field compared to the serum control condition.
Statistical analyses
All experiments were performed on at least three independent cultures, and each condition was performed in triplicate. Data are represented as mean ± standard error of the mean (SEM). Comparison between two groups was done using the Student’s t-test. Comparison between three or more groups was performed using one-way ANOVA followed by a Tukey post-hoc analysis. For CXCL1 and IL-6 ELISA assays, data were analyzed by 2 way ANOVA and Bonferroni post-hoc test. Significance was determined by a p value of less than 0.05.
RESULTS
IL-1α is elevated in the mouse brain after experimental ischemic stroke
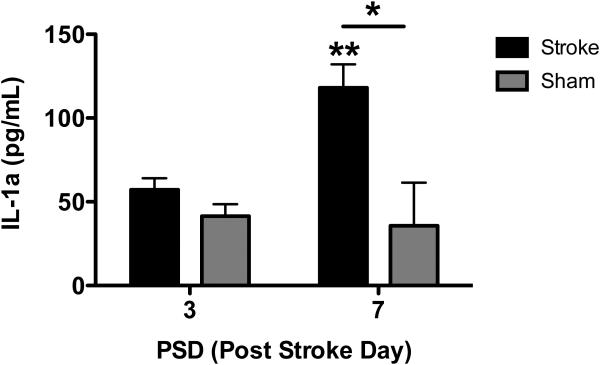
In order to investigate whether IL-1α was expressed in the brain after stroke to potentially play a role in post stroke recovery, we analyzed brains of stroked mice at several time points relative to the initiation (post-stroke day 3) and maintenance (post-stroke day 7) of post-stroke angiogenesis. We found that while IL-1α levels remain comparable to sham animals at post-stroke day 3, after 7 days IL-1α levels were significantly elevated (Figure 1). This demonstrates that IL-1α is elevated in the brain days after experimental stroke and could be a significant mediator of chronic post stroke processes.
Figure 1. IL-1α is chronically elevated after experimental stroke in mice.
Lysates from the brains of 3 month-old male C57Bl6 mice and their sham controls were collected at 3 and 7 days after experimental stroke and assayed for IL-1α by ELISA. IL-1α was significantly elevated at 7 days post stroke compared to day 7 sham controls*p<0.05, and day 3 sham controls **p<0.01. N=3
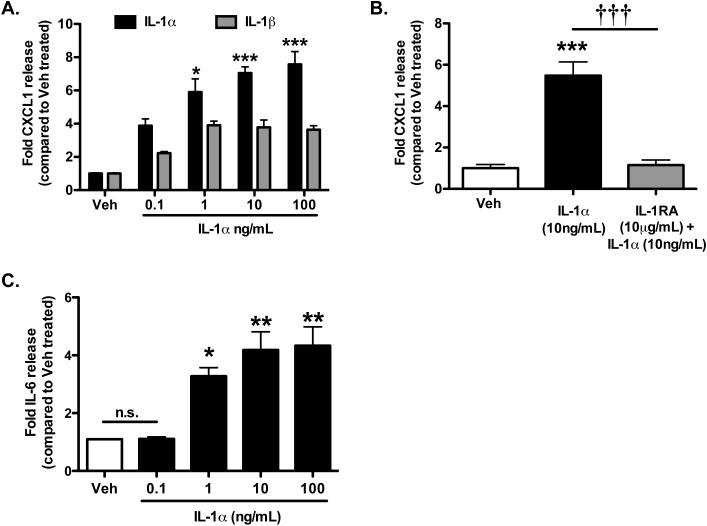
IL-1α increases brain endothelial cell activation
In order to determine the differential potency by which IL-1α and IL-1β might trigger pro-angiogenic responses in BEC cultures, we first investigated the effect of recombinant IL-1α or IL-1β on expression of CXCL1, a chemokine expressed upon endothelial cell activation and known to play a key role in recruitment of immune cells into the brain after acute injury (Losy et al. 2005) and to exert angiogenic actions (Vries et al. 2015; Wei et al. 2015). Both IL-1α and IL-1β induced marked increased expression of CXCL1 in BECs, and increased expression was concentration-dependent for both ligands (Figure 2A). Interestingly, optimum concentration of cytokines that induced maximum CXCL1 expression was found to be 1 ng/mL for both IL-1α and IL-1β, while a plateau was reached for the concentrations of 10 and 100 ng/mL. For all the concentrations tested, IL-1α was more potent than IL-1β at inducing CXCL1 expression, and this differential expression was significant for the concentrations of 1-100 ng/mL. Given these results, all of the following experiments tested the effect of IL-1α only. IL-1α-induced CXCL1 expression was completely inhibited by co-incubation with IL-1RA (Figure 2B), while IL-1RA alone had no effect (data not shown) demonstrating that these effects were mediated by the IL-1 receptor type 1 (IL-1R1). Finally, IL-1α induced significant release of IL-6 (known to exert angiogenic actions in brain endothelial cells (Gopinathan et al. 2015; Huang et al. 2004)) in a concentration-dependent manner, with optimum concentration of IL-1α found to be 10 ng/mL, while a plateau was reached for the concentration of 100 ng/mL (Figure 2C). These results demonstrate that IL-1α is a potent activator of BECs, and since both CXCL1 and IL-6 have been previously reported to mediate angiogenesis (Vries et al. 2015, Wei et al. 2015; Gopinathan et al. 2015; Huang et al. 2004), these results suggest that IL-1α might trigger angiogenic responses in endothelial cells.
Figure 2. IL-1α induces expression of CXCL-1 and IL-6 in BECs.
Lysates from Bend.5 endothelial cells, treated as labelled, were collected and assayed for CXCL-1 (A and B) and IL-6 (C) by ELISA. All cytokines were significantly increased in cultures treated with IL-1α and demonstrated a greater increase than IL-1β. Additionally, this effect was blocked in the presence of IL-1RA. *p<0.05, **p<0.01, ***p<0.001 vs. untreated or vehicle-treated cultures. †††p<0.001 vs. IL-1α (10ng/mL). N=4
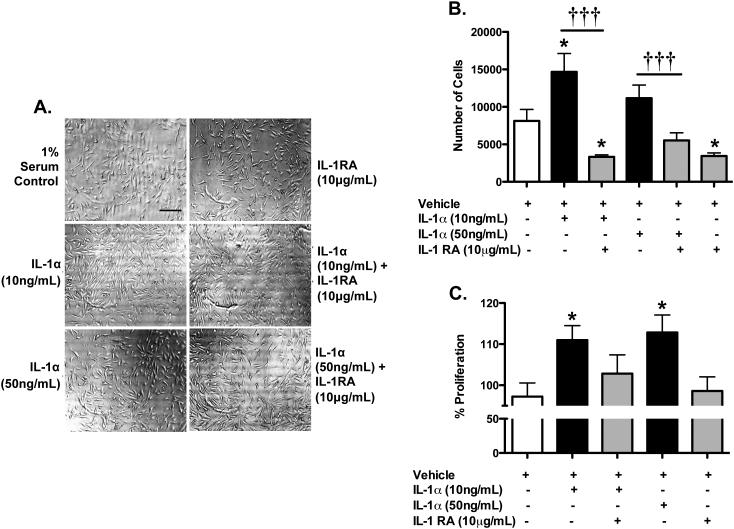
IL-1α increases proliferation of endothelial cells
We next investigated whether IL-1α could affect BEC proliferation, a known hallmark of endothelial angiogenic response in vitro, as measured directly by cell counts and indirectly by MTS assay. Visualisation of cultures using bright field microscopy found that IL-1α triggered cellular proliferation which was blocked by IL-1RA, but also demonstrated that no cytotoxicity occurred in response of any treatments (Figure 3A). We found that IL-1α (10 ng/mL or 50 ng/mL) increased cellular proliferation compared to untreated (1% serum control) cultures, which was inhibited by IL-1RA (Figure 3B); Quantification of cellular proliferation showed that 10 ng/mL IL-1α significantly increased endothelial proliferation by nearly two fold while 50 ng/mL IL-1α also increased endothelial proliferation, although this was not significant. Cellular proliferation in response to both IL-1α concentrations tested was significantly blocked by IL-1RA. Interestingly, IL-1RA alone appeared to significantly inhibit basal endothelial proliferation, suggesting that endogenous IL-1 actions might be involved.
Figure 3. IL-1α increases proliferation of BECs.
Trypan blue stained C57 BECs, treated for 24 h as labelled, were counted with a hemocytometer 24 h after treatment (A and B). Scale bar is 200 microns. C. Spectrophotometric MTS readings after 60, 90 and 120 min for cells, treated as labelled. Numbers represent the percent proliferation as compared to 1% serum control conditions.. *p<0.05 vs. vehicle-treated cultures. †††p<0.001 vs. IL-1α. N=3
Similarly, we found that IL-1α significantly increased endothelial proliferation compared to vehicle treatment, as measured by MTS assay (111.0 ± 3.528% for 10 ng/mL, 112.8 ± 4.278% for 50 ng/mL) (Figure 3C). We also found that the addition IL-1RA decreased IL-1α-induced endothelial proliferation, while IL-1RA alone had no effect. Collectively, these results indicate that IL-1α increases BEC proliferation in vitro and did not result in cytotoxicity.
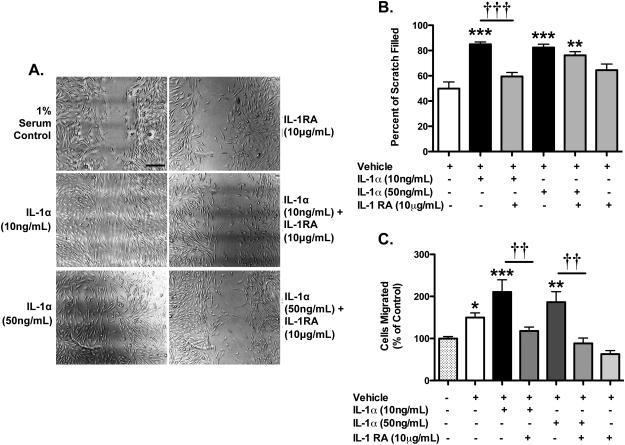
IL-1α increases BEC migration
We next investigated the potential effect of IL-1α on BEC migration, another hallmark of angiogenic responses in endothelial cells, using scratch and chemotactic migration assays. In the scratch assay, which measures cell migration into a “scratch” wound, 10 ng/mL or 50 ng/mL IL-1α significantly increases BEC migration into the wound (1.70 fold increase for 10 ng/mL and 1.65 fold increase for 50 ng/mL respectively) (Figure 4A and B). IL-1RA blocked IL-1α-induced endothelial cell migration, although this was only significant for the concentration of 10 ng/mL of IL-1α, while IL-1RA alone had no effect.
Figure 4. IL-1α increases migration of BECs.
Monolayers of C57 BECs were scratched and treated as labelled at the time of wounding. The area of the scratch was analysed and graphed as the percent of the wound filled in by the cells (shown in A, quantified in B). Scale bar is 200 microns. Quantification of chemotaxis assay showed increases in both the 1% serum and IL-1α conditions. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle-treated cultures. ††p<0.01 and †††p<0.001 vs. IL-1α. N=3
In order to confirm the potential effect of IL-1α to induce chemotactic migration in BECs, we performed chemotaxis chamber migration assays in which serum-starved cells migrate towards 1% serum across a porous membrane. Vehicle-treatment triggered a moderate increase in chemotaxic migration of endothelial cells (Figure 4C). However, IL-1α (10 ng/mL and 50 ng/mL) significantly increased the number of cells that migrate compared to 1% serum-directed controls. Finally, IL-1RA significantly blocked the pro-migratory activity of IL-1α indicating that IL-1α promotes chemotactic migration of BECs in an IL-1R1-mediated fashion.
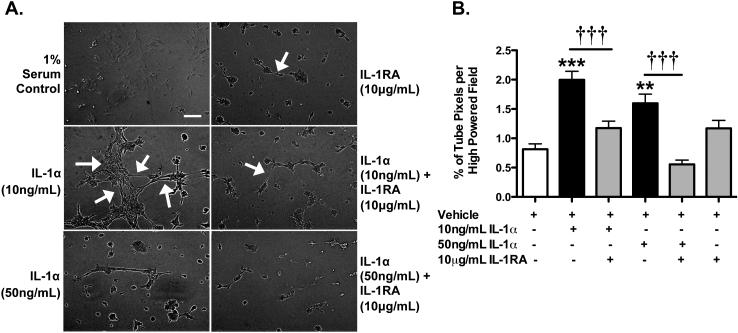
IL-1α promotes formation of capillary tube-like structures
Finally, we investigated whether IL-1α enhances capillary morphogenesis using the Matrigel assay, another stage of angiogenesis. Indeed, IL-1α enhanced the formation of capillary tube-like structures (2.45 fold for 10 ng/mL and 1.44 fold for 50 ng/mL) (Figure 5). This effect was inhibited by IL-1RA, while IL-1RA alone had no effect.
Figure 5. IL-1α increases BEC capillary-tube like morphogenesis on Matrigel Matrix.
A. Images of C57 BECs on Matrigel for 6 h, treated as labelled. Arrows indicate some of the capillary tube-like structures that were formed. B. Graph represents the % of capillary-tube-like positive pixels per high-powered field after 6 hours of treatment as labelled. Scale bar is 200 microns. **p<0.01, ***p<0.001 vs. untreated or vehicle-treated cultures. †††p<0.001 vs. IL-1α. N=3
DISCUSSION
Angiogenesis is an important component of post-stroke repair that may aid in the restoration of blood flow to ischemic brain regions and generate a neurovascular niche to guide and support neurogenesis (Ohab et al. 2006). In this study, we found that IL-1α is significantly elevated even a week after experimental stroke. Since IL-1 is an important inflammatory mediator known to trigger post-stroke inflammation by acting primarily on endothelial cells (Saini et al. 2011), and IL-1α has been suggested to selectively act on endothelial cells to exert neuroprotective and pro-angiogenic effects (Saini et al. 2012), we investigated the potential of IL-1α to enhance brain angiogenesis in vitro. Such pro-angiogenic activity, combined with our observation of chronic expression of IL-1α after experimental stroke, would support the hypothesis that IL-1α could be a key driver of brain post-ischemic injury angiogenesis and thereby represents a novel stroke therapeutic target. Our results demonstrated that IL-1α enhances key stages of angiogenesis, including endothelial activation, proliferation, migration and capillary morphogenesis, and induces important pro-angiogenic mediators (CXCL1 and IL-6) in an IL-1R1-dependent manner; First, we found that IL-1α was chronically elevated in the stroked brain. Next, we found that both IL-1α and IL-1β induced strong activation of endothelial cells, measured by expression of the chemokine CXCL1, although the effect of IL-1α was significantly higher to that of IL-1β, confirming the observations of our previous study (Saini et al. 2011) and that of others (Fleisher-Berkovich et al. 1999; Banks et al. 1999; Saadi et al. 2000) that IL-1α acts as a selective activator of endothelial cells. Importantly, although CXCL1 is known for its chemotaxis functions, this chemokine has been reported to have potent pro-angiogenic actions. Indeed, CXCL1 is known to induce angiogenesis in peripheral endothelial cells (Miyake et al. 2013), and the angiogenic actions of prostaglandin E2 is mediated by CXCL1 in disease (Wang et al. 2006). Finally, CXCL1 has been reported to promote angiogenesis by expressing neutrophil-derived vascular endothelial growth factor (VEGF) (Wang et al. 2006) adding to our hypothesis that IL-1α could mediate angiogenesis in the brain. This hypothesis is further supported by the fact that IL-1α induces strong expression of the cytokine IL-6, an established pro-angiogenic mediator in the CNS. Indeed, IL-6 is known to promote post-traumatic repair in the CNS (Swartz et al. 2001) and induces vasculogenesis of brain microvessel endothelial cells (Fee et al. 2000). Surprisingly, our data found that, while IL-1α induces strong IL-6 expression in endothelial cells, it failed to induce expression of VEGF (data not shown). However IL-6 is known to induce expression of VEGF in astrocytes (Loeffler et al. 2005). These observations taken together with our data suggest that IL-1α could exert pro-angiogenic effects, at least in part, by inducing IL-6 expression in endothelia, which in turns may activate neighbouring perivascular astrocytes that produce VEGF to subsequently induce angiogenesis. The potential significance of IL-6 in the pro-angiogenic effects of IL-1α is actively being investigated.
In further support of our hypothesis that IL-1α has pro-angiogenic actions on brain endothelial cells, we found that IL-1α induced strong BEC proliferation and migration, and promoted formation of tube-like structures, which are key features of angiogenesis and established angiogenic assay in vitro. Interestingly, in our BEC migration experiments, we noted that IL-1α both stimulated migration into a scratch wound and acted as a migration chemotactant, which let us speculate that IL-1α expressed after ischemic injury by peri-infarct astrocytes, microglia and other endothelial cells (Saini et al. 2011), like other angiogenic/chemotactant factors such as VEGF and angiopoetin, attracts migrating/angiogenic endothelial cells. Additionally, we found that IL-1R1 blockade with IL-1RA in the absence of IL-1α was sufficient to blunt some baseline angiogenic responses (proliferation and migration), suggesting that endogenously generated IL-1α may exert some angiogenic effects, further supporting the role of IL-1α in brain angiogenesis.
While our data suggest that CXCL1 and IL-6 could mediate IL-1α-induced angiogenesis, other mediators might be involved. Indeed, one possibility is that IL-1α enhances angiogenesis via causing brain endothelial cells to generate the pro-angiogenic extracellular matrix fragment perlecan LG3, as we have demonstrated in our previous studies (Saini et al. 2011). In the same study, we demonstrated that IL-1β did not exert this effect and in contrast, decreased LG3 levels below baseline levels (Saini et al. 2011; Clarke et al. 2012), further distinguishing the selective effects IL-1α on brain endothelial cells. Current studies to ascertain the mechanism(s) by which IL-1α generates LG3 and whether this could be responsible, at least in part, for its angiogenic effects, are ongoing.
Another important caveat to this study is that it was performed almost entirely in vitro using murine rather human cells and cell lines rather than primary cells. The potential role of IL-1α in brain angiogenesis in vivo is currently under investigation in our laboratory. Furthermore, while the current study demonstrates that IL-1α promotes brain angiogenesis in vitro under normal conditions (normoxia and normoglycemia), further studies are necessary to determine the experimental and therapeutic relevance of IL-1α as a key driver of brain angiogenesis after ischemic injury. For example, IL-1α dose-response and variable dosing schedule studies in experimental stroke could be performed to determine IL-1α’s safety and (angiogenic) therapeutic efficacy. Furthermore while IL-1RA is currently being considered as an anti-inflammatory therapeutic approach targeting the detrimental acute phase of inflammation (Pradillo et al. 2012), our results highlight the possibility that sustained IL-1α levels might be important to promote key repair mechanisms including angiogenesis. Indeed, our study suggests that refined IL-1RA administration (time window and amount to be administered) might be necessary or that selective inhibition of IL-1β to favour the potentially more beneficial effects of IL-1α could be achieved by co-administering IL-1RA followed by chronic low IL-1α regimens.
In summary, the present study demonstrates that the inflammatory cytokine IL-1α enhances several stages of brain angiogenesis in vitro in a receptor-mediated fashion. This novel observation adds further support to the idea that inflammatory signals in the brain, such as those that occur after stroke, may exert beneficial effects that could be therapeutically exploited.
Acknowledgements
This work was supported by the National Institute of Health (Grant R21NS085660) to GB and EP.
List of Abbreviations
- IL-1
interleukin 1
- IL-1α
interleukin 1 alpha
- IL-1β
interleukin 1 beta
- CXCL-1
chemokine (C-X-C motif) ligand 1
- IL-6
Interleukin 6
- IL-1R
interleukin 1 receptor
- IL-1RA
Interleukin 1 receptor antagonist
- LG3
laminin globular domain 3
- BBB
blood-brain barrier
- CNS
central nervous system
- CCA
mommon carotid artery
- MCA
middle cerebral artery
- MCAo
middle cerebral artery occlusion
- ELISA
enzyme-linked immunosorbent assay
- BEC
brain endothelial cell
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- ANOVA
analysis of variance
- VEGF
vascular endothelial growth factor
Footnotes
ARRIVE guidelines have been followed:
Yes
=> if No, skip complete sentence
=> if Yes, insert "All experiments were conducted in compliance with the ARRIVE guidelines."
Conflicts of interest: none
=> if 'none', insert "The authors have no conflict of interest to declare."
=> otherwise insert info unless it is already included
References
- Allen C, Thornton P, Denes A, McColl BW, Pierozynski A, Monestier M, Pinteaux E, Rothwell NJ, Allan SM. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol. 2012;189:381–392. doi: 10.4049/jimmunol.1200409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA. Characterization of Interleukin-1a Binding to Mouse Brain Endothelial Cells. Journal of Pharmacology and Experimental Therapeutics. 1999;291:665–670. [PubMed] [Google Scholar]
- Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- Clarke DN, Al Ahmad A, Lee B, et al. Perlecan Domain V induces VEGf secretion in brain endothelial cells through integrin alpha5beta1 and ERK-dependent signaling pathways. PLoS One. 2012;7:e45257. doi: 10.1371/journal.pone.0045257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Pinteaux E, Rothwell NJ, Allan SM. Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovasc Dis. 2011;32:517–527. doi: 10.1159/000332205. [DOI] [PubMed] [Google Scholar]
- Fee D, Grzybicki D, Dobbs M, Ihyer S, Clotfelter J, Macvilay S, Hart MN, Sandor M, Fabry Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine. 2000;12:655–665. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- Fleisher-Berkovich S, Danon A. IL-1alpha but not IL-1beta-induced prostaglandin synthesis is inhibited by corticotropin-releasing factor. Cytokine. 1999;11:239–243. doi: 10.1006/cyto.1998.0417. [DOI] [PubMed] [Google Scholar]
- Gopinathan G, Milagre C, Pearce OM, et al. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- Losy J, Zaremba J, Skrobanski P. CXCL1 (GRO-alpha) chemokine in acute ischaemic stroke patients. Folia Neuropathologica. 2005;43:97–102. [PubMed] [Google Scholar]
- Luheshi NM, Kovacs KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1alpha expression precedes IL-1beta after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation. 2011;8:186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Sawai H, Ma J, Xu D, Ochi N, Yasuda A, Takahashi H, Funahashi H, Takeyama H. IL-1alpha secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1alpha release and tumor cells' potential for liver metastasis. J Surg Oncol. 2009;99:361–367. doi: 10.1002/jso.21245. [DOI] [PubMed] [Google Scholar]
- Miyake M, Goodison S, Urquidi V, Gomes Giacoia E, Rosser CJ. Expression of CXCL1 in human endothelial cells induces angiogenesis through the CXCR2 receptor and the ERK1/2 and EGF pathways. Lab Invest. 2013;93:768–778. doi: 10.1038/labinvest.2013.71. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SL, Srikanth VK, Thrift AG. The large and growing burden of stroke. Current Drug Targets. 2007;8:786–793. doi: 10.2174/138945007781077418. [DOI] [PubMed] [Google Scholar]
- Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab. 2012;32:1810–1819. doi: 10.1038/jcbfm.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Grande B, Swana M, Nguyen L, et al. The acute-phase protein PTX3 is an essential mediator of glial scar formation and resolution of brain edema after ischemic injury. J Cereb Blood Flow Metab. 2014;34:480–488. doi: 10.1038/jcbfm.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Grande B, Varghese L, Molina-Holgado F, Rajkovic O, Garlanda C, Denes A, Pinteaux E. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J Neuroinflammation. 2015;12:15. doi: 10.1186/s12974-014-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial Cell Activation by Pore-Forming Structures: pivotal role for interleukin-1a. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- Saini MG, Bix GJ. Oxygen-glucose deprivation (OGD) and interleukin-1 (IL-1) differentially modulate cathepsin B/L mediated generation of neuroprotective perlecan LG3 by neurons. Brain Res. 2012;1438:65–74. doi: 10.1016/j.brainres.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini MG, Pinteaux E, Lee B, Bix GJ. Oxygen-glucose deprivation and interleukin-1alpha trigger the release of perlecan LG3 by cells of neurovascular unit. J Neurochem. 2011;119:760–771. doi: 10.1111/j.1471-4159.2011.07484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salven P, Hattori K, Heissig B, Rafii S. Interleukin-1a (IL-1a) promotes angiogenesis in vivo via VEGFR-2 pathway by inducting inflammatory cell VEGF synthesis and secretion. The FASEB Journal. 2002 doi: 10.1096/fj.02-0134fje. [DOI] [PubMed] [Google Scholar]
- Sapatino BV, Welsh CJ, Smith CA, Bebo BF, Linthicum DS. Cloned mouse cerebrovascular endothelial cells that maintain their differentiation markers for factor VIII, low density lipoprotein, and angiotensin-converting enzyme. In Vitro Cell Dev Biol Anim. 1993;29A:923–928. doi: 10.1007/BF02634230. [DOI] [PubMed] [Google Scholar]
- Swartz KR, Liu F, Sewell D, Schochet T, Campbell I, Sandor M, Fabry Z. Interleukin-6 promotes post-traumatic healing in the central nervous system. Brain Res. 2001;896:86–95. doi: 10.1016/s0006-8993(01)02013-3. [DOI] [PubMed] [Google Scholar]
- Vries MHM, Wagenaar A, Verbruggen SEL, Molin DGM, Post MJ. CXCL1 promotes arteriogenesis through enhanced monocyte recruitment into the peri-collateral space. Angiogenesis. 2015;18:163–171. doi: 10.1007/s10456-014-9454-1. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang H, Brown J, et al. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J Exp Med. 2006;203:941–951. doi: 10.1084/jem.20052124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZW, Xia GK, Wu Y, et al. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015;359:335–343. doi: 10.1016/j.canlet.2015.01.033. [DOI] [PubMed] [Google Scholar]