Abstract
Assessment of major adverse cardiovascular events (MACE) after liver transplantation (LT) has been limited by the lack of a multi-center study with detailed clinical information. An integrated database linking information from the University HealthSystem Consortium and the OPTN was analyzed using multivariate Poisson regression to assess factors associated with 30- and 90-day MACE after LT (2/2002–12/2012). MACE were defined as myocardial infarction (MI), heart failure (HF), atrial fibrillation (AF), cardiac arrest, pulmonary embolism and/or stroke. Of 32,810 recipients, MACE hospitalizations occurred in 8% and 11% of patients at 30 and 90 days. Recipients with MACE were older, and more likely to have a history of NASH, alcoholic cirrhosis (ETOH), MI, HF, stroke, AF and pulmonary and chronic renal disease than those without MACE. In multivariable analysis, age > 65 (Incidence rate ratio (IRR)=2.8 (1.8–4.4)), ETOH (IRR=1.6 (1.2–2.2)), NASH (IRR=1.6 (1.1–2.4)), pre-LT creatinine (IRR=1.1 (1.04–1.2)), baseline AF (IRR=6.9 (5.0–9.6)) and stroke (IRR=6.3 (1.6–25.4)) were independently associated with MACE. MACE were associated with lower 1-year survival post-LT (79% vs. 88%, p<.0001). In a national database, MACE occurred in 11% of LT recipients with a negative impact on survival. Pre-LT AF and stroke substantially increase risk of MACE, highlighting potentially high-risk LT candidates.
INTRODUCTION
Cardiovascular disease (CVD) is a major cause of morbidity and mortality after liver transplantation (LT)(1–3). The high prevalence of CVD-related morbidity and mortality is likely a result of several factors affecting LT candidates. The aging LT population is increasingly affected by significant medical comorbidities(4) that may result in adverse post-transplant outcomes(5, 6). Second, with increased prioritization of the sickest LT candidates for transplant, there is a growing critical illness burden among those with high model-for-end-stage-liver-disease (MELD) scores and an increased proportion of ventilator-dependent patients at the time of transplant(7). Such patients may be disproportionately affected by subclinical cardiac disease and a blunted cardiovascular response to the hemodynamic stress of LT resulting in arrhythmia and incident heart failure. Third, over the past decade there has been a marked rise in the prevalence of CVD comorbid conditions, such as diabetes, among LT candidates related to the ongoing obesity epidemic in the United States(8, 9). In fact, it is well established that nonalcoholic steatohepatitis (NASH), an obesity-related cause of end-stage liver disease that is associated with increased CVD morbidity and mortality(10, 11), is likely to become the most common indication for LT over the next decade(12, 13).
The prevalence and predictors of major adverse cardiovascular events (MACE, including myocardial infarction, atrial fibrillation, pulmonary embolism, heart failure, cardiac arrest and/or stroke) after LT are not well established. Previous investigations of MACE after LT have been limited by single center data with inherent variability in candidate selection, or they often predate recent advances in surgical technique and anesthesia(2, 3, 14–19). In addition, there is substantial heterogeneity across studies in the definition of MACE and the majority focus solely on ischemic heart disease events(3, 14, 16, 19). Thus, little is known about the impact of non-coronary heart disease, including arrhythmia, heart failure and stroke, on LT outcomes. In one small single center study, preexisting atrial fibrillation (AF) was shown to increase risk of intraoperative cardiac events and cardiovascular morbidity after LT(17), and among the general population AF is associated with an increased risk of stroke, heart failure, and all-cause mortality representing a substantial healthcare burden with high rates of re-hospitalization and healthcare utilization(20). Utilizing data from the Organ Procurement and Transplantation Network (OPTN) we recently demonstrated that CVD in general has surpassed infection and graft failure as the leading cause of early mortality in the United States, accounting for over 40% of deaths within 30 days of LT(1). However, the OPTN database is limited in its ability to capture CVD morbidity. Thus, the objectives of the current study were to evaluate the prevalence and factors associated with MACE, including both ischemic and non-ischemic events, following LT using an integrated multi-center database that supplements OPTN registry data with national administrative billing claims data from the University HealthSystem Consortium (UHC). We hypothesized that MACE after LT are common and that non-coronary events, which are not considered by current pre-operative risk assessment algorithms, account for the majority of MACE after LT. Identification and incorporation of potentially modifiable risk factors for MACE after LT into validated risk algorithms may improve candidate selection and organ utilization.
PATIENTS AND METHODS
Protocol, Design, Data Sources and Inclusion Criteria
The Northwestern University Institutional Review Board approved this project and the Health Resources and Services Administration (HRSA) of the United States Department of Health and Human Services (HHS) through the Organ Procurement and Transplantation Network (OPTN) approved the study protocol. A retrospective cohort study was conducted including data from LT recipients transplanted between February 1, 2002 and December 31, 2011 with follow up data through December 31, 2012. Clinical data were drawn from the OPTN standard transplant analysis and research (STAR) files (created on March 15, 2013). These data were then linked to clinical data from the University HealthSystem Consortium (UHC) clinical database/resource manager (CD/RM).
The UHC is an alliance of 117 academic medical centers and 300 of their affiliated hospitals representing approximately 90% of the nation’s nonprofit academic medical centers. UHC’s database included self-reported billing claims data from 68 U.S. transplant centers for this study cohort. The database allows for comparison of clinical performance of member-hospitals as well as within-hospital comparisons. The information is based primarily on data submitted from the UB-04 billing forms which includes patient demographics, International Classification of Diseases, 9th revision (ICD-9) diagnostic codes as well as assignment into Medicare severity diagnosis-related groups (DRG) from 2007 onward (8).
The linkage between OPTN data and UHC data was completed using date of transplant, date of birth, Medicare ID of the transplant center, and recipient’s sex. Duplicate matches were verified by residential zip codes based on previously published methodology(21). All direct identifiers were removed before the final data set was available for analysis.
For the present study, inclusion was restricted to adult patients (age ≥ 18 years of age) who received a first liver or liver–kidney transplant for chronic liver disease and who had the transplant performed in a hospital affiliated with the UHC. The matched cases (n=32,810) represent 60.0% of all 54,697 transplant procedures performed in the United States during this period (2002–2011). Donor and recipient demographic characteristics of this restricted population were similar to the overall national transplant cohort defined by the OPTN registry (data not shown).
Clinical Outcome and Covariate Definitions
The primary outcomes were 30 or 90-day MACE, defined as a discharge ICD-9 code within the first 3 diagnosis positions that indicated myocardial infarction (MI), heart failure (HF), atrial fibrillation (AF), cardiac arrest, pulmonary embolism (PE) and/or stroke during the initial transplant admission or subsequent hospitalization at UHC member hospital (Supplementary Table 1). The discharge diagnosis was used in an attempt to capture in-hospital as well as rehospitalization events. The secondary outcomes were 1-year and long-term patient survival. Patients were censored at time of death, date of last follow up, or time of re-transplantation. Prevalent comorbid conditions at the time of transplant were assessed by admission ICD-9 codes present on admission at the initial transplant admission and/or Medicare severity DRG. Obesity was defined as body mass index (BMI) ≥ 30 k/m2 or an ICD-9 code at transplant admission for obesity (ICD-9=278.00, 278.01, 278.02 or 278.03). Transplantation for nonalcoholic steatohepatitis (NASH) was defined as a primary listing diagnosis for NASH or cryptogenic cirrhosis with at least one risk factor for the metabolic syndrome (pretransplant obesity, diabetes, hypertension and/or dyslipidemia).
Potential risk factors for MACE after LT were examined based on a priori clinical hypotheses. These included known traditional CVD risk factors (e.g. diabetes status) as well as transplant-specific critical illness indicators known to contribute to competing mortality risk. Recipient risk factors evaluated included age at transplant, sex, race/ethnicity (Black, non-Hispanic White, Asian and Hispanic), socioeconomic status, BMI, etiology of liver disease (including diseases known to increase CVD risk, such as NASH and hepatitis C), history of comorbid CVD conditions (diabetes, ischemic heart disease, cerebrovascular disease, cardiac dysrhythmias, hypertension, pulmonary embolism, peripheral vascular disease, renal failure), laboratory values at time of transplant (creatinine, albumin, sodium, INR, alanine aminotransferase (ALT), bilirubin), hepatocellular carcinoma, calculated model for end-stage liver disease (MELD) score at the time of transplant, waitlist time, functional capacity prior to transplant, complications of end-stage liver disease (ascites, encephalopathy, portal vein thrombosis, etc.), and hospitalization, dialysis and ventilator status at transplant. Donor risk factors included age, sex, BMI, donor type (living, deceased, donation after cardiac death), and donor risk index. Transplant related variables included transplant center region, organ allocation type, cold ischemia time (CIT), steroid induction, and use of a calcineurin inhibitor (Supplementary Table 2).
Statistical Methods
Clinical characteristics of primary LT recipients from the integrated dataset were described using frequency counts and percentages for categorical variables and means ± standard deviations for continuous variables. Chi-square tests were used to examine the relationship between the characteristics and MACE. Eleven candidate variables that were significant (p<0.05) in univariate analysis were entered into a multivariable Poisson regression model. Seven covariates were selected for the final model based on significance and additive contribution to the model based on Bayesian Information Criteria (BIC). Further covariates did not improve model fit. Poisson multivariate regression analysis was also used to assess the relationship between any MACE after LT and recipient all-cause mortality. Kaplan-Meier with long-rank test assessed long-term mortality. The assumption of proportional hazards was assessed graphically. Cox proportional hazard analysis was then used with multivariable adjustment for variables known to effect post-transplant mortality, including recipient age, sex, race, BMI, education level, insurance status, MELD score, indication for transplant, donor risk index and CIT to assess the effect of prevalent MACE on 1-year and long-term patient survival. Missing data that were categorized as ‘‘other’’ or ‘‘unknown’’ was excluded from analysis. An alpha level of 0.05 was used for all significance tests. All analysis was performed using SAS 9.3 (SAS institute, Cary, NC).
RESULTS
Clinical Characteristics of the Study Cohort
During the period of the study we identified 32,810 LT recipients within the UHC database with an OPTN LT record between February 1, 2002 and December 31, 2011. Clinical characteristics of the study cohort are shown in Table 1. Mean age of the study sample was 55 ± 10 years, 32% were female, and 73% were non-Hispanic White. The majority of the patients were transplanted for hepatitis C, followed by hepatocellular carcinoma and alcohol. The average MELD score at transplant for this sample was 20 ± 11. Hypertension was the most common preexisting CVD condition and 34% of the study sample met criteria for obesity, 24% had diabetes, and 14% had chronic kidney disease at the time of LT. Patients in the sample experienced a median of 1 post-transplant hospitalization during the first year after the initial transplant event: 4,440 (14%) recipients were readmitted within 30 days and 6,095 (19%) recipients within 90 days of LT.
Table 1.
Demographic and Clinical Characteristics of Liver Transplant Recipients Included in the Study (2002–2011)
| Characteristic | All first liver transplants N=32,810 |
|---|---|
| Age, years, mean ± SD | 55.2 ± 9.9 |
| 18–44, % | 14.9 |
| 45–64, % | 73.5 |
| 65+, % | 11.7 |
| Sex (female), % | 32.6 |
| Recipient race & ethnicity, % | |
| Non-Hispanic White | 73.1 |
| Non-Hispanic Black | 9.6 |
| Hispanic | 10.9 |
| Asian | 5.4 |
| Other | 1.0 |
| Primary indication for transplant, % | |
| Hepatitis C | 24.2 |
| Hepatocellular carcinoma | 20.2 |
| Alcohol | 17.5 |
| NASH | 9.7 |
| Cryptogenic | 1.5 |
| Hepatitis B | 2.3 |
| Other | 24.7 |
| Insurance type, % | |
| Private | 62.0 |
| Medicare | 20.6 |
| Medicaid | 13.8 |
| Other | 3.5 |
| Calculated MELD score at LT, mean ± SD | 20.0 ± 11.1 |
| CVD comorbidities present at LT, % | |
| Hypertension | 35.3 |
| Thromboembolism, any | 11.7 |
| Pulmonary embolism | 0.8 |
| Ischemic heart disease | 7.4 |
| Myocardial Infarction | 2.5 |
| Cardiac dysrhythmia, any | 14.7 |
| Atrial fibrillation | 6.0 |
| Heart Failure | 3.1 |
| Hyperlipidemia | 1.4 |
| Peripheral vascular disease | 0.3 |
| Stroke | 1.4 |
| Other medical comorbidities present at LT, % | |
| Obesity | 34.1 |
| Diabetes | 23.6 |
| Chronic kidney disease | 14.0 |
| Asthma/COPD | 10.1 |
| Pulmonary hypertension | 3.3 |
| Obstructive sleep apnea | 1.7 |
| Complications of ESLD at LT, No (%) | |
| Ascites at transplant | 40.3 |
| Variceal Bleed | 20.6 |
| Hepatic Encephalopathy | 17.5 |
| Transhepatic intravenous portosystemic shunt (TIPS) | 8.3 |
| Hepatorenal syndrome | 14.6 |
| Portal Vein Thrombosis | 6.3 |
| Spontaneous bacterial peritonitis (SBP) | 2.7 |
| Hepatocellular carcinoma | 3.1 |
| Hepatopulmonary syndrome | 0.3 |
| Charlson Comorbidities, % | |
| 0 | 34.9 |
| 1 | 40.1 |
| 2 | 19.8 |
| 3 | 4.3 |
| 4 | 0.8 |
| 5+ | 0.1 |
| Simultaneous liver-kidney, % | 7.0 |
| Living donor, % | 4.5 |
Abbreviations: No, number; ESLD, end-stage liver disease; COPD, chronic obstructive pulmonary disease; LT, liver transplant; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis; SD, standard deviation
Prevalence and characteristics associated with MACE after liver transplant
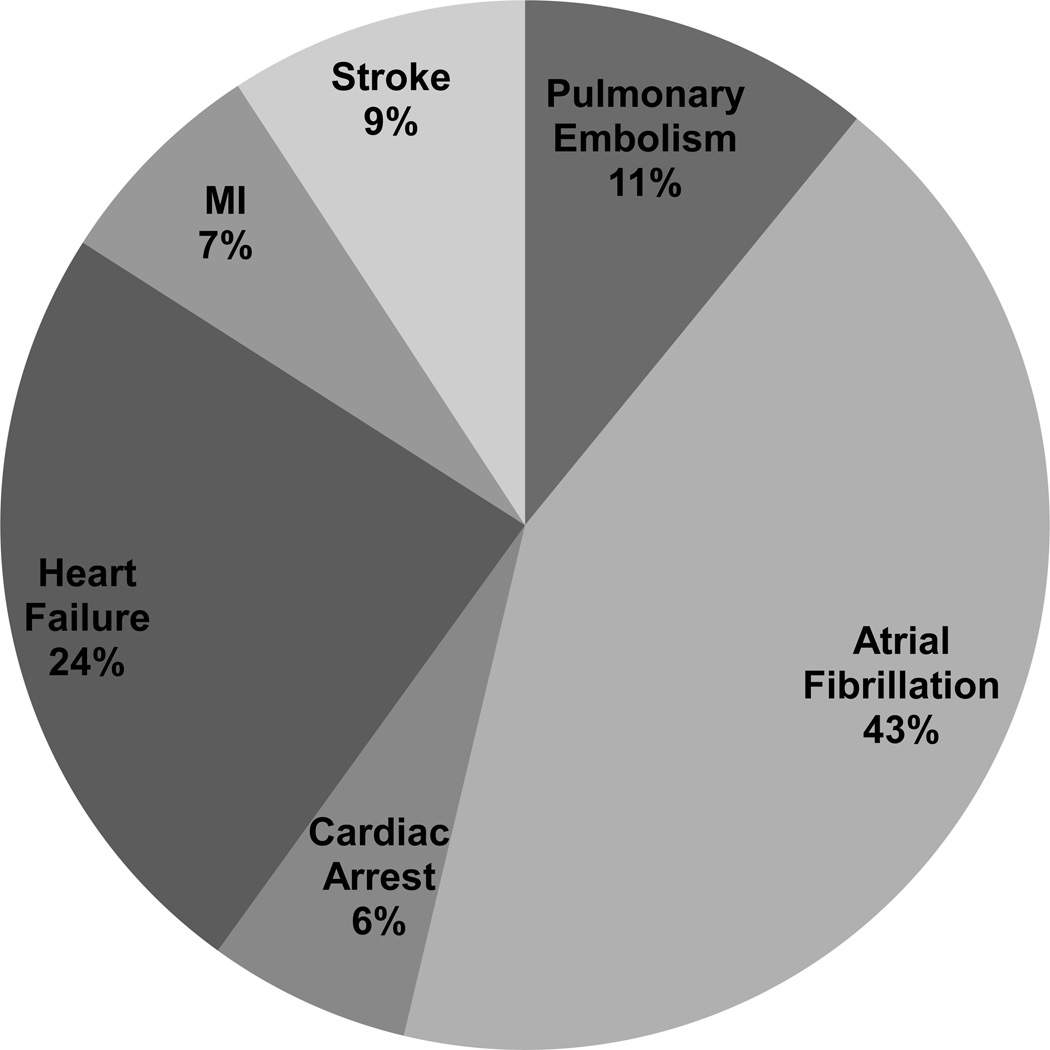
MACE occurred in 368 (8%) and 474 (11%) patients who were readmitted at 30 and 90 days, respectively. The most common cause of an early (within 90-days of LT) MACE was AF (43%) followed by HF (24%) and PE (11%). MI only accounted for 7% of all MACE after LT. Distribution was similar for 30-day MACE (Figure 1).
Figure 1. Distribution of early MACE after liver transplantation.
Among 32,810 LT recipients, 4,440 were admitted within 30 days and 6,095 within 90 days of LT. MACE occurred in 368 (8%) and 474 (11%) patients at 30 and 90 days, respectively. The most common cause of an early (within 90-days of LT) MACE was atrial fibrillation followed by heart failure and pulmonary embolism. Distribution was similar for 30-day MACE.
Abbreviations: MACE, major adverse cardiovascular event; LT, liver transplantation; MI, myocardial infarction.
LT recipients with early MACE were older, more likely to be non-Hispanic white and have a diagnosis of NASH or alcohol-induced cirrhosis (Table 2). They also had poorer functional status and were more likely to be hospitalized prior to transplant than recipients without an early MACE after LT (Table 2). LT recipients with an early MACE were more likely to have hepatic encephalopathy and hepatopulmonary syndrome (p<0.01 for both). Calculated MELD score at transplant was higher in LT recipients with an early MACE (21.8 ± 11.8) compared to those without a MACE (20.0 ± 11.1, p<0.0008). LT recipients with an early MACE had a higher prevalence of pre-transplant ischemic heart disease, MI, HF, and dysrhythmia, including AF (Table 2). There was a higher prevalence of pulmonary comorbidities, such as asthma, pulmonary hypertension and obstructive sleep apnea, among recipients who experienced MACE. In addition, these recipients were more likely to have respiratory failure requiring ventilator support at the time of transplant. Finally, LT recipients with an early MACE had higher mean creatinine (1.9 vs. 1.6 mg/dL, p<.0001) and prevalence of chronic renal disease (19% vs. 14%, p=.0018) than those without MACE. No notable differences were found in donor factors, cold ischemia time, recipient immune prophylaxis or discharge status between those with and without a MACE (Table 3).
Table 2.
Comparison of recipient characteristics stratified by 30 or 90-day major adverse cardiovascular events (MACE) after liver transplantation
| Characteristic | No 90-day MACE N = 32,442 |
+30-day MACE N = 368 |
+90-day MACE n = 474 |
P value* |
| Recipient Age, % | < .0001 | |||
| 18–44 | 15.0 | 7.6 | 7.4 | |
| 45–64 | 73.5 | 71.7 | 73.2 | |
| 65+ | 11.6 | 20.7 | 19.4 | |
| Female, % | 32.6 | 32.3 | 32.7 | .96 |
| Race & ethnicity, % | .08 | |||
| Non-Hispanic White | 73.0 | 79.4 | 78.3 | |
| Non-Hispanic Black | 9.6 | 9.0 | 9.5 | |
| Hispanic | 11.0 | 6.8 | 7.2 | |
| Asian | 5.4 | 4.6 | 4.6 | |
| Other | 1.0 | 0.3 | 0.4 | |
| Highest education level, % | .26 | |||
| Less than high school | 4.1 | 3.5 | 3.4 | |
| High school | 40.1 | 35.6 | 37.1 | |
| College+ | 38.5 | 44.8 | 42.8 | |
| Unknown | 17.2 | 16.0 | 16.7 | |
| Primary indication for transplant, % | .0004 | |||
| Hepatitis C | 24.3 | 19.4 | 19.4 | |
| Hepatocellular carcinoma | 20.2 | 17.1 | 17.1 | |
| Alcohol | 17.4 | 22.2 | 22.2 | |
| NASH | 9.6 | 13.4 | 13.9 | |
| Other | 28.5 | 27.2 | 27.4 | |
| Payor Status, % | .0004 | |||
| Private | 62.1 | 59.2 | 61.6 | |
| Medicare | 20.5 | 28.3 | 26.8 | |
| Medicaid | 13.9 | 9.8 | 8.9 | |
| Other* | 3.5 | 2.3 | 2.7 | |
| Functional status at transplant, % | .03 | |||
| Independent | 44.8 | 37.5 | 38.8 | |
| Partially dependent | 20.9 | 26.1 | 24.3 | |
| Totally dependent | 24.2 | 27.2 | 27.6 | |
| Unknown | 10.1 | 9.2 | 9.3 | |
| Medical condition at transplant, % | .003 | |||
| Not hospitalized | 71.3 | 66.0 | 66.0 | |
| Hospitalized not in ICU | 17.2 | 17.5 | 17.5 | |
| In ICU | 11.5 | 16.5 | 16.5 | |
| Calculated MELD score at transplant, mean |
20.0 ± 11.1 | 21.9 ± 11.7 | 21.8 ± 11.8 | .0008 |
| Days on waiting list, mean | 209.5 ± 360.5 | 234.3 ±448.6 | 230.5 ±189.5 | .21 |
| Simultaneous liver-kidney, % | 7.0 | 10.3 | 9.5 | .04 |
| CVD risk factors at transplant, % | ||||
| Obesity | 34.0 | 36.7 | 39.9 | .007 |
| Hypertension | 35.2 | 39.7 | 40.1 | .03 |
| Diabetes | 23.6 | 21.5 | 22.2 | .45 |
| Chronic kidney disease | 14.0 | 20.1 | 19.0 | .0018 |
| Cardiac dysrhythmia, any | 14.3 | 41.9 | 40.9 | < .0001 |
| Atrial fibrillation | 5.6 | 33.2 | 32.5 | < .0001 |
| Ischemic heart disease | 7.3 | 13.3 | 13.3 | < .0001 |
| Prior myocardial infarction | 2.4 | 5.2 | 5.3 | <.0001 |
| Heart failure | 3.0 | 14.4 | 12.9 | < .0001 |
| Stroke or TIA | 1.4 | 2.2 | 1.9 | .37 |
| Hyperlipidemia | 1.3 | 1.6 | 1.9 | .29 |
| Peripheral Vascular Dz | 0.3 | 0 | 0 | .27 |
| Thromboembolism | 11.7 | 13.6 | 13.9 | .13 |
| Pulmonary Embolism | 0.8 | 1.6 | 1.5 | .08 |
| Current/former smoker | 15.2 | 18.3 | 18.3 | .08 |
| Family history of CVD | 1.1 | 1.4 | 1.7 | .24 |
| Characteristic | No MACE N = 32,442 |
+30-day MACE N = 368 |
+90-day MACE n = 474 |
P value* |
| Pulmonary Comorbidities, % | ||||
| Pulmonary Hypertension | 3.3 | 6.3 | 6.3 | .0002 |
| Respiratory failure on ventilator | 6.1 | 8.4 | 9.5 | .002 |
| Asthma/COPD | 10.0 | 13.9 | 14.1 | .0032 |
| Obstructive sleep apnea | 1.7 | 4.1 | 4.0 | .0001 |
| Complications of ESLD, % | ||||
| Ascites at transplant | 40.2 | 43.5 | 44.7 | .05 |
| Variceal Bleed | 20.6 | 22.8 | 21.7 | .55 |
| Hepatic Encephalopathy | 17.4 | 21.2 | 24.5 | <.0001 |
| TIPS | 8.3 | 7.6 | 7.4 | .47 |
| Hepatorenal syndrome | 14.6 | 17.9 | 19.6 | .002 |
| Portal Vein Thrombosis | 6.2 | 8.7 | 8.7 | .03 |
| SBP | 2.7 | 4.1 | 4.0 | .09 |
| Hepatocellular carcinoma | 3.1 | 2.2 | 1.9 | .15 |
| Hepatopulmonary syndrome | 0.3 | 1.1 | 1.1 | .006 |
| Charlson comorbidities, % | < .0001 | |||
| 0 | 35.0 | 28.8 | 29.3 | |
| 1 | 40.1 | 35.6 | 35.4 | |
| 2 | 19.7 | 26.1 | 26.4 | |
| 3 | 4.3 | 7.1 | 6.5 | |
| 4 | 0.8 | 2.5 | 2.3 | |
| 5+ | 0.1 | 0 | 0 | |
| Laboratory Values at time of transplant, mean ± SD |
||||
| Creatinine (mg/dL) | 1.6 ± 1.4 | 1.9 ± 1.8 | 1.9 ± 1.7 | < .0001 |
| ALT (U/L) | 178.1 ± 586.1 | 111.7± 296.4 | 119.4 ± 322.0 | .02 |
| Total bilirubin (mg/dL) | 8.3 ± 11.0 | 8.7 ±10.8 | 8.8 ± 10.8 | .30 |
| Albumin | 3.0 ± 0.7 | 3.1 ± .72 | 3.0 ± .7 | .03 |
| INR | 1.9 ± 1.7 | 2.0 ± 1.2 | 2.0 ± 1.2 | .45 |
| Sodium | 135.9 ± 5.2 | 136.2 ± 5.4 | 136.4 ± 5.4 | .06 |
| BMI (kg/m2) at transplant, mean | 28.2 ± 28.1 | 28.5 ± 28.0 | 28.7 ± 5.9 | .03 |
chi-square or Fisher’s exact test for categorical variables, t test for continuous variables for 90-day MACE. Findings were similar for 30-day MACE
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESLD, end-stage liver disease; ICU, intensive care unit; INR, international normalized ratio; LOS, length of stay; MACE, major adverse cardiovascular event MELD, model for end-stage liver disease score; NASH, nonalcoholic steatohepatitis; No, number; SBP, spontaneous bacterial peritonitis; SD, standard deviation; TIA, transient ischemic attack; TIPS, transhepatic intravenous portosystemic shunt
Table 3.
Comparison of donor and transplant characteristics stratified by recipient 30 or 90-day major adverse cardiovascular events (MACE) after liver transplantation
| Characteristic | No 90-day MACE N = 32,442 |
+30-day MACE N = 368 |
+90-day MACE n = 474 |
P value* |
|---|---|---|---|---|
| Organ sharing, No (%) | .30 | |||
| Local | 75.2 | 72.3 | 70.7 | |
| Regional | 18.8 | 20.1 | 21.9 | |
| National | 5.9 | 7.6 | 7.4 | |
| Donor Factors | ||||
| Age, mean, years | 41.2 ± 16.9 | 41.8 ±16.8 | 42.6 ± 16.7 | .07 |
| Sex (female) | 41.3 | 40.0 | 39.9 | .02 |
| Donor Risk Index, mean | 3.2 ± 6.0 | 3.0 ± 2.1 | 3.0 ± 1.9 | .40 |
| DCD donor | 4.61 | 4.4 | 4.0 | .54 |
| Donor BMI | 27.0 ± 6.0 | 27.2 ± 6.0 | 27.4 ± 6.1 | .12 |
| Living Donor | 4.5 | 3.3 | 3.2 | .17 |
| CDC high risk donor | 9.9 | 12.7 | 11.6 | .43 |
| Cold ischemia time, mean, min | 7.0 ± 3.6 | 6.9 ± 3.3 | 7.0 ± 3.1 | .78 |
| Recipient initial immune prophylaxis, No (%) |
||||
| Calcineurin Inhibitor | 91.9 | 93.5 | 93.5 | .27 |
| Steroids | 92.4 | 93.8 | 93.7 | .33 |
chi-square or Fisher’s Exact test for categorical variables, t test for continuous variables for 90-day MACE. Findings were similar for 30-day MACE
Abbreviations: ALT, alanine aminotransferase; CDC, centers for disease control; DCD, donation after cardiac death; MACE, major adverse cardiovascular event; Min, minutes; SD, standard deviation;
Risk Factors for early MACE after liver transplant and impact on patient survival
In sex-adjusted multivariate analysis, age > 65 (Incidence rate ratio (IRR)=2.8 (95% confidence interval (95% CI): 1.8–4.4)), alcoholic cirrhosis (IRR=1.6, 95%CI: 1.2–2.2), nonalcoholic steatohepatitis (IRR=1.6, 95% CI: 1.1–2.24), pre-transplant creatinine (IRR=1.1, 95% CI: 1.04–1.2), AF (IRR=6.9, 95% CI: 5.0–9.6) and stroke (IRR=6.3, 95% CI: 1.6–25.4) independently predicted 30-day MACE after LT (Table 4). Findings were similar for 90-day MACE. Other variables significant in univariate analysis including MELD score, prior history of MI, HF, asthma/COPD, obstructive sleep apnea, pulmonary hypertension, hepatic encephalopathy, dialysis status, hospitalization status and functional status were not significant in multivariable models and addition of these variables did not improve model fit.
Table 4.
Multivariable predictors of 30 and 90-day MACE after liver transplantation
| 30-day MACE | 90-day MACE | |||
|---|---|---|---|---|
| Incidence rate ratio (IRR) |
95% confidence interval |
Incidence rate ratio (IRR) |
95% confidence interval |
|
| Recipient female sex | 1.0 | .82–1.1 | 1.0 | .84–1.3 |
| Recipient age | ||||
| <45 | Ref | Ref | Ref | Ref |
| 45–64 | 1.8 | 1.2–2.7 | 2.0 | 1.4–2.8 |
| 65+ | 2.8 | 1.8–4.4 | 2.9 | 1.9–4.3 |
| Atrial fibrillation pretransplant | 6.9 | 5.0–9.6 | 6.1 | 4.5–8.3 |
| Stroke pretransplant | 6.3 | 1.6–25.4 | 4.8 | 1.2–19.2 |
| Alcohol-induced cirrhosis | 1.6 | 1.2–2.2 | 1.5 | 1.2–2.0 |
| Nonalcoholic steatohepatitis | 1.6 | 1.1–2.4 | 1.7 | 1.2–2.4 |
| Creatinine, at transplant (per dL) | 1.1 | 1.04–1.2 | 1.1 | 1.03–1.14 |
Multivariate Poisson regression analysis
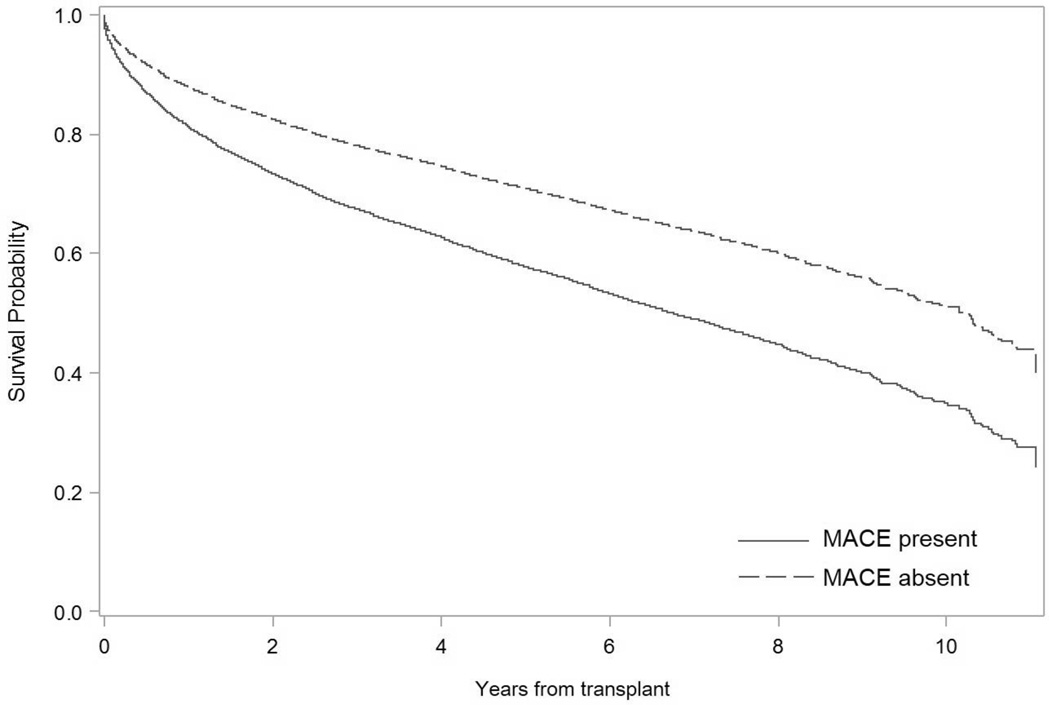
The 1-year mortality rate was 12.2% (n=5,420). Those with an early MACE had lower 1-year survival post-LT than those without an event (79.1% vs. 88.4%, log-rank p<.0001). The most common cause of 1-year mortality within the cohort was infection (35.2%), followed by cardiovascular-related death (18.2%). Of note, 1-year cardiovascular-related mortality was significantly more common among those with a MACE compared to those without (24.7% vs. 12.4%, p=.0008). In multivariable survival analysis adjusted for recipient age, sex, race, BMI, education level, insurance status, MELD score, indication for transplant, donor risk index and cold ischemic time, early MACE were associated with greater long-term mortality (HR=1.62, 95% CI: 1.36–1.92 for 30-day MACE; HR=1.54, 95% CI: 1.32–1.79 for 90-day MACE, Figure 2). There was no significant interaction between MACE and transplant center (p=0.67) for long-term mortality.
Figure 2. Long-term recipient survival in those with and without a MACE after liver transplantation.
Cox proportional hazard survival analysis adjusted for recipient age, gender, race, body mass index, education level, insurance status, MELD score, indication for transplant, donor risk index and cold ischemic time. 30-day and 90-day MACE were associated with lower long-term recipient survival (HR=1.62, 95% CI: 1.36–1.92 for 30-day MACE; HR=1.54 95% CI: 1.32–1.79 for 90-day MACE). Patients were censored at time of death or date of last follow up.
Abbreviations: MACE, major adverse cardiovascular event
DISCUSSION
This study provides the first multicenter assessment of major cardiovascular events after liver transplantation using a combined national database that captures 60% of the LT population in the United States. We have identified that MACE occur in approximately 10% of inpatient admissions within 90 days of LT and that the majority are non-coronary events. In addition, a significant proportion of deaths after LT are related to cardiovascular disease and MACE are a significant risk factor for these deaths resulting in 10% lower 1-year survival and decreased long-term survival after LT. We also demonstrate that pre-transplant AF and stroke, both clinical conditions in which perioperative risk might be mitigated, substantially increase the risk of MACE. When compared to ischemic heart disease, these conditions have been relatively understudied in the LT population. Risk factor reduction for AF and stroke includes smoking cessation, blood pressure reduction, statin use and anticoagulation in certain situations. The physiologic conditions of end-stage liver disease and concern for postoperative bleeding may impede the initiation of some of these measures, however, there are no prospective studies evaluating the safety or efficacy of the measures among a LT population. Thus, future prospective studies aimed at determining whether aggressive risk factor reduction, both pre-transplant and particularly in the early postoperative period, can decrease MACE and improve post-LT outcomes are needed.
A preoperative CVD evaluation is undertaken in all potential LT candidates prior to transplant listing, mainly to screen for significant obstructive coronary artery disease, severe HF and/or severe pulmonary hypertension which are considered absolute contraindications to liver transplantation(22). Despite exclusion of these high-risk patients, the rate of early MACE after LT was nearly 8% in this national sample. This is higher than the early MACE rate after other types of intraabdominal surgery ranging from 0.1% in laparoscopic cholecystectomy(23) to 0.2% after Whipple(24) and is in line with early MACE rates after kidney transplantation, historically the highest risk transplant population for MACE, which ranges from 3.3% to 13.4%(25, 26). The early MACE rate reported in the current study is consistent with previously published single-center studies that have reported MACE rates after LT ranging from 7% to 15%(2, 14, 18). To our knowledge we are the first to provide a multicenter estimate that may provide more accurate and precise data on the true prevalence of early MACE in the current era of transplantation.
The leading underlying types of early MACE were primarily non-coronary events, including AF, HF, thromboembolism, and stroke. This suggests that some LT candidates may have subclinical CVD and may not be identified as high risk when using standard risk algorithms for non-cardiac surgery that were primarily designed to detect underlying coronary heart disease(27). One plausible explanation for these findings is a high prevalence of pretransplant underlying cirrhotic cardiomyopathy, which is characterized by increased cardiac output, electrophysiological abnormalities and a compromised ventricular response to stress, which may place LT recipients at risk for post-operative MACE(28). In addition, poor functional status was a univariate predictor of increased MACE and may be a marker of accumulating physiologic declines that make LT candidates less able to deal with stressors, including surgery. Prehabilitation is intervention to enhance functional capacity before surgery, aimed at improving the patient’s tolerance to upcoming physiologic stress(29). The role of prehabilitation in decreasing risk of MACE after LT warrants further study.
Pretransplant history of AF, a potential marker of underlying cirrhotic cardiomyopathy, was highly prevalent (~33% vs. 7%) among LT recipients with an early MACE compared to those without an event. Atrial fibrillation is the most common cardiac arrhythmia with prevalence estimates ranging from 0.95% to 2.3% in the general population(30) and AF significantly increases cardiovascular and cerebrovascular morbidity, as well as all-cause mortality(31). In a recent single-center study, the prevalence of AF among LT recipients was reported at 4.5% and patients with AF had a higher incidence of intraoperative cardiac events and a higher cardiovascular morbidity rate though there was no impact of AF on overall graft or patient survival(17). Thus, AF may be a marker of risk for future decompensation and should be considered as part of the risk profile in candidate selection.
A prior history of stroke was also identified as a substantial predictor of early MACE in multivariable analysis, and posttransplant stroke accounted for 9% of all events. Though ischemic and hemorrhagic stroke are relatively rare after LT, a higher risk of cerebrovascular complications has been reported in older recipients and in those with pre-transplant diabetes, hypertension, and hyperlipidemia(32). It is notable that a substantial proportion of our study population had a pre-transplant diagnosis of hypertension (40%) and there was a trend towards hypertension as a significant predictor of early MACE (p=0.07). In addition, hepatic encephalopathy, which is associated with dysregulation of cerebral blood flow autoregulation(33), was also more prevalent among recipients with an early MACE. Taken collectively these findings warrant further investigation into the mechanisms and risk factors for stroke among LT recipients.
Finally, conditions associated with hepatic steatosis, including both NASH and alcohol-induced liver disease, were independently associated with early MACE in the final multivariate model. Patients with NASH have multiple established risk factors for CVD including insulin resistance, hypertension, atherogenic dyslipidemia and obesity(34, 35). NASH patients also have an increased prevalence of chronic kidney disease, which is another known risk factor for CVD(36). Finally, NASH is associated with many other emerging and nontraditional CVD risk factors(37–39). Thus, it is not surprising that CVD is the leading cause of morbidity and mortality among patients transplanted for NASH(10, 11, 40, 41). Excessive alcohol consumption may not only lead to cirrhosis but also alcohol-induced cardiomyopathy, which is the main cause of secondary non-ischemic dilated cardiomyopathy in the western world(42, 43). Heart failure was the second leading cause of early MACE in our study sample and it is plausible that the long-term effects of chronic alcohol use and systemic inflammation due to NASH may account for a significant proportion of these observed HF events.
Our study has several limitations. First, our analysis may be subjected to reporting error or bias inherent to large registry and administrative claims databases. In addition, in the current study MACE assessment was limited to events that occurred at a UHC member hospital. Therefore, not all MACE may have been captured if a recipient presented to a non-member organization. However, LT recipients are very likely to be readmitted or transferred to their transplant hospital within the first year. Our ascertainment of MACE were limited to those listed in the top 3 discharge diagnosis codes in order to maximize code specificity for MACE(44). Thus, our estimates may underestimate the true incidence of MACE particularly if events occurred during the index transplant hospitalization where other transplant-related complications may be coded higher in the taxonomy. We also acknowledge that the current analysis may be limited due to the lack of precise measurement of pre-operative CVD risk variables on individual recipients, such as preoperative cardiovascular testing, laboratory values, such as lipids and troponin levels, and medication use, which are not currently available within these databases. Finally, certain CVD risk factors, such as recipient family history of CVD or thromboembolism are historically unreliable based on claims data and may not be accurately captured here(45).
Identifying LT-specific MACE risk factors has important policy implications for both Medicare reimbursement, candidate selection, and for organ allocation(46). The Scientific Registry of Transplant Recipients (SRTR) risk prediction models are used by the Centers for Medicare & Medicaid Service (CMS) to certify transplant centers for reimbursement. Transplant centers are required to achieve or exceed 1-year expected graft and patient survival as determined by these risk-adjusted models (47). Due to the ramifications of these evaluations for CMS certification, centers may be motivated to restrict higher risk patients from access, who might still benefit from transplantation. The current study has rigorously evaluated the available national data and identified an additional key predictor variable, atrial fibrillation, which is not currently collected by OPTN. Future prospective studies focused on determining whether this variable, and any additional CVD variables, should be collected by OPTN and therefore included in the risk-adjusted SRTR performance measures are needed. As we enter a new era of effective treatment for hepatitis C virus (HCV), transplantation rates for HCV are predicted to decline and therefore transplantation for NASH and ETOH, which both highly associated with MACE, will increase in relative, as well as absolute importance over the coming decade(12). Given this new reality, large-scale, multi-center studies should prospectively examine the effects of functional status on post-transplant cardiovascular complications and evaluate the effect of “prehabilitation” e.g. increasing functional capacity in anticipation of transplantation as has been evaluated for other major intra-abdominal surgeries(48, 49).
CONCLUSION
We have identified patients who are at high risk of early MACE after liver transplantation using a large integrated database representative of a national sample of U.S. LT recipients. Importantly, early MACE have a significant impact on 1-year post-transplant survival and pretransplant atrial fibrillation is a substantial predictor of early MACE that is not currently captured by the OPTN registry. Our findings highlight an opportunity to improve care, enhance recipient selection, and maximize organ utilization among liver transplant candidates.
Supplementary Material
Acknowledgments
The data reported here have been supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network (OPTN) and the University HealthSystem Consortium (UHC). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the OPTN, the U.S. Government or the UHC.
GRANTS AND FINANCIAL SUPPORT:
This work was supported by the National Institutes of Health (1 F32 HL116151-01), the American Liver Foundation (New York, NY), and an Alpha Omega Alpha Postgraduate Award. Dr. VanWagner is currently supported by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number KL2TR001424.
Abbreviations
- AF
Atrial fibrillation
- ALT
Alanine Aminotransferase
- BMI
Body mass index
- CVD
Cardiovascular disease
- CIT
Cold ischemic time
- COPD
Chronic Obstructive Pulmonary Disease
- DRG
Diagnosis-related group
- ESLD
End-stage liver disease
- HCV
Hepatitis C virus
- HF
Heart failure
- HHS
U.S. Department of Health and Human Services
- HRSA
U.S. Health Resources and Services Administration
- ICD9
International Classification of Diseases, 9th revision
- ICU
Intensive care unit
- IL6
Interleukin-6
- INR
International normalized ratio
- IRR
Incidence rate ratio
- LT
Liver transplantation
- MACE
Major adverse cardiac event
- MELD
Model for end-stage liver disease
- MI
Myocardial infarction
- NASH
Nonalcoholic steatohepatitis
- OPTN
Organ Procurement and Transplant Network
- PE
Pulmonary embolism
- PVT
Portal Vein Thrombosis
- STAR
Standard transplant analysis and research files
- SRTR
Scientific registry for transplant recipients
- TNF
Tumor necrosis factor
- UHC
University HealthSystem Consortium
Footnotes
DISCLOSURES
The authors of this manuscript have no conflicts of interest to disclose.
REFERENCES
- 1.VanWagner LB, Lapin B, Levitsky J, Wilkins JT, Abecassis MM, Skaro AI, et al. High early cardiovascular mortality after liver transplantation. Liver Transpl. 2014;20(11):1306–1316. doi: 10.1002/lt.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, et al. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18(3):370–375. doi: 10.1002/lt.22468. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SD, Morris JK, Cramb R, Gunson BK, Neuberger J. Cardiovascular morbidity and mortality after orthotopic liver transplantation. Transplantation. 2002;73(6):901–906. doi: 10.1097/00007890-200203270-00012. [DOI] [PubMed] [Google Scholar]
- 4.Annual Report of the U.S. Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR): Transplant Data 1998–2011. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [Google Scholar]
- 5.Audet M, Piardi T, Panaro F, Cag M, Ghislotti E, Habibeh H, et al. Liver transplantation in recipients over 65 yr old: a single center experience. Clin Transplant. 2010;24(1):84–90. doi: 10.1111/j.1399-0012.2009.00972.x. [DOI] [PubMed] [Google Scholar]
- 6.Zetterman RK, Belle SH, Hoofnagle JH, Lawlor S, Wei Y, Everhart J, et al. Age and liver transplantation: a report of the Liver Transplantation Database. Transplantation. 1998;66(4):500–506. doi: 10.1097/00007890-199808270-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wedd JP, Harper AM, Biggins SW. MELD score, allocation, and distribution in the United States. Clinical Liver Disease. 2013;2(4):148–151. doi: 10.1002/cld.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saab S, Lalezari D, Pruthi P, Alper T, Tong MJ. The impact of obesity on patient survival in liver transplant recipients: a meta-analysis. Liver Int. 2015;35(1):164–170. doi: 10.1111/liv.12431. [DOI] [PubMed] [Google Scholar]
- 9.Wong RJ, Cheung R, Perumpail RB, Holt EW, Ahmed A. Diabetes mellitus, and not obesity, is associated with lower survival following liver transplantation. Dig Dis Sci. 2015;60(4):1036–1044. doi: 10.1007/s10620-014-3469-8. [DOI] [PubMed] [Google Scholar]
- 10.VanWagner LB, Lapin B, Skaro AI, Lloyd-Jones DM, Rinella ME. Impact of Renal Impairment on Cardiovascular Disease Mortality After Liver Transplantation for Nonalcoholic Steatohepatitis Cirrhosis. Liver Int. 2015 doi: 10.1111/liv.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56(5):1741–1750. doi: 10.1002/hep.25855. [DOI] [PubMed] [Google Scholar]
- 12.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation. 2009;120(13):1189–1194. doi: 10.1161/CIRCULATIONAHA.108.847178. [DOI] [PubMed] [Google Scholar]
- 15.Fouad TR, Abdel-Razek WM, Burak KW, Bain VG, Lee SS. Prediction of cardiac complications after liver transplantation. Transplantation. 2009;87(5):763–770. doi: 10.1097/TP.0b013e318198d734. [DOI] [PubMed] [Google Scholar]
- 16.Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011;17(1):23–31. doi: 10.1002/lt.22140. [DOI] [PubMed] [Google Scholar]
- 17.Bargehr J, Trejo-Gutierrez JF, Patel T, Rosser B, Aranda-Michel J, Yataco ML, et al. Preexisting atrial fibrillation and cardiac complications after liver transplantation. Liver Transpl. 2015;21(3):314–320. doi: 10.1002/lt.24060. [DOI] [PubMed] [Google Scholar]
- 18.Nicolau-Raducu R, Gitman M, Ganier D, Loss GE, Cohen AJ, Patel H, et al. Adverse cardiac events after orthotopic liver transplantation: a cross-sectional study in 389 consecutive patients. Liver Transpl. 2015;21(1):13–21. doi: 10.1002/lt.23997. [DOI] [PubMed] [Google Scholar]
- 19.Josefsson A, Fu M, Bjornsson E, Castedal M, Kalaitzakis E. Pre-transplant renal impairment predicts posttransplant cardiac events in patients with liver cirrhosis. Transplantation. 2014;98(1):107–114. doi: 10.1097/01.TP.0000442781.31885.a2. [DOI] [PubMed] [Google Scholar]
- 20.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Salvalaggio PR, Dzebisashvili N, MacLeod KE, Lentine KL, Gheorghian A, Schnitzler MA, et al. The interaction among donor characteristics, severity of liver disease, and the cost of liver transplantation. Liver Transplantation. 2011;17(3):233–242. doi: 10.1002/lt.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raval Z, Harinstein ME, Skaro AI, Erdogan A, DeWolf AM, Shah SJ, et al. Cardiovascular risk assessment of the liver transplant candidate. J Am Coll Cardiol. 2011;58(3):223–231. doi: 10.1016/j.jacc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Rao A, Polanco A, Qiu S, Kim J, Chin EH, Divino CM, et al. Safety of Outpatient Laparoscopic Cholecystectomy in the Elderly: Analysis of 15,248 Patients Using the NSQIP Database. J Am Coll Surg. 2013;217(6):1038–1043. doi: 10.1016/j.jamcollsurg.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Yeo CJ, Cameron JL, Sohn TA, Lillemoe KD, Pitt HA, Talamini MA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–257. doi: 10.1097/00000658-199709000-00004. discussion 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salerno MP, Piselli P, Rossi E, Favi E, Gargiulo A, Spagnoletti G, et al. Metabolic syndrome and cardiovascular disease in kidney transplantation. Transplant Proc. 2011;43(4):1067–1068. doi: 10.1016/j.transproceed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Claes K, Bammens B, Evenepoel P, Kuypers D, Coosemans W, Darius T, et al. Troponin I is a predictor of acute cardiac events in the immediate postoperative renal transplant period. Transplantation. 2010;89(3):341–346. doi: 10.1097/TP.0b013e3181bc405e. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 28.Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2013 doi: 10.1038/nrgastro.2013.210. [DOI] [PubMed] [Google Scholar]
- 29.Rumer KK, Saraswathula A, Melcher ML. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? Curr Opin Organ Transplant. 2016 doi: 10.1097/MOT.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 30.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364. doi: 10.1016/s0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 32.Gaynor JJ, Moon JI, Kato T, Nishida S, Selvaggi G, Levi DM, et al. A cause-specific hazard rate analysis of prognostic factors among 877 adults who received primary orthotopic liver transplantation. Transplantation. 2007;84(2):155–165. doi: 10.1097/01.tp.0000269090.90068.0f. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Villamayor AJ, Merritt W, Pustavoitau A, Latif A, Bhambhani R, et al. Continuous cerebral blood flow autoregulation monitoring in patients undergoing liver transplantation. Neurocrit Care. 2012;17(1):77–84. doi: 10.1007/s12028-012-9721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 36.Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9(7):372–381. doi: 10.1038/nrgastro.2012.79. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12(3):394.e1–402.e1. doi: 10.1016/j.cgh.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 38.VanWagner LB, Ning H, Lewis CE, Shay CM, Wilkins J, Carr JJ, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: The Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235(2):599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology. 2015 doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49(4):608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2014 doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 42.Lazarevic AM, Nakatani S, Neskovic AN, Marinkovic J, Yasumura Y, Stojicic D, et al. Early changes in left ventricular function in chronic asymptomatic alcoholics: relation to the duration of heavy drinking. J Am Coll Cardiol. 2000;35(6):1599–1606. doi: 10.1016/s0735-1097(00)00565-9. [DOI] [PubMed] [Google Scholar]
- 43.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121(5):1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- 44.O'Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring Diagnoses: ICD Code Accuracy. Health Serv Res. 2005;40(5 Pt 2):1620–1639. doi: 10.1111/j.1475-6773.2005.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 46.VanWagner LB, Skaro AI. Program-specific reports: implications and impact on program behavior. Curr Opin Organ Transplant. 2013;18(2):210–215. doi: 10.1097/MOT.0b013e32835f07f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abecassis MM, Burke R, Cosimi AB, Matas AJ, Merion RM, Millman D, et al. Transplant center regulations--a mixed blessing? An ASTS Council viewpoint. Am J Transplant. 2008;8(12):2496–2502. doi: 10.1111/j.1600-6143.2008.02434.x. [DOI] [PubMed] [Google Scholar]
- 48.Mayo NE, Feldman L, Scott S, Zavorsky G, Kim do J, Charlebois P, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 49.Dunne DF, Jack S, Jones RP, Jones L, Lythgoe DT, Malik HZ, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg. 2016 doi: 10.1002/bjs.10096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.