Abstract
Background
Pituitary carcinomas (PC) are uncommon neuroendocrine tumors, accounting for 0.1% of all pituitary tumors. The diagnosis of PC is based on the presence of metastases from a pituitary adenoma, and not by local invasion or pathological features alone. PC is typically resistant to therapy, with a median overall survival (OS) of only 31 months. There is no standard treatment for PC, but maximal safe resection and radiation are performed when possible. Encouraging preliminary data on the use of temozolomide (TMZ)-based therapy has been previously reported.
Methods
We report the response to therapy and safety of radiation with concurrent temozolomide (RT/TMZ) in 2 adult patients with heavily pretreated PC and extraneural metastases.
Results
Both patients had prior history of pituitary macroadenoma. At the time of diagnosis of PC, Ki-67% was 24.2% and 10%, with positive p53 staining in one case. Metastatic sites included lymph nodes, liver and bone. Case-1 received RT/TMZ to the tumor bed in the skull base and to the metastases in the cervical lymph nodes. Case-2 received RT/TMZ to recurrent tumor involving portacaval lymph nodes. Both patients achieved excellent long-term control of the sites of treated extraneural metastases, with no significant acute or delayed toxicity.
Conclusions
RT/TMZ was safely delivered and might provide sustained control of extraneural metastases in PC. Although this retrospective report has limitations, RT/TMZ can be considered as a therapeutic option for the management of extraneural metastases in PC.
Keywords: Pituitary carcinoma, pituitary macroadenoma, temozolomide, capecitabine, chemoradiation
Introduction
Pituitary carcinomas (PC) are uncommon neuroendocrine tumors (NET), accounting for only 0.1% of all pituitary tumors [1] and representing a treatment challenge. The diagnosis of PC is based on a clinical presentation of a pituitary tumor with documented metastases to the subarachnoid space, neuraxis or rest of the body [1,2]. Radiographic or pathologic findings of local invasion into surrounding sellar structures such as dura, bone, blood vessels or nerve sheaths are not sufficient to make the diagnosis of PC [1]. Quantitative analysis of Ki-67 labeling index and p53 expression by immunohistochemistry (IHC) are useful tests that correlate with the degree of invasiveness in pituitary tumors [3]. However, there are no pathognomonic IHC or molecular markers to predict malignant behavior [4,5]. A new proposed clinicopathological classification of pituitary tumors attempts to better predict the tumor biology by accounting for tumor size, immunocytochemical type, invasion and proliferation [6]. PC is typically resistant to therapy, with a median overall survival of only 31 months [1,7]. There is no standard treatment for PC, but maximal safe resection and radiation are performed when possible. Traditional cytotoxic chemotherapy has also been used (eg. cis-platinum and etoposide-based regimens) but overall response is relatively poor [1,8].
In recent years, encouraging preliminary data on the efficacy of temozolomide (TMZ)-based chemotherapy for NETs (including PC), alone or in combination with capecitabine, has been reported [7,9–13]. However, optimal schedule and duration of treatment have not been established. To our knowledge, the safety and efficacy of radiation with concurrent TMZ (RT/TMZ) for treatment of extraneural metastases from PC has not been previously reported. The aim of this study is to report our experience with RT/TMZ for treatment of extraneural metastatic disease in two patients with PC (Table).
Table. Demographics, histopathology, treatment and outcome in the two study patients with Pituitary Carcinoma.
Demographics, histopathology, treatment and outcome in two patients with pituitary carcinoma and extraneural metastases successfully treated with radiotherapy and concurrent temozolomide.
| Case | Sex | Age at diagnosis of PA (y) |
Age (y) at onset of PC/last follow-up |
Clinical presentation PC |
Invasion of primary tumor |
Metastases | Tumor Phenotype | Radiographic and pathological findings (IHC) |
Therapy of PC | PFS (m) | OS (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 53 | 69/71 | Facial/ear pain, exacerbated with mastication |
Right CS, infratemporal fossa dura/epidural space, trigeminal nerve sphenoid sinus, nasal region, maxillary sinus |
Cervical/mediastinal and hilar lymph nodes, left ischium |
Clinically Nonfunctional |
Macroadenoma Ki67 24.2% P53 (+) |
• ChemoRT (tumor bed and neck) with TMZ | + 24 | + 27 |
| 12 cycles of TMZ 100 mg/m2/day, days 10 to 14 and capecitabine 1500 mg/m2 in divided doses twice daily, days 1– 14 every 28-day cycle | |||||||||||
| 2 | M | 36 | 40/45 | Retro-orbital pain, abdominal pain, recurrent Cushing syndrome |
Right CS, right orbit |
Liver, portacaval lymph nodes |
ACTH-secreting | Macroadenoma Ki67 10% P53 (−) ACTH stain (+) |
• 15 cycles of TMZ 200 mg/m2/day, days 1–5 every 28–day cycle | 17 | + 51 |
| • 2 cycles of TMZ 100 mg/m2/day, days 10–14 and capecitabine 1000 mg QAM and 1500 QPM daily, days 1–14 every 28-day cycle |
4 | ||||||||||
| • Right orbital exenteration + RT | 11 | ||||||||||
| Percutaneous microwave frequency ablation of liver metastasis | |||||||||||
| • ChemoRT (portacaval lymph nodes) with TMZ followed by 6 cycles of TMZ 150 mg/m2/day, days 1–5 every 28-day cycle |
9 | ||||||||||
| • Partial percutaneous microwave frequency ablation of liver metastasis and 2 cycles of TMZ 150 mg/m2/day, days 1–5 every 28-day cycle |
2 | ||||||||||
| • 4 cycles of TMZ 100 mg/m2/day, days 10–14 and capecitabine 1500 mg/m2 in divided doses twice daily, days 1–14 every 28- day cycle |
|||||||||||
| • Phase I clinical trial: mTOR Inhibitor and IL-1 receptor antagonist |
Abbreviations: ACTH = Adrenocorticotropic hormone; CS = cavernous sinus; F = female; IHC = immunohistochemistry; M = male; (m) = months; OS = overall survival; PA = pituitary adenoma; PFS = progression-free survival since PC diagnosis; RP = retroperitoneum; RT = radiation therapy; TMZ = temozolomide; (y) = years; (+) = present; (−) = absent.
Clinical Presentation
Case #1
A 56-year-old woman presented with persistent headache and bitemporal hemianopsia, with workup revealing a clinically non-functional pituitary macroadenoma. She underwent transsphenoidal surgical resection. Pathology revealed a null cell adenoma of the pituitary gland. Five years later, the tumor recurred. She was treated with Gamma-Knife radiosurgery, receiving 25 Gy in a single fraction to the area of recurrent disease. She remained asymptomatic for 5 more years, when magnetic resonance imaging (MRI) showed extensive pituitary tumor recurrence. An endocrine evaluation documented central hypothyroidism. She was started on thyroid hormone supplementation and underwent a second resection. Pathologic findings confirmed pituitary adenoma with atypical features, positive synaptophysin and negative p53 expression by IHC, and a Ki-67 index of 9.1%. She received radiation with a total dose of 45 Gy in 25 fractions over five weeks using intensity-modulated radiation therapy (IMRT).
Two years later, MRI showed again extensive recurrence in the right masticator space, extending through the floor of the middle cranial fossa with dural elevation and invasion of the posterior right orbit (Fig.1). She underwent right neck compartmental lymph node dissection and infratentorial fossa dissection, followed by immediate reconstruction of the skull base defect. Pathology showed strong tumor cell cytoplasmic positivity for synaptophysin. The pituitary hormone panel was negative for prolactin (PRL), adrenocorticotropic hormone (ACTH), growth hormone (GH), thyrotropin (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Immunostaining for p53 protein was weakly positive in scattered nuclei with a Ki-67 index of 24.2%. PC was diagnosed based on the presence of metastatic disease involving a cervical lymph node with extranodal extension in the right upper internal jugular region. Additionally, staging with 18F-fluorodeoxyglucose positron emission tomography–computed tomography (FDG PET-CT) revealed FDG-avid mediastinal and hilar lymph nodes, with bronchoscopy-guided hilar lymph node biopsy confirming metastatic disease. Post operatively, the patient started RT with concurrent TMZ (at 75 mg/m2/day) to the tumor bed and the right neck, for the treatment of cervical lymph node metastasis, to a total dose of 54 Gy in 28 fractions. She had no significant hematological or non-hematological toxicities from the TMZ nor any significant acute radiation toxicity.
Figure 1. Case 1: Radiologic findings of surgical site and extraneural metastases before and after chemoradiation with temozolomide.
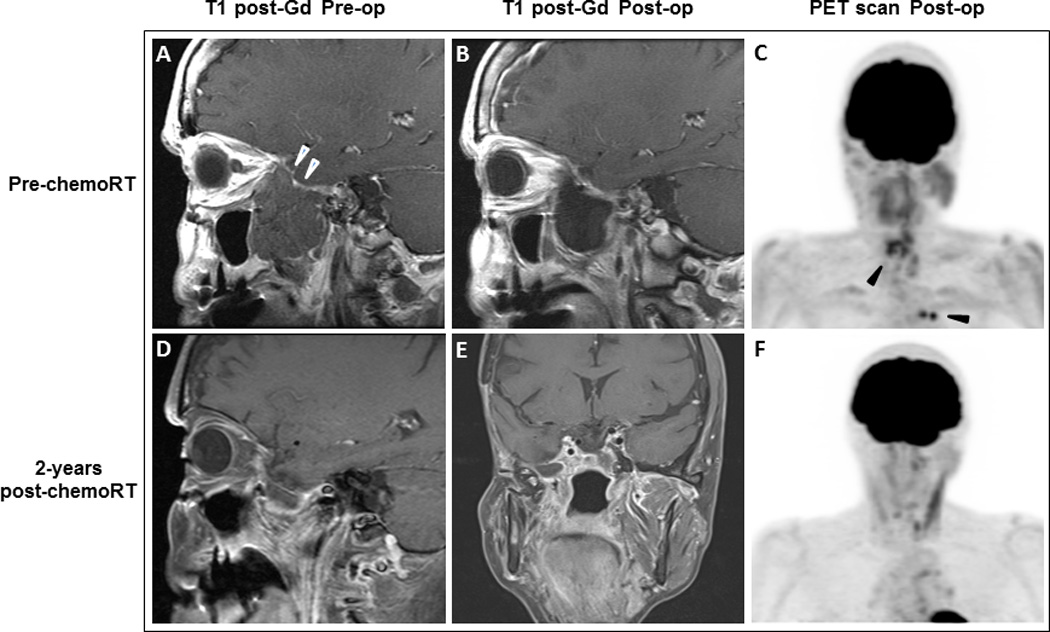
(A) Pre-operative sagittal image with T1-weighted gadolinium-enhanced MRI demonstrates a non-enhancing mass measuring 4.7 x 3.7 x 5.4 cm (white arrowheads) involving the lateral wall of the right sphenoid sinus, nasopharynx, right middle cranial fossa, and masticator space. (B) MRI shows postoperative changes in the right masticator space. All of the previously seen masticator space disease has been resected. A large fluid space is present below the temporal lobe. (C) PET scan for treatment planning purposes shows FDG-avid soft tissue density in the left posterior ethmoid air cells and the region of the right sphenoid sinus (suspicious for residual carcinoma) as well as in the mediastinum and hilar lymph nodes (black arrowheads). MRI (D and E) and PET scan (F) 2 years after chemoradiation shows stable findings in the masticator space and reduction of activity in the left posterior ethmoid sinus as well as the mediastinal and left hilar nodes.
Her post-chemoradiation staging studies revealed reduced metabolic activity in the cervical lymph nodes, stable foci of hypermetabolism in the ethmoid region and chest, and a small, metabolically active lucent lesion in the left ischium suggestive of bone metastasis. She was started on combined chemotherapy with capecitabine and TMZ [11]. She completed 12 cycles and has since been on surveillance for one additional year with no evidence of disease recurrence (Fig.1). PET-CT scan at completion of chemotherapy showed metabolic response to therapy with reduction of activity in the cervical lymph nodes, left posterior ethmoid sinus as well as the mediastinal and left hilar nodes. No new sites of FDG avid disease were identified.
Case #2
A 36-year-old man presented with bitemporal hemianopsia and Cushingoid features. Imaging revealed a pituitary macroadenoma, for which he underwent a transsphenoidal gross total resection. Pathology confirmed an ACTH-secreting pituitary adenoma with negative p53 expression by IHC and only rare positively MIB-1 nuclei staining, indicating a relatively low proliferative rate (Ki-67 index). He then received 50.4 Gy in 28 fractions of external beam radiotherapy. Within a span of 3 years the patient had 2 local recurrences for which he was treated with further surgery followed by a second (45 Gy in 25 fractions) and third course of radiation (45 Gy in 25 fractions), respectively. He developed hypopituitarism and was started on endocrine replacement therapy as well as on ketoconazole and cabergoline for hypercortisolism.
The patient was then stable for 2 years. He then presented with abdominal pain and CT of abdomen showed multiple liver metastases. A core needle biopsy revealed a metastatic intermediate grade neuroendocrine carcinoma, immunoreactive to ACTH and synaptophysin staining with a Ki-67 index of 10%. The patient was started on TMZ at 200 mg/m2/day, days 1–5 every 28-day cycle. After completing 15 cycles, he had a complete response of the liver metastases, but MRI revealed progression of tumor in the right inferior orbit. Capecitabine was added to TMZ; however, after only 2 cycles, disease progression was noted with growth of the right orbital lesion provoking orbital pain, ophthalmoplegia, proptosis, vision loss, numbness in right V3 distribution, and worsening Cushing disease. He underwent right orbital exenteration with pathology confirming neuroendocrine carcinoma. Post operatively he was treated with fractionated external beam radiation therapy (45 Gy in 25 fractions) to the right orbit.
Six months later, he developed biochemical and clinical recurrence of his Cushing syndrome and whole-body staging FDG PET/CT scan revealed new FDG avid portacaval adenopathy (Fig. 2). He underwent laparoscopic bilateral retroperitoneoscopic adrenalectomy to provide better control of his severe Cushing syndrome, and subsequently started stereotactic body radiotherapy to the portacaval lymph nodes to a total dose of 70 Gy in 10 fractions using a stereotactic technique, inspiration breath hold respiratory gating and daily CT image guidance, with concurrent TMZ at 75 mg/m2/day. He tolerated treatment well with mild fatigue as the only side effect. After chemoradiation, he restarted TMZ at 200 mg/m2/day, on days 1 through 5 every 28-day cycle, and completed 6 cycles. He had a complete response and continued having excellent tumor control of his portacaval adenopathy, but developed a new solitary liver metastasis, for which he received multiple interventions, including radiofrequency ablation, combination of capecitabine and TMZ, and more recently, experimental therapy on a Phase I clinical trial. Otherwise, he remains with stable orbital and residual sellar disease, and without evidence of progression in the portacaval lymph nodes 30 months post completion of chemoradiation.
Figure 2. Case 2: Radiologic findings of portacaval adenopathies before and after chemoradiation with temozolomide.
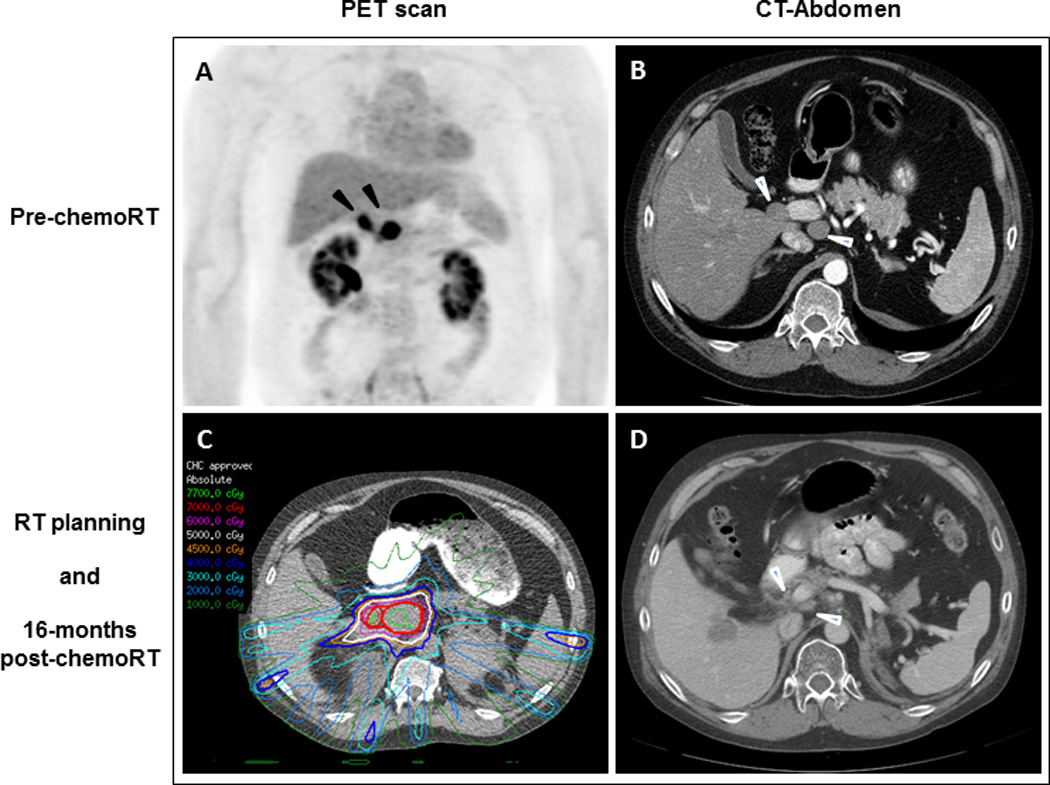
(A) PET scan shows 2 FDG-avid portacaval lymph nodes (black arrowheads). (B) CT of abdomen confirms the 2 masses measuring 2.1 x 2.1 cm and 3.0 x 3.6 cm (white arrowheads). (C) Radiation therapy planning. (D) 16 months after chemoradiation to the portacaval region, the adenopathy has decreased in size (white arrowheads) but unfortunately the hepatic metastasis has increased.
Discussion
PC typically presents in the third to fifth decade of life in patients with preexisting pituitary adenomas [14]. Reported latency periods from the diagnosis of pituitary adenoma vary from 4 months to 18 years, with a mean interval of 6.6 years [15]. It has been suggested that certain tumor subtypes such as PRL-secreting tumors progress to carcinoma earlier than ACTH-secreting tumors (4.7 vs. 9.5 years), but these data are derived from small series. [16]. No clear gender predilection for PC has been reported, despite the fact that PRL- and ACTH-secreting tumors - the most commonly encountered carcinoma subtypes - are more common in females [16–19]. The standard treatment for PC is surgical resection, if feasible, and radiation therapy. Chemotherapy has been used, but overall response to classic agents is relatively poor [1,7]. However, more recently, TMZ-based chemotherapy regimens have shown encouraging antitumor activity [13,20–23].
We report two patients with locally aggressive pituitary tumors and extraneural metastases (Case 1 -cervical lymph nodes- and Case 2 -portocaval lymph nodes) whose extraneural metastases were successfully treated with concomitant TMZ and radiation therapy. In these two cases, PC had previously progressed despite initial standard treatment including maximal safe resection and repeated courses of radiation therapy, and one of the cases progressed even through TMZ-based chemotherapy. Both cases had a prior history of pituitary macroadenoma, with one being non-functional and the other an ACTH-secreting tumor. Pathology revealed a high Ki-67 index in metastatic tumor tissue in both cases (24.2% and 10%) with positive p53 staining in one case.
Chemoradiation, i.e., the use of chemotherapy delivered concurrently with radiation, is used as part of a multimodality approach for local control in many solid tumors [24]. Radiation with concurrent TMZ plus or minus other anticancer drugs has been also used for treatment of brain metastases, and has exhibited encouraging activity in several phase II clinical trials [25]. A phase II randomized trial reported significant improvement in response rate when TMZ was administered in combination with radiation in patients with previously untreated brain metastases [26]. Chemotherapy with sequential and/or concurrent radiation therapy has been reported in the treatment of extraneural metastases from medulloblastoma and glioblastoma [27,28] with variable clinical responses. However, there is no prior reported experience on the safety and efficacy of radiation with concurrent TMZ for treatment of extraneural metastases from PC. Both patients reported here received radiation with concurrent TMZ for the extraneural metastases during the course of their disease, without significant local or systemic toxicity and excellent long-term control of the treated extraneural metastases. No late toxicities from chemoradiation have been identified after +24 and +30 months of surveillance. Both patients received high doses per fraction using stereotactic techniques that seems to have ablated the disease at treated sites. This approach may be important to overcome the radio resistance to standard doses of chemoradiation that has been seen with differentiated, functional NETs.
This anecdotal experience and the retrospective nature of the data collection limit formal conclusions regarding the role of radiation with concurrent TMZ for the treatment of extraneural metastases of PC. Additionally, it is not possible to determine if radiation alone would have provided similar benefit. However, in both cases combined therapy was safely delivered and provided sustained control of the extraneural metastases despite prior failure of radiation alone (Case 1) or sequential radiation and chemotherapy -temozolomide and capecitabine- (Case 2) to control other sites of disease. Prospective clinical trials would be required to unequivocally demonstrate safety, clinical benefits and long-term disease control with this treatment approach; however, the rarity of PC makes prospective research very challenging. Despite these limitations, this report suggests that radiation with concurrent TMZ can be a safe and might be an effective treatment in patients with PC with extraneural metastases. Further studies into the role of concurrent TMZ when radiation is initially delivered for locally advanced pituitary tumors should also be pursued.
Acknowledgments
Funding: No targeted funding reported.
Footnotes
Conflict of Interest: The authors report no conflicts of interest.
References
- 1.Heaney AP. Pituitary carcinoma: difficult diagnosis and treatment. J Clin Endocrinol Metab. 2011;96(12):3649–3660. doi: 10.1210/jc.2011-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheithauer BW, Kovacs KT, Laws ER, Jr, Randall RV. Pathology of invasive pituitary tumors with special reference to functional classification. J Neurosurg. 1986;65(6):733–744. doi: 10.3171/jns.1986.65.6.0733. [DOI] [PubMed] [Google Scholar]
- 3.Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER., Jr p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996;38(4):765–770. discussion 770-771. [PubMed] [Google Scholar]
- 4.Kaltsas GA, Nomikos P, Kontogeorgos G, Buchfelder M, Grossman AB. Clinical review: Diagnosis and management of pituitary carcinomas. J Clin Endocrinol Metab. 2005;90(5):3089–3099. doi: 10.1210/jc.2004-2231. [DOI] [PubMed] [Google Scholar]
- 5.McCutcheon IE, Pieper DR, Fuller GN, Benjamin RS, Friend KE, Gagel RF. Pituitary carcinoma containing gonadotropins: treatment by radical excision and cytotoxic chemotherapy: case report. Neurosurgery. 2000;46(5):1233–1239. doi: 10.1097/00006123-200005000-00042. discussion 1239-1240. [DOI] [PubMed] [Google Scholar]
- 6.Trouillas J, Roy P, Sturm N, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013;126(1):123–135. doi: 10.1007/s00401-013-1084-y. [DOI] [PubMed] [Google Scholar]
- 7.Morokuma H, Ando T, Hayashida T, et al. A case of nonfunctioning pituitary carcinoma that responded to temozolomide treatment. Case Rep Endocrinol. 2012;2012:645914. doi: 10.1155/2012/645914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaltsas GA, Mukherjee JJ, Plowman PN, Monson JP, Grossman AB, Besser GM. The role of cytotoxis chemotherapy in the management of aggressive and malignant pituitary tumors. J Clin Endocrinol Metab. 1998;83:4233–4238. doi: 10.1210/jcem.83.12.5300. [DOI] [PubMed] [Google Scholar]
- 9.Fine RL, Fogelman DR, Schreibman SM. Effective treatment of neuroendocrine tumors with temozolomide and capecitabine. ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23(16S):4216. [Google Scholar]
- 10.Lim S, Shahinian H, Maya MM, Yong W, Heaney AP. Temozolomide: a novel treatment for pituitary carcinoma. Lancet Oncology. 2006;7(6):518–520. doi: 10.1016/S1470-2045(06)70728-8. [DOI] [PubMed] [Google Scholar]
- 11.Thearle MS, Freda PU, Bruce JN, Isaacson SR, Lee Y, Fine RL. Temozolomide (Temodar®) and capecitabine (Xeloda®) treatment of an aggressive corticotroph pituitary tumor. Pituitary. 2011;14:418–424. doi: 10.1007/s11102-009-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fadul CE, Kominsky AL, Meyer LP, et al. Long-term response of pituitary carcinoma to temozolomide: report of two cases. J Neurosurg. 2006;105(4):621–626. doi: 10.3171/jns.2006.105.4.621. [DOI] [PubMed] [Google Scholar]
- 13.Losa M, Bogazzi F, Cannavo S, et al. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126(3):519–525. doi: 10.1007/s11060-015-1991-y. [DOI] [PubMed] [Google Scholar]
- 14.Kontogeorgos G. Classification and pathology of pituitary tumors. Endocrine. 2005;28(1):27–35. doi: 10.1385/ENDO:28:1:027. [DOI] [PubMed] [Google Scholar]
- 15.Roncaroli F, Scheithauer BW, Horvath E, et al. Silent subtype 3 carcinoma of the pituitary: a case report. Neuropathol Appl Neurobiol. 2010;36(1):90–94. doi: 10.1111/j.1365-2990.2009.01043.x. [DOI] [PubMed] [Google Scholar]
- 16.Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804–812. doi: 10.1002/(sici)1097-0142(19970215)79:4<804::aid-cncr18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Kaltsas GA, Grossman AB. Malignant pituitary tumors. Pituitary. 1998;1(1):69–81. doi: 10.1023/a:1009975009924. [DOI] [PubMed] [Google Scholar]
- 18.Scheithauer BW, Fereidooni F, Horvath E, et al. Pituitary carcinoma: an ultrastructural study of eleven cases. Ultrastruct Pathol. 2001;25(3):227–242. doi: 10.1080/019131201300343865. [DOI] [PubMed] [Google Scholar]
- 19.Brown RL, Muzzafar T, Wollman R, Weiss RE. A pituitary carcinoma secreting TSH and prolactin: a non-secreting adenoma gone awry. Eur J Endocrinol. 2006;154(5):639–643. doi: 10.1530/eje.1.02141. [DOI] [PubMed] [Google Scholar]
- 20.Rivera E, Meyers C, Groves M, et al. Phase I study of capecitabine in combination with temozolomide in the treatment of patients with brain metastases from breast carcinoma. Cancer. 2006;107(6):1348–1354. doi: 10.1002/cncr.22127. [DOI] [PubMed] [Google Scholar]
- 21.Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. doi: 10.1007/s00280-012-2055-z. [DOI] [PubMed] [Google Scholar]
- 22.Gulati AP, Krantz B, Moss RA, et al. Treatment of multiple endocrine neoplasia 1/2 tumors: case report and review of the literature. Oncology. 2013;84(3):127–134. doi: 10.1159/000342961. [DOI] [PubMed] [Google Scholar]
- 23.Zacharia BE, Gulati AP, Bruce JN, et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery. 2014;74(4):E447–E455. doi: 10.1227/NEU.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt B, Lee HJ, Ryeom S, Yoon SS. Combining Bevacizumab with Radiation or Chemoradiation for Solid Tumors: A Review of the Scientific Rationale, and Clinical Trials. Curr Angiogenes. 2012;1(3):169–179. doi: 10.2174/2211552811201030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W, Zhou L, Qian JQ, Qiu TZ, Shu YQ, Liu P. Temozolomide for treatment of brain metastases: A review of 21 clinical trials. World J Clin Oncol. 2013;5(1):19–27. doi: 10.5306/wjco.v5.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20(17):3644–3650. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 27.Mahoney DH, Jr, Steuber CP, Sandbach JF, Fernbach DJ. Extraneural metastases from medulloblastoma: long-term survival after sequentially scheduled chemotherapy and radiotherapy. Med Pediatr Oncol. 1986;14(6):329–331. doi: 10.1002/mpo.2950140611. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton JD, Rapp M, Schneiderhan T, et al. Glioblastoma multiforme metastasis outside the CNS: three case reports and possible mechanisms of escape. J Clin Oncol. 2014;32(22):e80–e84. doi: 10.1200/JCO.2013.48.7546. [DOI] [PubMed] [Google Scholar]


