Abstract
Pathogenic fungi are a major causative group for opportunistic infections (OIs). AIDS patients and other immunocompromised individuals are at risk for OIs, which if not treated appropriately, contribute to the mortality associated with their conditions. Several studies have indicated that the majority of HIV-positive patients contract fungal infections throughout the course of their disease. Similar observations have been made regarding the increased frequency of bone marrow and organ transplants, the use of antineoplastic agents, the excessive use of antibiotics, and the prolonged use of corticosteroids among others. In addition, several pathogenic fungi have developed resistance to current drugs. Together these have conspired to spur a need for developing new treatment options for OIs. To aid this effort, this article reviews the biological targets of current and emerging drugs and agents that act through these targets for the treatment of opportunistic fungal infections.
Keywords: Antifungal agents, Antifungal drug target, Anti-opportunistic infection, Biological target, Fungal infections, Mechanism of action, Opportunistic infection targets, Target of antifungal agents
INTRODUCTION
The continuing need to develop drugs that treat opportunistic infections (OIs) is related to several factors including the capacity of OIs to cause serious and life threatening illnesses in immunocompromised patients, emerging opportunistic pathogens, limited availability of effective antifungal drugs and the emergence of fungal strains resistant against drugs on the market [1]. The major infectious pathogens are fungi, but bacteria also contribute to opportunistic infections. The most common opportunistic pathogenic fungi include the Candida species, Cryptococcus neoformans (sometimes referred to as Filobasidiella neoformans), the Aspergillus species, Histoplasma capsulatum, Coccidioides imitis and the Fusarium species [2–4]. As a result of the increasing prevalence of these life threatening systemic mycoses, [5] there is a continuing need for novel antifungal agents not only to treat the common pathogens but also for resistant fungi associated with the immunosuppressant conditions used during solid organ transplants [6]. Classified as eukaryotes, fungal cells present several similarities with mammalian cells including the fact that their cell nucleus contains DNA organized into chromosomes. Fungal cells also have distinct cytoplasmic organelles and biosynthetic pathways analogous to mammalian cells. While the so called “magic-bullet concept” worked fairly well for bacteria, due to the similarities to mammalian cells, its application to fungi has been less certain [1]. These similarities have created additional problems in the design of drugs with selective toxicity to fungal cells [7, 8]. As a consequence, no therapeutic drug was available for treatment of fungal infections until the discovery of amphotericin B in 1953 [9]. This was followed by the development of flucytosine in 1957 [10], azoles in the 1960s [11] and later the triazoles.
More recently, with the advent of the AIDS epidemic, the increasing number of patients living with suppressed immune systems and the lack of effective drugs for the treatment of systemic fungal infections, there has been resurgence in the interest in the development of new antifungal agents [12, 13]. Other conditions such as the increased frequency of bone marrow and organ transplants, prolonged use of corticosteroids, the use of antineoplastic agents, abusive use of antibiotics [14] and the emergence of fungal resistance to currently available agents have made the need for new and effective antifungal agents a high priority [15–18].
An ideal antifungal agent should meet several criteria including the following: a) To have a broad spectrum of activity against a variety of yeast and filamentous fungi, b) to be fungicidal rather than fungistatic, c) to be directed at a specific fungal target and spare interference with host targets including enzymes such as Cytochrome P450, which is responsible for most drug interactions, d) to have multiple delivery methods, particularly oral availability, to enable prolonged out-patient care, and e) to have minimal side effects or toxicities. It is clear that obtaining or designing a drug that satisfies the above criteria is a major challenge and requires the identification of biological targets that are unique to fungi. Thus, the primary objective of this article is to review the current biological targets in fungi, the drugs that target them and their mechanisms of action so as to aid antifungal drug development.
FUNGAL CELL MEMBRANE AS A DRUG DEVELOPMENT TARGET
Polyene Antifungal Agents
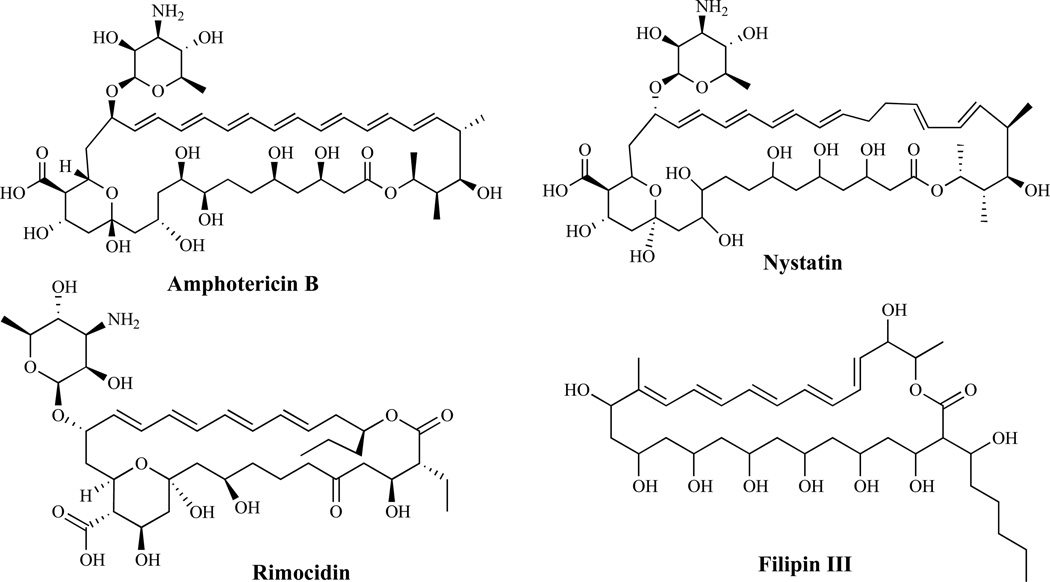
The polyene antibiotics (Fig. 1) produced by the Streptomyces species are fungicidal and have the broadest spectrum of activity of any clinically useful antifungal compounds [19, 20]. Agents in this class act by forming a complex with ergosterol in the plasma membrane [21], causing membrane disruption [22], increased permeability, leakage of cytoplasmic contents and ultimately cell death [23]. Recent evidence [24] suggests that they also cause oxidative damage, which may contribute to their fungicidal activity [25]. The clinically useful polyenes including amphotericin B, nystatin, natamycin (pimaricin), rimocidin, filipin and candicin, all have a higher affinity for ergosterol than its mammalian counterpart, cholesterol, and are thus, relatively less toxic to mammalian cells [26]. The acute and chronic side effects of amphotericin B may be reduced in newer formulations, such as liposomes [20, 27, 28], lipid complexes [29] and colloidal dispersions [30]. The major goal of developing newer formulations has been to obtain a compound with lower toxicity with at least similar efficacy compared to the parent compound. Ambisome is an amphotericin B liposomal formulation composed of high transition temperature phospholipids and cholesterol. The formulation is designed to incorporate amphotericin B securely into a liposomal bilayer such that upon binding to the cell wall, the liposome is disrupted, and the drug is released and binds to ergosterol after being transferred through the cell wall. By this mechanism of action, the integrity of the liposome in mammalian cells is maintained, and thus, resulting in minimal toxicity to the host [31].
Fig. (1).
Polyene Antifungal Agents.
Azole Antifungal Agents
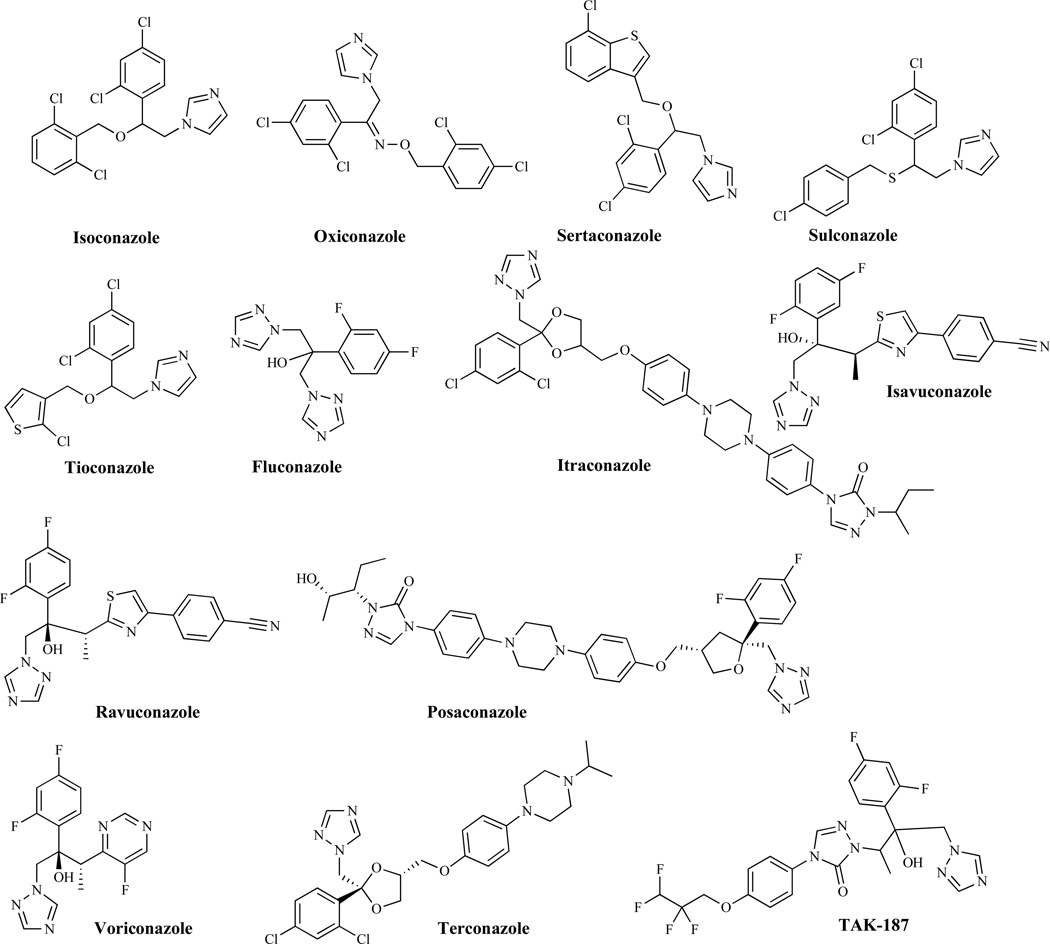
The first azole derivatives (Fig. 2) were discovered in the late 1960s. These are totally synthetic and currently are the most rapidly expanding group of antifungal compounds [32, 33]. They are classified as imidazoles or triazoles on the basis of whether they have two or three nitrogens in the five-membered azole ring respectively. Depending on the particular compound, azole antifungal agents have fungistatic and broad-spectrum activity against most yeasts and filamentous fungi. Inhibition of ergosterol biosynthesis (Fig. 3) [34] at the C-14 demethylation stage is the basis of their mechanism of action [35]. Azole antifungal agents form, through their azole ring, a stoichiometric complex with the heme iron of P-450 demethylase (DM), which can be measured spectrophotometrically by the red shift of the Soret band of heme from 417 to 447 nm [36]. The resulting ergosterol depletion and the accumulation of lanosterol and other 14-methylated sterols interfere with the “bulk” functions of ergosterol as a membrane component [33, 37, 38]. In addition, the resulting disruption of the structure of the plasma membrane makes it more vulnerable to further damage, including the alteration of the activity of several membrane bound enzymes, such as those associated with nutrient transport and chitin synthesis [39], growth, and proliferation [40]. Imidazoles including, miconazole, ketoconazole, clotrimazole, econazole, bifonazole, butoconazole, fenticonazole, isoconazole, oxiconazole, sertaconazole, sulconazole and tioconazole which are used primarily as topical agents, also interact and damage the cell membrane directly at higher concentrations and are fungicidal and toxic [41]. The other class of the azoles, the triazoles, are newer, less toxic and more effective and include fluconazole, itraconazole, isavuconazole, ravuconazole, posaconazole, voriconazole and terconazole [41]. Several related agents are reported in the literature and include non-azole inhibitors of P-450DM that apparently have not been pursued further [42]. Other notable agents include SCH-56592 (posaconazole), a triazole which was first presented at the 1995 Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) meeting by Schering-Plough [43]; TAK-187, an optically active azolone triazole [1]; ER-30346, a thiazole, which was shown in vitro to be more potent than itraconazole against C. albicans, C. parapsilosis, C. glabrata, Trichosporon beigelli, A. fumigatus, and C. neoformans [44]; and T-8581, a triazole amide [45], which is highly water-soluble and active against C. neoformans and C. albicans.
Fig. (2).
Azole Antifungal Agents.
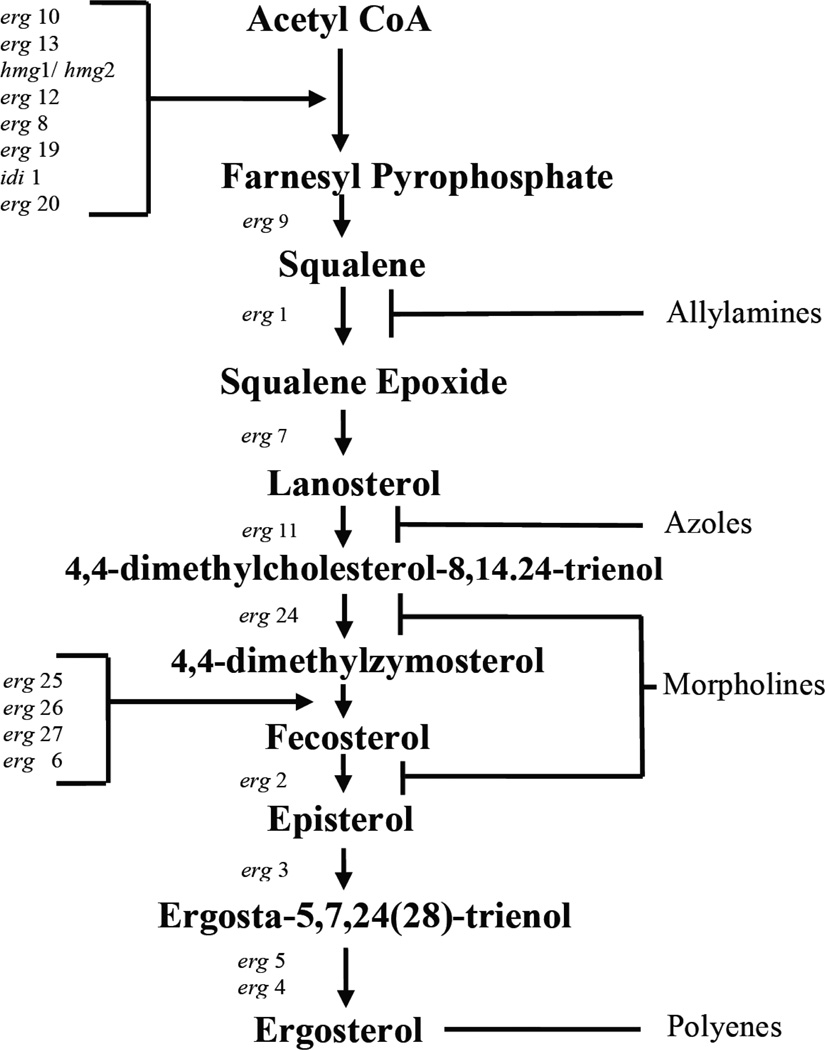
Fig. (3).
Linear model of the ergosterol biosynthetic pathway adapted from Saccharomyces cerevisiae. Significant intermediates are in boldface, genes involved in the step of biosynthesis appear on the left and inhibitors are shown on the right. CoA = coenzyme A [34].
Allylamine, Thiocarbamate and Morpholine Antifungal Agents
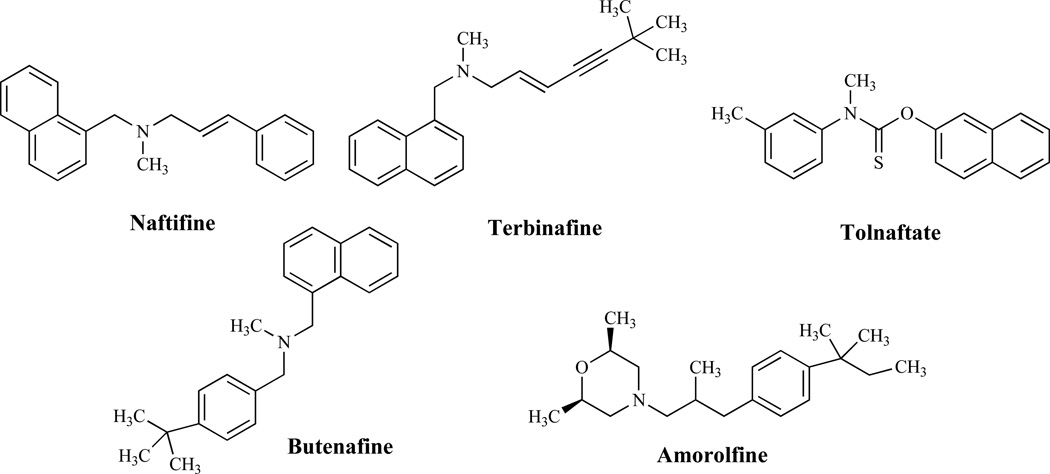
The mechanism of action of allylamines and thiocarbamates (Fig. 4) is described as a reversible, noncompetitive inhibition of squalene epoxidase [46], an enzyme, which together with (2,3)-oxidosqualene cyclase, is responsible for the cyclization of squalene to lanosterol. The resulting ergosterol depletion and squalene accumulation as indicated earlier affect membrane structure and functions, such as nutrient uptake [47, 48]. Allylamines such as naftifine and terbinafine along with tolnaftate, a thiocarbamate, have found utility as antifungal agents in the clinic [48]. The benzylamine, butenafine, has a mechanism of action similar to that of allylamines and, in addition, causes direct membrane effects in ergosterol-depleted cells [49].
Fig. (4).
Allylamines, Thiocarbamates and Morpholines.
The morpholines are totally synthetic and, with the exception of amorolfine, are agricultural fungicides [50]. Amorolfine, a morpholine antifungal agent, acts on the ergosterol pathway by inhibiting two enzymes, Δ14 reductase and Δ7–Δ8 isomerase [50, 51]. However, the antifungal activity of morpholines is probably due solely to sterol Δ14 reductase inhibition [52]; Δ14 reductase is an essential enzyme [53] while Δ7–Δ8 isomerase is not considered as such [54]. Interestingly, the morpholine fenpropimorph also inhibits cholesterol biosynthesis in mammalian cells, although it appears to affect the demethylation of lanosterol rather than sterol reductases or isomerases [55].
Inhibitors of Sphingolipid Biosynthesis
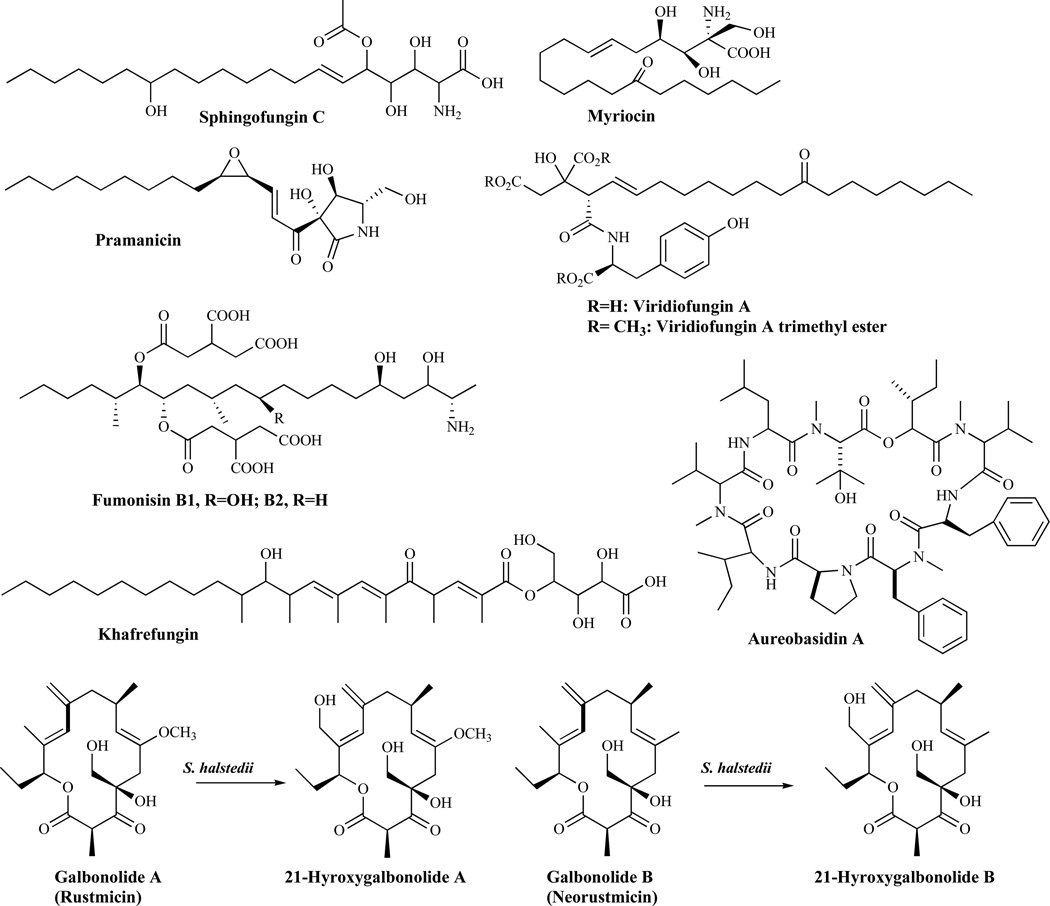
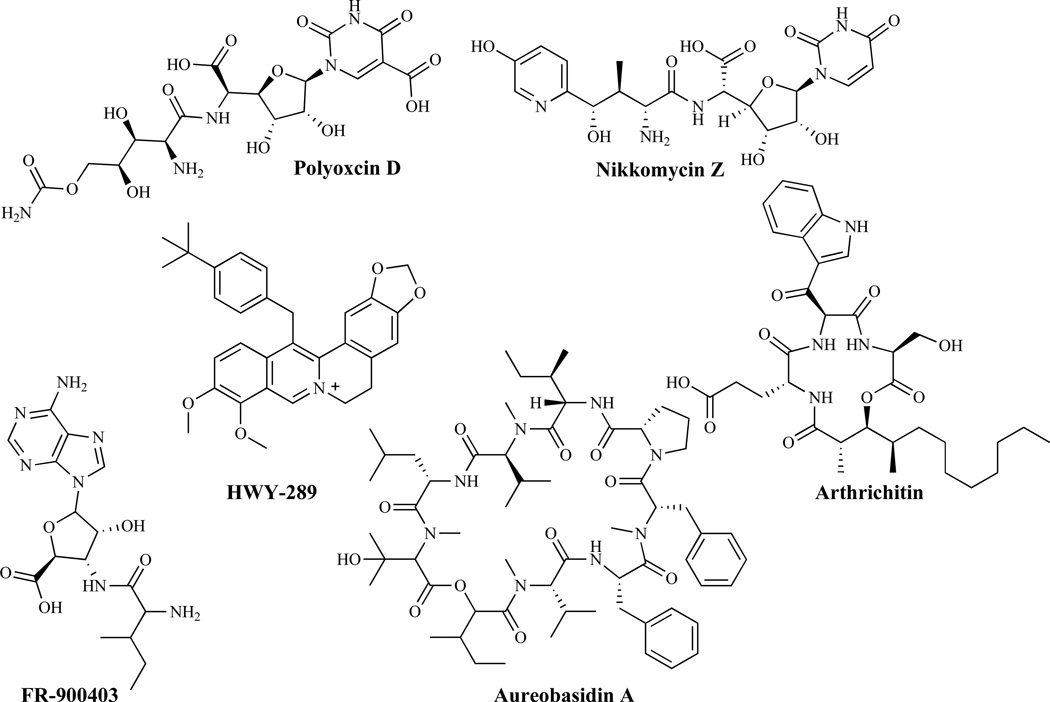
Sphingolipids are essential membrane components of both mammalian and fungal cells and they are localized primarily on the outer leaflet of the fungal cytoplasmic membrane [56]. The first committed step in the biosynthesis of sphingolipid is the condensation of serine and fatty acyl-Coenzyme A (acyl-CoA, usually palmitoyl-CoA), catalyzed by serine palmitoyltransferase to form the long-chain base, ketodihydrosphingosine. Serine palmitoyltransferase is the target of several known natural product inhibitors including: sphingofungins, lipoxamicin, myriocin, pramanicin and viridiofungins. The other enzyme in the sphingolipid biosynthetic pathway, the sphingamine N-acetyltransferase (ceramide synthase), is inhibited by such agents as fumonisins. All early steps of sphingolipid biosynthesis have mammalian counterparts and thus, are less attractive for the development of antifungal agents. The first fungal-specific enzyme in sphingolipid biosynthesis is inositol phosphoceramide (IPC) synthase [57]. It catalyzes the transfer of phosphatidylinositol from glycerophosphatidylinisitol to the C1 hydroxyl of ceramide to yield inositol phosphoceramide. IPC synthase has been suggested to be an attractive fungicidal target [58] and is the target for agents such as aureobasidin A, khafrefungin, rustmicin, 21-hydroxyrustrin, galbonolide, 21-hydroxygalbonolide and pleofungin A (Fig. 5).
Fig. (5).
Inhibitors of Sphingolipid Synthesis.
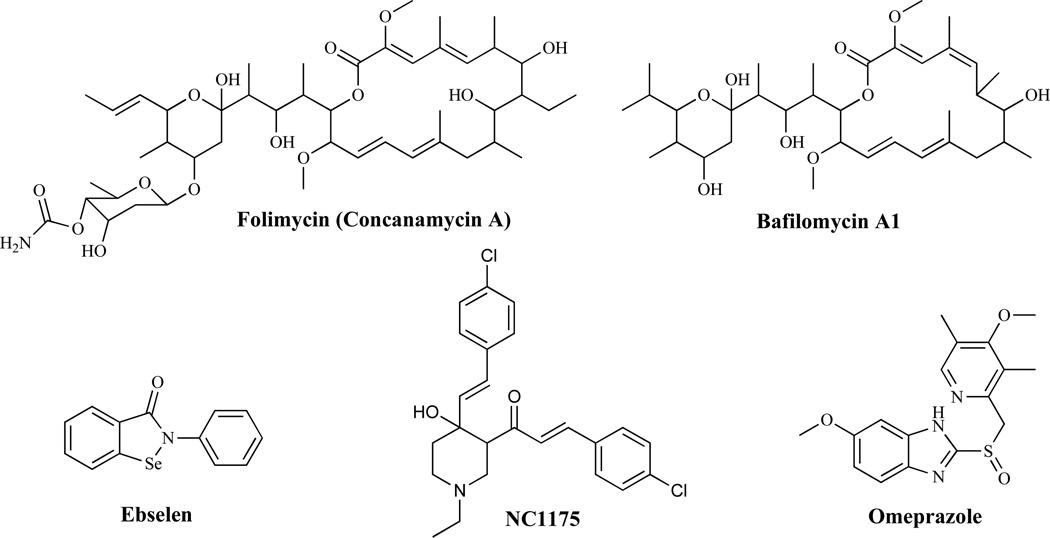
Inhibitors of Proton ATPases
Proton ATPases are involved in electrochemical proton gradient maintenance and intracellular pH regulation. Plasma membrane H1 ATPase is an abundant and essential enzyme [59] and given that there are significant differences between the fungal and mammalian enzymes, plasma membrane H1 ATPase offers a great opportunity for exploitation as a rational drug design target. Fungal proton ATPase inhibitors (Fig. 6) include: folimycin, an agent structurally related to bafilomycins [60], which acts by inhibiting the vesicular H1 ATPase (V-ATPase) and bafilomycins, which generally block acidification of intracellular organelles and thereby affect intracellular protein trafficking and translocation to the cell surface. The H+-ATPase encoded by the PMA1 gene in C. neoformans has emerged as a particularly interesting target with the discovery of the fungicidal effects of the ATPase antagonist, ebselen [61], and a conjugated styryl ketone, NC1175 [62]. Monk and Perlin [63] have previously reported that the anti-gastric ulcer drug omeprazole (a cysteine-modifying agent which inhibits the gastric K+ H+-ATPase) inhibited C. albicans growth, although high concentrations of the drug were needed to have such an effect. They further demonstrated that the inhibition of C. albicans growth was correlated with the inhibition of the H+-ATPase of the organism.
Fig. (6).
Proton ATPase Inhibitors.
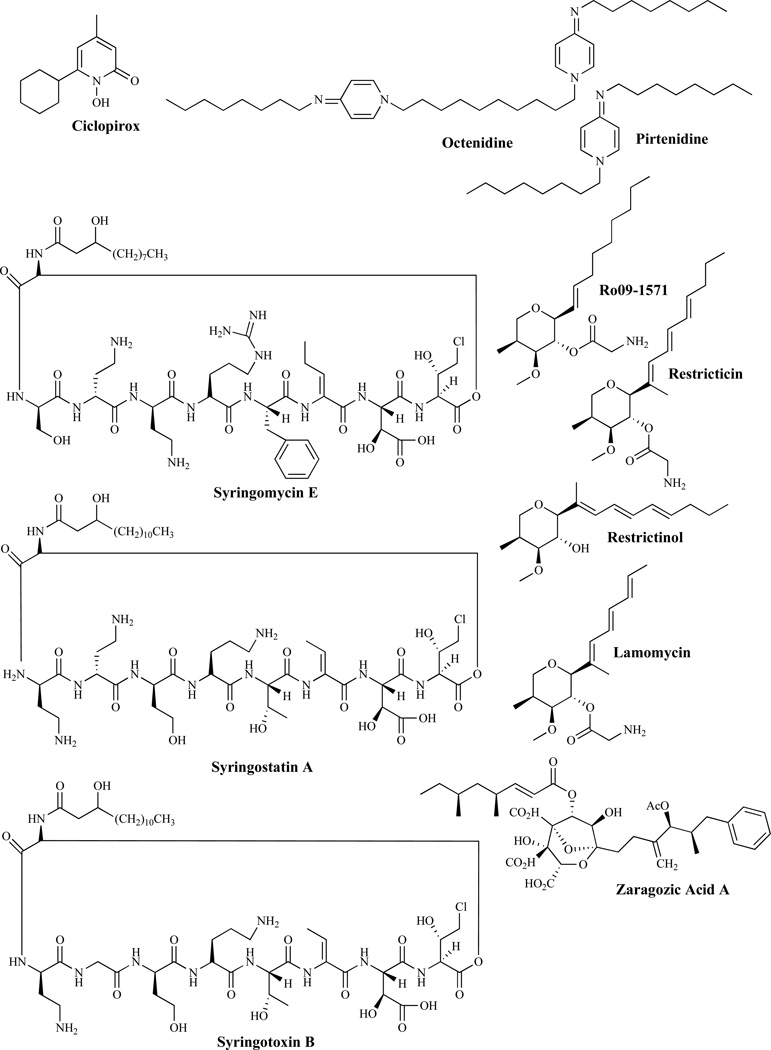
Other Antifungal Agents Interfering With the Cell Membrane
Ciclopirox (Fig. 7) is a broad-spectrum antifungal agent used in the treatment of fungal infections of the nails and hair [64] but which also has antibacterial and anti-inflammatory properties. The mechanism of action is thought to be through the chelation of polyvalent metal cations, such as Fe3+ and Al3+. These cations are cofactors of many enzymes, including cytochromes and their inhibition may lead to the disruption of the biosynthesis of ergosterol. Ciclopirox is also thought to act by modifying the fungal plasma membrane, resulting in the disorganization of internal structures [64]. Octenidine and pirtenidine are reported to have antifungal activity against C. albicans and act by inhibiting ergosterol biosynthesis through the inhibition of 14-α-demethylase, [65] (Fig. 3). Syringomycins are members of the Pseudomonas syringae pv. syringae group [66]. Syringomycin E (SE), the abundant form, alters several membrane functions including membrane potential, protein phosphorylation, H1-ATPase activity, and cation transport fluxes. Syringomycins (Fig. 7) also appear to bind to ergosterol in yeasts [67]. Sorenson et al. have published a study on fungicidal properties of several agents [68] which includes syringostatin A, SE, and syringotoxin B. Ro 09-1571 was designed as an analog of restricticin with in vitro activity close to that of ketoconazole against C. albicans and A. fumigatus and similar to fluconazole against C. neoformans [1]. Restricticin, restricticinol and lanomycin are reported to act by inhibiting C-14 α-demethylase [69]. SP-19502, isolated from the plant species I. Alata and A. djalonensis [70], has potent antifungal activity probably by inhibiting 2,3-oxidosqualene-lanosterol cyclase [71]. Zaragozic acid has been found to inhibit squalene synthase [72] while several pyrimidinium compounds were reported to have antifungal activity by acting as squalene cyclase inhibitors [73].
Fig. (7).
Other Antifungal Agents That Interfere With the Cell Membrane.
FUNGAL CELL WALL AS A DRUG DEVELOPMENT TARGET
Fungal cells, like several other microorganisms are enclosed in a cell wall that provides structural support and protection to the cell. Mammalian cells however lack a cell wall, making the fungal cell wall a desirable target for the development of antifungal agents [74, 75]. β-glucan, chitin and mannoproteins are components of the cell wall and disruption of their synthesis can lead to an ineffective cell wall unable to fully protect the cell [76]. In C. albicans, it has been suggested that β-glucan and chitin are associated with the strength and shape of the cell wall, while mannoproteins are responsible for the porosity, its antigenicity and adhesion [77]. Many of these targets and the drugs that act on them have previously been extensively reviewed [78].
Glucan Biosynthesis as a Target
Glucans are polysaccharides of D-glucose monomers and β-(1,3)-glucan synthase is the enzyme which catalyzes their synthesis. At least two functional components have been identified in the enzyme: a catalytic component and a regulatory component. Inhibition of glucan synthesis is thought to be the mode of action of glucan biosynthesis inhibitors (Figs. 8a, b). Specifically, inhibition results in a loss of the enzymatic activity of 1,3-β-glucan synthase and this in turn compromises the structure and osmolarity of the cell wall [79, 80].
Fig. (8).
Fig. (a). Inhibitors of Glucan Biosynthesis.
Fig. (b). Inhibitors of Glucan Biosynthesis.
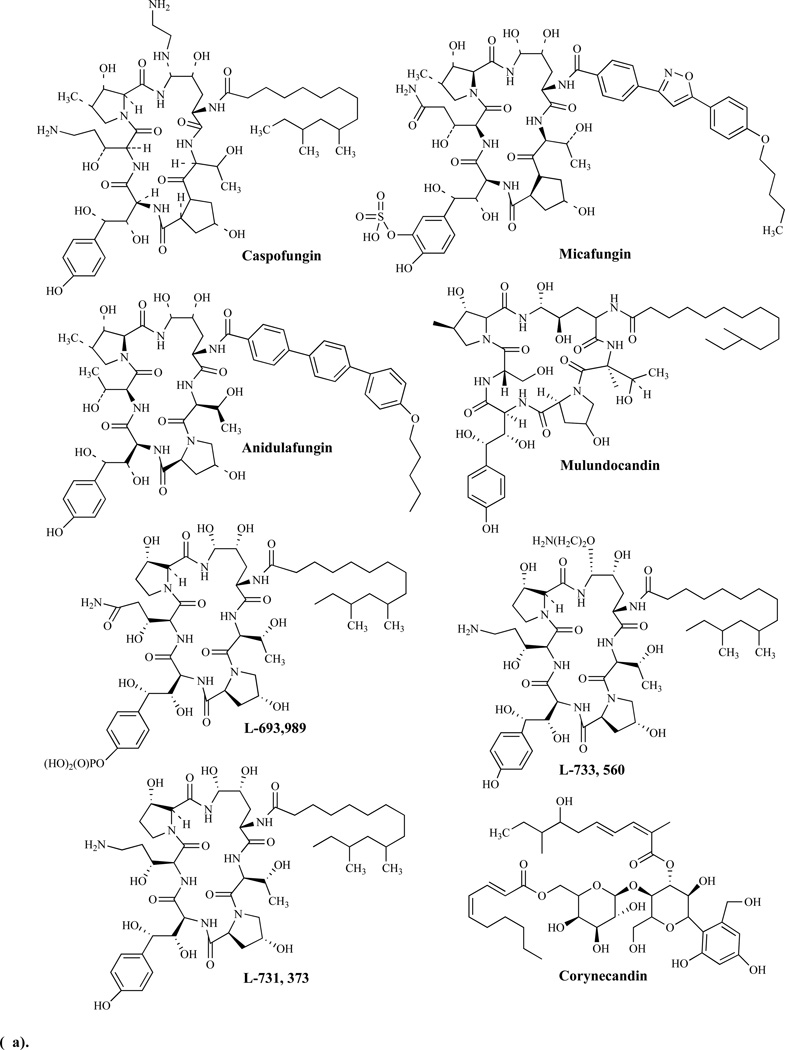
Caspofungin is the first of the approved first in-class echinocandin antifungal agents in clinical use. Its introduction was quickly followed by micafungin and anidulafungin. These agents have been shown to target the fungal cell wall by inhibiting the synthesis of β-1,3-D-glucan, the key and critical cell wall component of many pathogenic fungi including Candida spp. [81, 82]. Since then several additional echinocandins have been introduced into clinical use (Figs. 8a, b).
Echinocandins as a group are fungicidal against Candida spp. and fungistatic against molds, such as Aspergillus fumigatus. While they are highly effective against most Candida spp., including azole-resistant strains and biofilms, the echinocandins as a group have a rather limited antifungal spectrum and are thus, limited in their clinical utility.
Echinocandins are fatty acid derivatives of cyclic hexapeptides, while the related papulacandins are fatty acid derivatives of the disaccharide β-(1,4)-galactosylglucose [83]. As a family, the echinocandins include the echinocandins, pneumocandins, aculeacins, mulundocandin and WF11899. Pneumocandin analogs including L-693,989 (a phosphate ester of pneumocandin A); L-733,560; L-743,872; L-731,373 and L-773,560, derivatives of pneumocandin B0, have been shown to be more potent when compared to the lead compound [84].
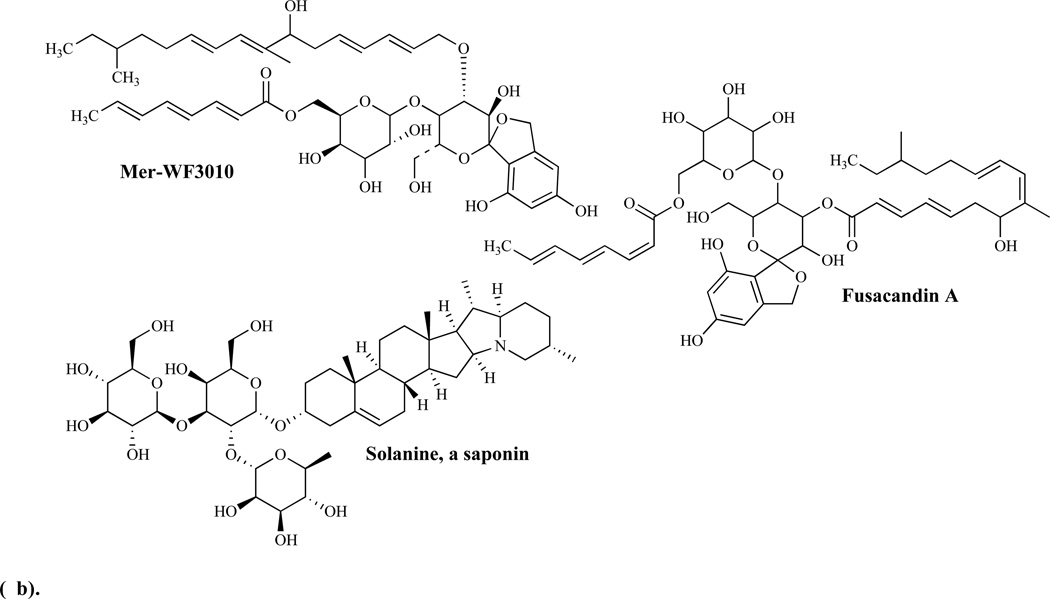
Mulundocandin and deoxymulundocandin have been shown to be active against A. niger and C. albicans [85]. The WF11899 group containing WF11899 A, B, and C have demonstrated anti-Candida activities in vivo in a murine model of systemic infection and were more effective than cliofungin or fluconazole [86]. Papulacandin analogs include: a) corynecandin [87] isolated from cultures of Coryneum modonium, a glycolipid analogous to the structural variant of papulacandin named chaetiacandin, b) Mer-WF3010, isolated from the culture broth of Phialophora cyclaminis [88], and c) fusacandin, another structural variant of chaetiacandin isolated from Fusarium sambucinum [89]. Fusacandin is a trisaccharide and unlike the other analogs which are disaccharides, has demonstrated poor activity in animal models, prompting attempts to find analogs with improved activity in vivo using structure–activity relationship (SAR) studies [90]. Saponins, another group of natural product glycosides, have been reported to inhibit both glucan and chitin synthesis [91].
Chitin Biosynthesis as a Target
Chitin is a polymer of β-(1,4)-linked N-acetylglucosamine (GlcNAc) residues [92] and serves as an essential structural component of the fungal cell wall. Its synthesis is catalyzed by chitin synthase and three such chitin synthases (Chs 1, 2 and 3) have been identified in C. albicans and S. cerevisiae [93]. Chitin synthase 1 (Chs1) plays only a nonessential repair role in C. albicans. Chitin synthase 2 (Chs2) is engaged in septum formation while Ch3 participates in cell wall maturation and bud ring formation [94]. Inhibitors of Chs (Fig. 9) include polyoxins and nikkomycins produced by streptomycetes. These have been recently reviewed [95].
Fig. (9).
Inhibitors of Chitin Biosynthesis.
The inhibitors act as analogs of the UDP-GlcNAc substrate which are transported by peptide permeases into the cell, and bind to the catalytic site of the chitin synthase [96]. HWY-289 is another potent inhibitor of the chitin synthase isozymes CaChs1 and CaChs2. Arthrichitin has a broad-spectrum of activity against Candida spp., Trychophyton spp. and a number of phytopathogens through the inhibition of fungal chitin and glucan synthases but demonstrates a greater potency against chitin synthase [97]. The low in vitro potency of arthrichitin however has prevented its clinical development. Structural modifications could potentially lead to an improvement of activity [98]. FR-900403 differs structurally from nikkomycins and polyoxins but was found to be active against C. albicans and inactive against filamentous fungi [99]. Aureobasidins, produced by Aureobasidium pullulans [100], differ structurally from the echinocandins and their mode of action is believed to be a disruption of the actin assembly and delocalization of chitin in cell walls leading to lysis. A different study however, has shown that aureobasidin A targets sphingolipid biosynthesis [58].
An associated enzyme in chitin biosynthesis is chitinase. Chitinase has been suggested to play an essential role in the remodeling of the fungal cell wall and hence can serve as an important target for drug design and development. Consistent with this thought, a co-crystal structure of AfChiA1 with acetazolamide has been used to explore the identification of inhibitors of chitinase as new antifungal agents [101]. There has also been a report of a screening-based discovery of chitinase inhibitors as antifungal agents against Aspergillus fumigatus [102].
Mannoprotein Biosynthesis as a Target
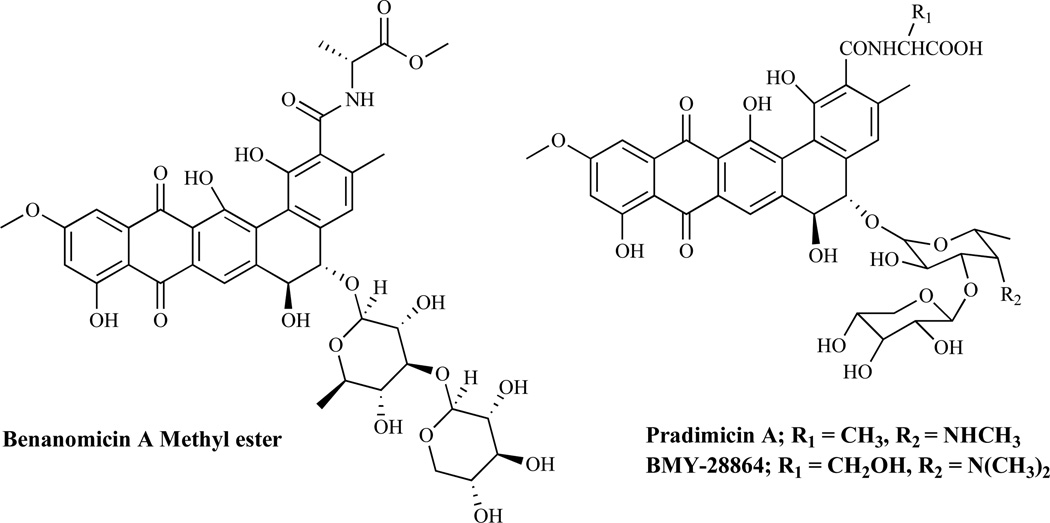
Mannoproteins are interstitial components of fungal cell walls. Structurally, these are complex chains of mannose units linked to proteins through N-acetylglucosamine and asparagine residues. Mannoproteins may be involved in cell-cell recognition and reproductive processes. Available evidence suggests that prevention of N- or O-glycosylation of sensitive proteins is lethal to fungi [1]. The mechanism of action of mannoprotein biosynthesis inhibitors (Fig. 10) apparently involves complexation of their carboxyl group with the saccharide moiety of the cell surface mannoproteins. Agents such as benanomicins, pradimicins, and benzonaphthacene quinones are among the known inhibitors of mannoprotein biosynthesis.
Fig. (10).
Mannoprotein Biosynthesis Inhibitors.
FUNGAL NUCLEIC ACID AND PROTEIN BIOSYNTHESIS AS A DRUG DEVELOPMENT TARGET
As a consequence of the similarities between eukaryotes and mammalian cells, DNA/RNA and protein synthesis have been difficult targets for antifungal drug development. More recently however, as important differences between the cell types become known, attempts have been made to exploit the differences for the development of new antifungal agents.
Interference with Normal Metabolic Processes
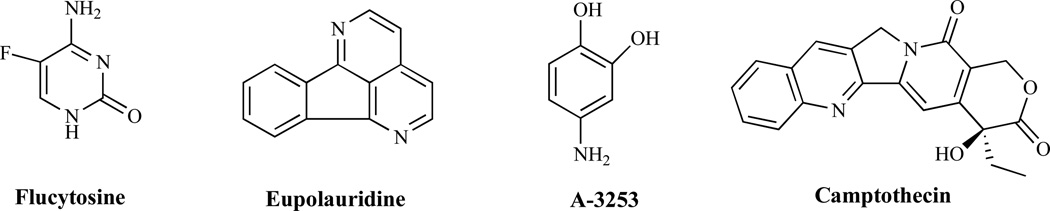
Targeting normal metabolic processes can be a viable strategy for drug development against fungal pathogens. This will require designing compounds that structurally resemble key metabolites such that they are mistakenly utilized by fungi but not mammalian cells. The drug flucytosine or s5-fluorocytosine (5-FC) (Fig. 11) provides an obvious example. It was originally developed in the 1950's as a potential antineoplastic agent but was found to be ineffective against tumors [15]. Subsequent testing led to its identification as an antimetabolite antifungal agent. Transported inside susceptible fungal cells by a cytosine permease, cytosine deaminase converts 5-FC into 5-fluorouracil (5-FU). Subsequent phosphorylation and incorporation into RNA, leads to miscoding and disruption of protein synthesis. Additionally, phosphorylated 5-FU is converted to its deoxynucleoside and is believed to block DNA synthesis by inhibiting thymidilate synthase [103] leading to the disruption of DNA replication.
Fig. (11).
Antimetabolites and Topoisomerase Inhibitors.
Inhibitors of Topoisomerase
Topoisomerases (TOP) I and II are involved in the replication, transcription, repair, and chromosomal segregation [104] in cells. Topoisomerase II is an essential enzyme [105] and the mammalian topoisomerase II is the target of the known anticancer drugs anthracyclines and epipodophylotoxins. Targeting DNA gyrase, the equivalent enzyme in bacteria, has led to the identification and clinical utility of quinolones such as ciprofloxacin. While not considered an essential enzyme, fungal topoisomerase I has been successfully targeted in the development of inhibitors such as camptothecin. Fungal TOP I inhibitors apparently form complexes with the enzyme and ultimately derail the oncoming replication fork [106]. The alkaloid eupolauridine (Fig. 11) inhibits the growth of C. albicans and it has been suggested that these actions may be through the stabilization of the cleavage complex formed by the C. albicans topoisomerase [107a]. Fortunately, it has been demonstrated that the response of the C. albicans TOP I to eupolauridine is greater than that of the human enzyme. Specific targeting of the fungal TOP I has also been demonstrated using the aminocatechol A-3253 which is less effective against human Top I [107b]. In contrast, camptothecin demonstrates greater activity against the human rather than fungal Top I. The combined observations suggest differences exist that could be exploited for drug design purposes. It is not entirely clear whether fungal topoisomerase II, the other topoisomerase, could serve as a selective target relative to its mammalian counterpart.
Inhibitors of Elongation Factors
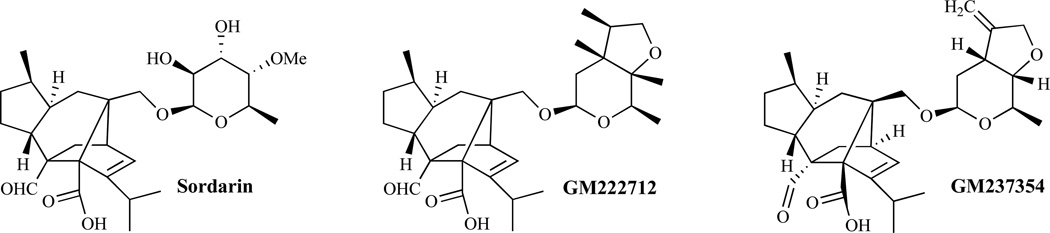
Elongation factor 1 (EF-1) and elongation factor 2 (EF-2) are required for the polypeptide chain elongation reactions in the synthesis of proteins in both fungal and mammalian cells [108]. Elongation factor 3 (EF-3) required by fungi but not by mammalian cells [109, 110] is present in most fungi including C. albicans and P. carinii and is essential for cell viability [111]. EF-3 has ATPase activity and is specifically required by the yeast 40S ribosomal subunit. EF-3 may also be involved in the translocation of the growing peptide although its exact function in the elongation cycle remains unclear. Sordarins are believed to act by inhibiting the protein synthesis elongation cycle in yeasts. The inhibition occurs without affecting the protein synthesis machinery in mammals [112]. The mechanism of action of sordarins appears to involve the inhibition of EF-2 [113]. The structure-affinity relationship for binding to EF-2 from different species is influenced by the nature of the sugar residue of the glycoside. BE31405, whose sugar is a rigid tricyclic ring system, has a broader spectrum of activity compared to sordarin. GM222712 and GM237354, analogs (Fig. 12) of sordarins, reportedly display in vitro activities against several pathogenic fungi [114].
Fig. (12).
Elongation Factor Inhibitors.
Inhibitors of Protein Farnesyltransferase (PFT)
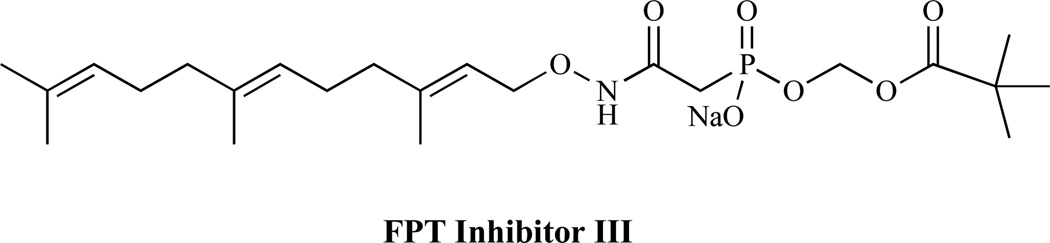
Many small G proteins require post-translational modification to allow functional association to the cell membrane. This process often involves the enzymatic addition of hydrophobic prenyl groups to a conserved cysteine residue near the C-terminus of the protein. The enzymes that catalyze these reactions include protein farnesyltransferase and protein geranylgeranyltransferases. Cryptococcus neoformans requires post-translational modification with Ras and Rho proteins in order to undergo normal growth and differentiation. Inhibition of farnesyltransferase has been shown to result in dose-dependent cytostasis of C. neoformans, as well as prevention of hyphal differentiation [115]. FPT inhibitor III (Fig. 13) is reported to produce antifungal activity by inhibiting the protein farnesyltransferase.
Fig. (13).
A Protein-farnesyltransferase Inhibitor
Inhibitors of N-myristoyltransferase (NMT)
Another post-translational modification involves the synthesis of N-myristoylated proteins in a process described as myristoylation. This involves the co-translational transfer of myristate from CoA to the amino-terminal glycine of proteins [116]. The reaction is essential in C. neoformans [117] and other fungi [118] and is catalyzed by myristoyl CoA:protein-N-myristoyltransferase (NMT). An analog of myristic acid with an oxygen atom replacing a methylene at position 4 had shown in vitro fungicidal activity. In view of the fact that there has been a demonstration of differences in substrate specificities of fungal and mammalian NMTs, fungal NMTs may serve as desirable targets for drug design against pathogenic microorganisms that require N-myristoylation.
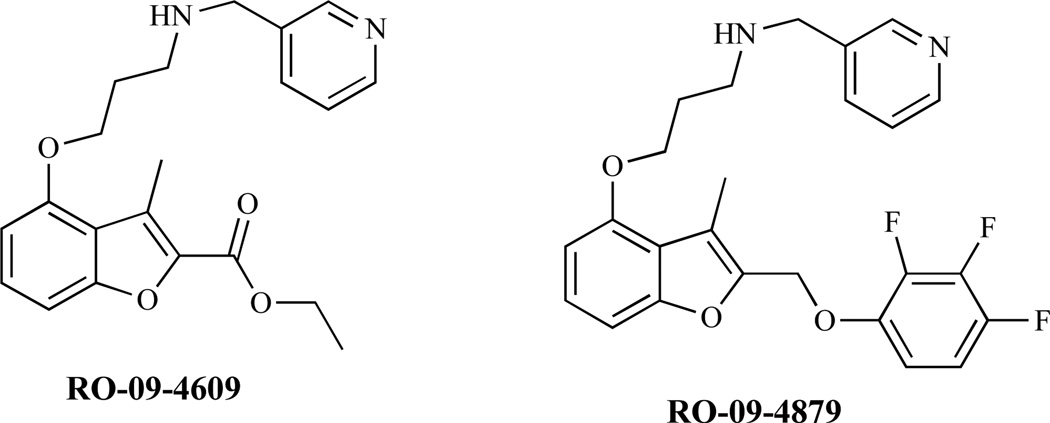
Enactins and neoenactins are antibiotics with antifungal activity. They are reported to act through inhibition of NMT [1, 119 a–d]. Analogs of 2-bromotetradecanoic acid [120], putative inhibitors of NMT have demonstrated in vitro activity against yeasts including S. cerevisiae, C. albicans and C. neoformans and filamentous fungi such as A. niger. The benzofuran derivatives RO-09-4609 and RO-09-4879 have been reported to be fungal NMT inhibitors. Several of these inhibitors (Fig. 14) have been reported to show high selectivity over human NMT and have exhibited antifungal activity in vivo.
Fig. (14).
N-myristoyltransferase (NMT) Inhibitors.
Inhibitors of Nucleic Acid Biosynthesis
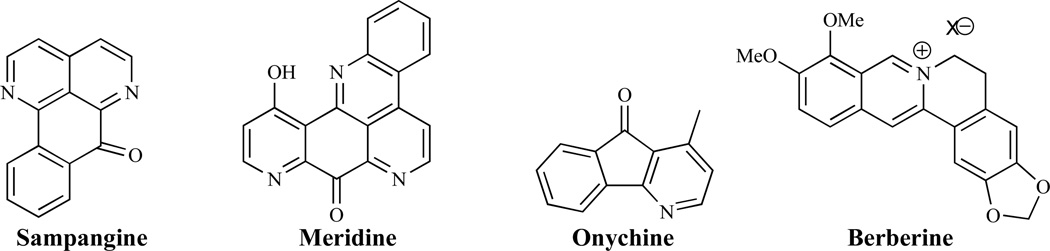
Several natural products including sampangine, meridine, onychine and berberine (Fig. 15) have been reported to have antifungal activity by interfering with fungal cell nucleic acid biosynthesis [121, 122].
Fig. (15).
Inhibitors of Nucleic Acid Biosynthesis.
Inhibitors of Amino Acids Biosynthesis
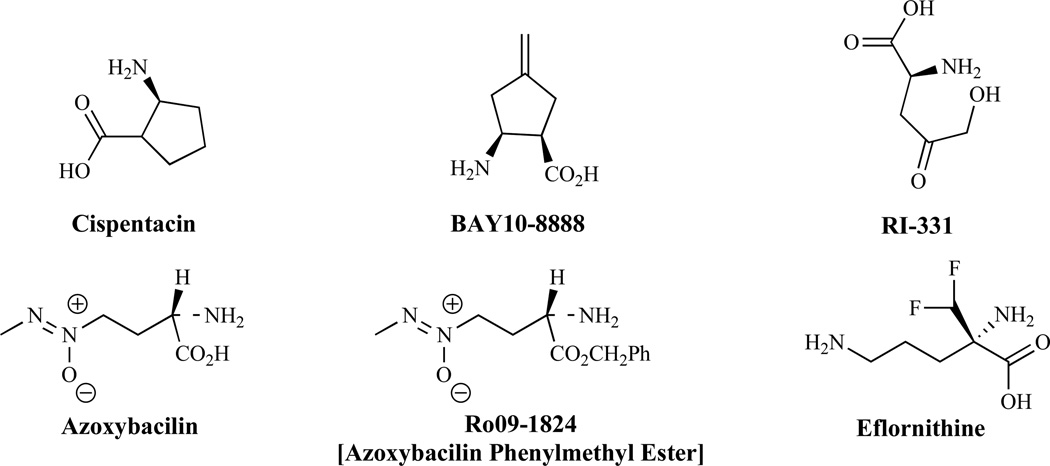
Studies have suggested that cispentacin [123], an antifungal agent with in vivo activity against multiple targets, interferes with amino acid synthesis [124]. Cispentacin (Fig. 16) and FR109615 [125] are unnatural cyclic β-amino acids. These compounds and the analog BAY108888, appear to have a dual mode of action. The suggested mechanism of action involves interference with amino acid transport and cellular regulation of amino acid metabolism. Other amino acid analogs with antifungal activity are: RI-331, which inhibits homoserine dehydrogenase [126], a desirable target for drug design as a mammalian counterpart has not been reported, and azoxybacilin, which inhibits the biosynthesis of sulfur-containing amino acids. Azoxybacilin interferes with the induction of gene expression by low levels of methionine in the sulfate assimilation (SA) pathway, but has weak activity in vivo, perhaps because of a poor pharmacokinetic profile. Compounds not antagonized by methionine, such as Ro091824, have been obtained and reportedly are antagonized to a lesser extent [127].
Fig. (16).
Inhibitors of Amino Acid and Polyamine Biosyntheses.
Inhibitors of Polyamine Biosynthesis
Ornithine decarboxylase, an enzyme involved in the biosynthesis of polyamine and a target of anticancer chemotherapeutic agents, is a potential antifungal target [128]. Eflornithine, a difluoromethyl analog of ornithine (Fig. 16), displays some antifungal activity [129]. P. carinii ornithine decarboxylase is far less susceptible to eflornithine [130] than the mammalian enzyme, suggesting differences in the active sites, although it remains unclear whether targeting the enzyme for rational drug design purposes is a viable option.
Inhibitors of Folic Acid Biosynthesis
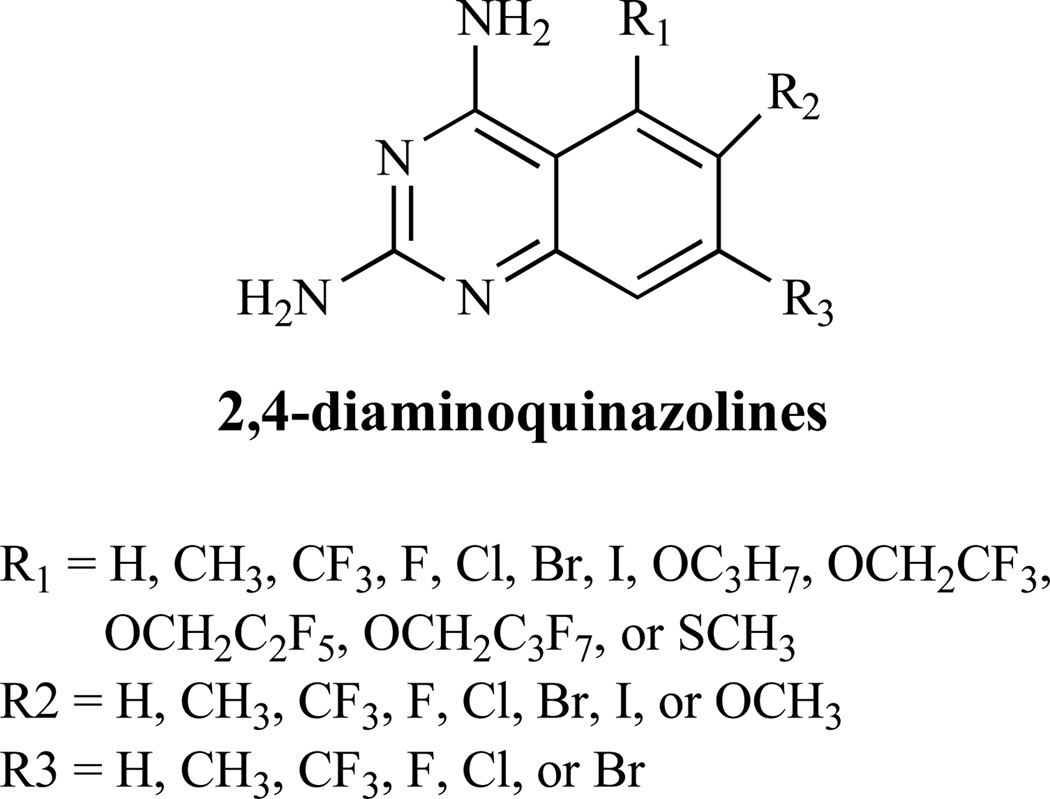
The diaminoquinazoline structures (Fig. 17), are reported to inhibit the dihydrofolate reductase enzyme, which catalyzes the reduction of dihydrofolic acid to tetrahydrofolic acid, and thus interferes with DNA biosynthesis [131].
Fig. (17).
Inhibitors of Folic Acid Biosynthesis.
OTHER CELLULAR FUNCTIONS AS DRUG DEVELOPMENT TARGETS
Inhibitors of Microtubule Aggregation
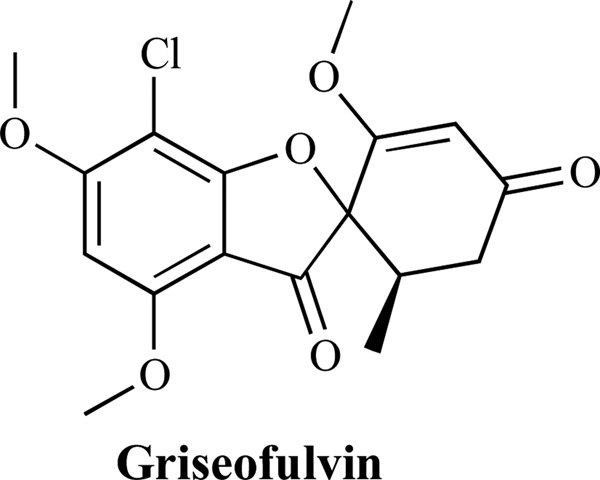
Microtubules are polymers of α- and β-tubulin dimers [132]. Microtubule aggregation-disaggregation plays a key role in cell morphology and growth. Griseofulvin (Fig. 18) inhibits microtubule aggregation by interacting with β-tubulin, a protein highly conserved in eukaryotes. Nevertheless, there appears to be differences between mammalian and fungal tubulins; for example, colchicine binds preferentially to mammalian tubulin [133].
Fig. (18).
An Inhibitor of Microtubule Aggregation.
Inhibitors of Signal Transduction Pathways
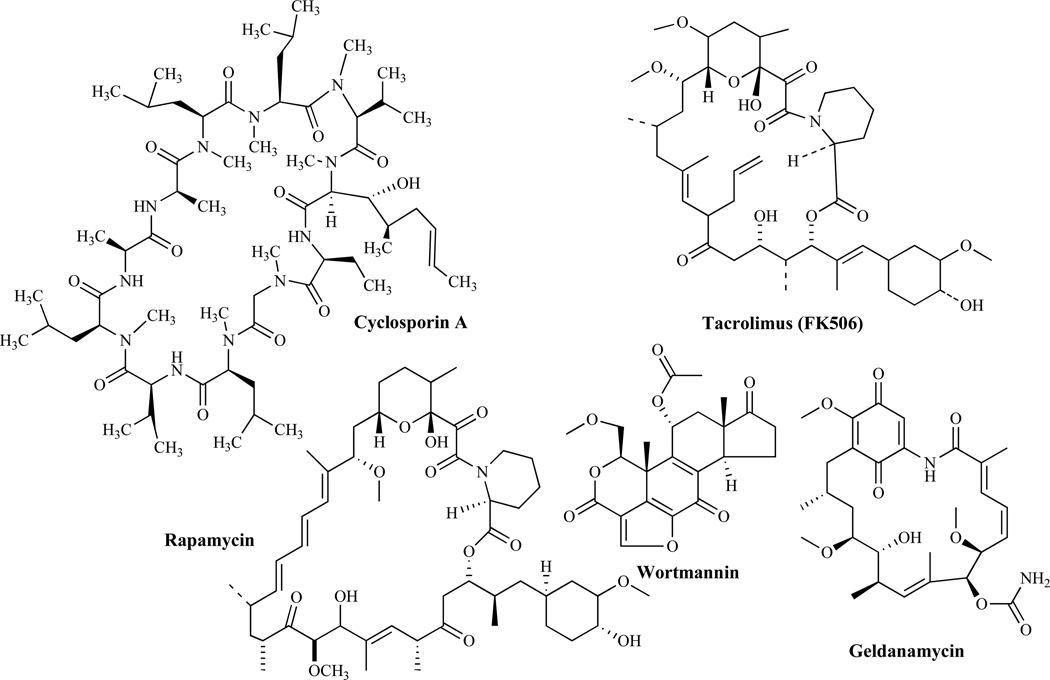
The signal transduction cascades in fungi have become very attractive targets since their components are now emerging as potential targets for the development of new antifungal agents. A review by Cardenas et al. [134] has provided insight on the mechanism of action of several natural products (Fig. 19) including cyclosporin A (CsA), tacrolimus (FK506), rapamycin, wortmannin and geldanamycin.
Fig. (19).
Inhibitors of Signal Transduction Pathways.
Inhibitors of Calcineurin-dependent Signaling
Calcineurin is critical in maintaining the perfect homeostasis of the cell under stress conditions. In response to increases in intracellular calcium, calmodulin activates calcineurin which is required to control cell survival [135]. CsA and FK-506 are immunosuppressive agents that have been shown to inhibit fungal calcineurin [136]. Mechanistically, they are believed to diffuse across the plasma membrane forming complexes with cyclophilin A (CyPA) and FKBP12, respectively. Cyclophilin A (CyPA) and FKBP12 are cytosolic immunophilins that catalyze cis-trans prolyl isomerization, required for protein folding. When FK-506 binds to FKBP12, inhibition of prolyl-isomerase is observed. Inhibition of prolyl-isomerase is also observed when CsA binds to CyPA and forms a drug-immunophilin complex [134]. As a result of the essential role of calcineurin in fungal virulence, its inhibitors are expected to have several therapeutic uses in a variety of clinical settings. To develop CsA and FK-506 as clinically useful antifungal agents however, will require the design of analogs selective for the antimicrobial activities but that do not produce immunosuppression. Indeed, there have been indications that antifungal agents with reduced immunosuppressive properties have been identified [134b]. Because calcineurin inhibitors have also been observed to demonstrate significant synergism with azoles or echinocandins, they could provide another therapeutic window for the treatment of fungal infections and especially for the treatment of resistant fungal pathogens [136].
Inhibitors of Target of Rapamycin (TORs) Dependent Signaling
TORs regulate cell growth and are targets for the drug rapamycin [136, 137]. Rapamycin (sirolimus) has been shown to bind to the FKBP receptor to yield a rapamycin-FKBP complex that binds to the TOR protein, and blocks signal transduction. In a number of human and plant pathogens, rapamycin blocks filamentation, a conserved mechanism of action among eukaryotes [136]. With several studies suggesting a globally conserved role for the TOR protein in regulating growth and proliferation, targeting its essential functions is expected to result in an effective antifungal activity [137].
Inhibitors of Phosphoinositide 3-Kinases (PI-3-kinases)-dependent Signaling
Wortmannin (Fig. 19) is a hydrophobic steroid-related product of the fungus Talaromyces wortmanni that inhibits signal-transduction pathways [138]. In the yeast S. cerevisiae, although the sole PI-3 kinase (VPS34) is only inhibited at very high concentrations of Wortmannin, PI-4 kinase STT4 is potently inhibited by wortmannin at much lower concentrations.
Inhibitors of HSP90 and HSP90-dependent Signaling
Geldanamycin is a benzoquinone ansamycin, natural-fermentation product that was originally thought to be a direct tyrosine-protein-kinase inhibitor. However, subsequent studies revealed that geldanamycin and two other structurally related analogues (herbimycin and macbecin) bind to and inhibit the 90 kDa heat-shock protein HSP90 instead [139]. HSP90, HSP70 and many of the associated chaperones are highly conserved between yeast and humans. Steroid-receptor and oncogenic kinase functions in yeasts are HSP90 dependent. However, geldanamycin is not toxic to wild-type yeast strains. It has been recently discovered that yeast mutants lacking HSP90-associated chaperones become sensitive to geldanamycin, suggesting that the mechanism of geldanamycin action is conserved between yeast and human, and involves the inhibition of HSP90-dependent signaling cascades that are required for cell function. These findings will permit further genetic dissection of HSP90-dependent signaling cascades and of the mechanisms of action of geldanamycin (Fig. 19).
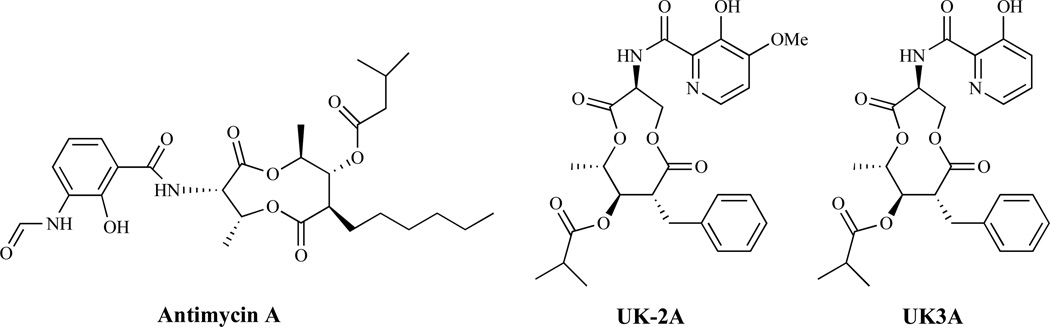
Inhibitors of Electron Transport
A series of related antibiotics, including antimycin A, UK-2A and UK3A (Fig. 20), have been shown to inhibit incorporation of nucleic acid precursors, amino acids, glucose and mannose into acid-insoluble material in a dose-dependent manner, making it difficult to identify the specific mode of antifungal action [140]. UK-2A and UK-3A however, are known to inhibit mitochondrial electron transport and interfere with in vitro respiration in both yeast and rat liver mitochondria [140].
Fig. (20).
Inhibitors of Electron Transport.
MISCELLANEOUS ANTIFUNGAL AGENTS
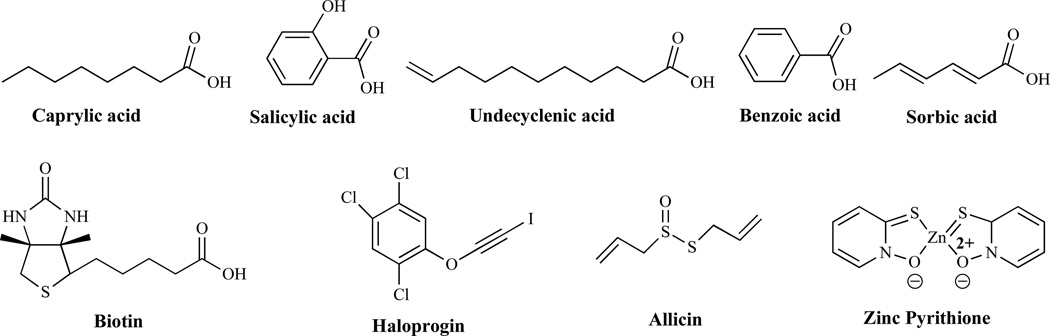
Organic acids such as caprylic acid, salicylic acid, undecylenic acid, propionic acid, and benzoic acid (Fig. 21) exhibit antifungal activity by interacting with non-specific components in the cell membrane. Reports over the years have indicated that short and medium-chain organic acids have antimicrobial properties [141]. In addition, common aromatic acids such as benzoic acid, have found utility as preservatives in foods on the basis of their antifungal properties [142]. Sorbic acid is an antimycotic agent which demonstrates broad spectrum activity against yeast and fungal molds [143]. Biotin, essential for the activity of many important metabolic reactions, plays a key role in maintaining healthy skin, hair, sweat glands, nerves and bone marrow. With primary activity in the mitochondria, biotin is a cofactor in at least four key carboxylase enzymes. Its activity is required for normal neuronal and hematopoietic function. In a study seeking to validate biotin biosynthesis as a potential target for the development of new antibiotics, the authors have demonstrated that de novo biotin synthesis is essential for M. tuberculosis to establish and maintain a chronic infection [144]. In another study, analogs of biotin have been synthesized and have demonstrated antimicrobial activity through the inhibition of biotin protein ligase (BPL) [145]. Taken together, there appears to be a great potential for targeting biotin biosynthesis, not only in antibacterial drug development but perhaps fungal BPL could serve as a potential drug development target for new antifungal agents.
Fig. (21).
Miscellaneous Antifungal Agents.
Haloprogin, which is used to treat athlete’s foot and other fungal infections, was marketed over the counter primarily to treat tinea infections of the skin. Its mechanism of action is unknown [146]. Allicin, the main parent antifungal compound in garlic, appears to have a mode of action related to its ability to cross the cell membrane and combine with sulfur-containing groups in amino acids and proteins and interfering with cell metabolism. Thus, it appears that human cells are poisoned by allicin derivatives due to their interaction with glutathione. In addition to their biochemical mechanism, these derivatives appear to stimulate cellular immunity, an important ability lacking in conventional antifungal chemotherapy [147]. Tea tree oil, citronella oil, lemon grass, orange oil, palmarosa oil, patchouli, lemon myrtle, neem seed oil and coconut oil all exhibit antifungal activity, although their mechanisms of action are currently unknown. However, it is hypothesized that they might act by altering membrane properties and compromising membrane-associated functions [148]. Olive leaf acts by interfering with the pathogen's amino acid properties and prevents the pathogen from reproducing and creating more microbes in the body and directly stimulating their phagocytosis [149]. Zinc pyrithione (ZnP), (Fig. 21), also known as pyrithione zinc, is an antifungal agent best known for its use in treating dandruff and seborrheic dermatitis. It is believed to act through the disruption of membrane transport by blocking the proton pump that energizes the transport mechanism. Fungi are capable of inactivating pyrithione in low concentrations [150].
AGENTS EXHIBITING ANTIFUNGAL ACTIVITY, WITH UNKNOWN MECHANISM OF ACTION
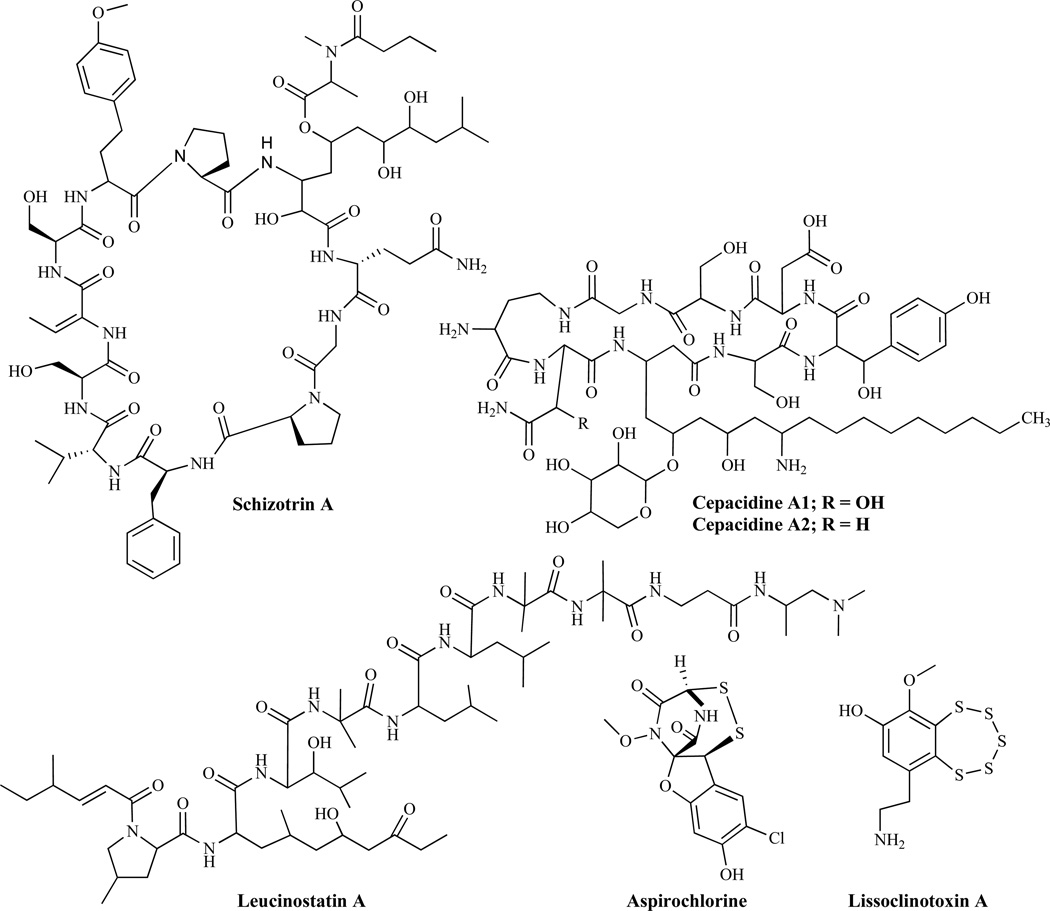
Schizotrin A (Fig. 22) is a cyclic undecapeptide produced by cyanobacterium [151] which has activity against C. albicans and C. tropicalis and has also been found to inhibit the radial growth of F. oxysporum. Cepacidines, comprising cepacidines A1 and A2 (Fig. 22) have shown potent antifungal properties comparable to amphotericin B [152]. However, the mechanisms by which these activities occur have not been reported.
Fig. (22).
Agents Exhibiting Antifungal Activity, with Unknown Mechanism of Action.
Leucinostatin-trichopolyn Group
Leucinostatin A (Fig. 22) represents a group of products of submerged cultures of Penicillium lilacinum [153]. Several leucinostatins isolated from Paecilomyces marquandii (Massee) Hughes, have been reported to display biological activities including antifungal activity against C. neoformans and Candida species [154]. Other natural products, including trichopolyns A and B from Trichoderma polysporum [155] and helioferins A and B, products of Mycogone rosea, inhibit C. albicans [156]. Histatins, produced by human salivary glands, are naturally occurring peptides that have shown several activities including antifungal effects against pathogens such as C. albicans and C. neoformans. While their antifungal mechanism of action is not fully understood, some studies have suggested interaction of Histatin H5 with heat-shock-related proteins in C. albicans [157]. Thiarubrine A and B classified as dithiins, are natural products isolated from plants of the family Compositae that have shown antifungal activity [158] while aspirochlorine, lissoclinotoxin A and related analogs were reported to have potent antifungal activities [159].
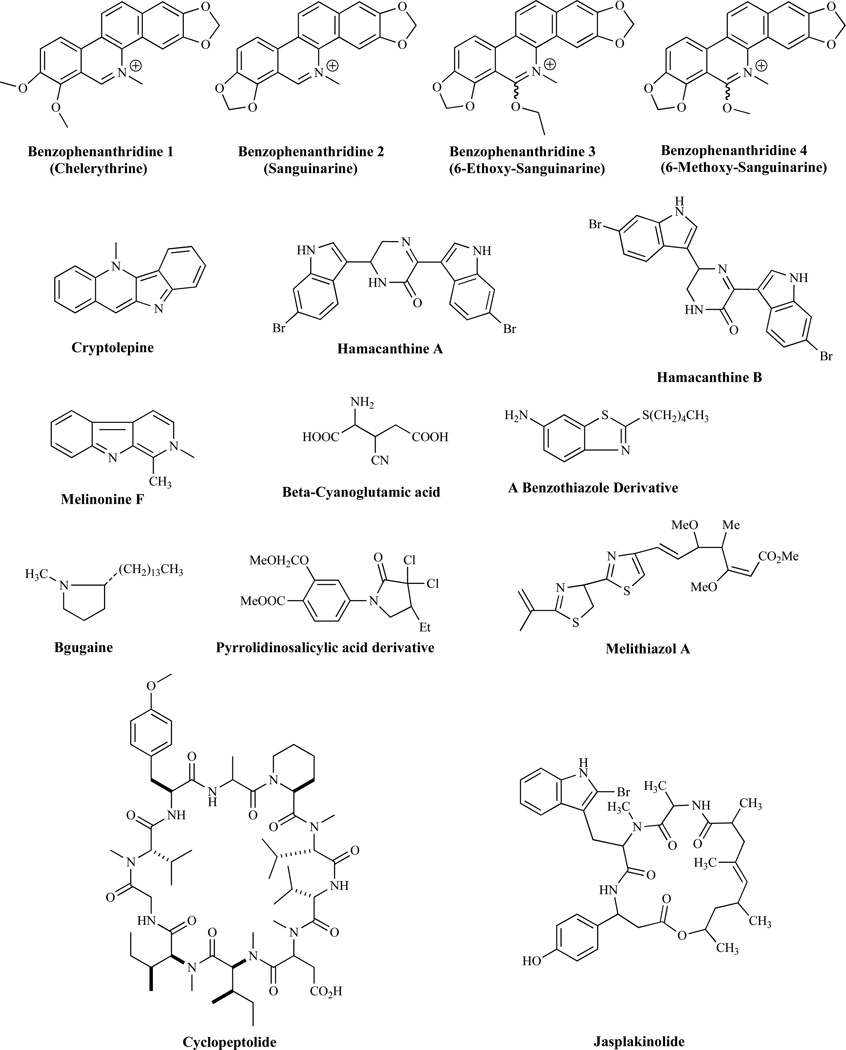
Benzophenanthridines 1, 2, 3 and 4 (Fig. 23) have also been reported to have moderate antifungal activity [160]. Cryptolepine, an indoloquinoline alkaloid with a variety of biological activities including antifungal activities [161], has served as a scaffold for the development of novel antifungal agents [162–172]. 3-Dimethylallylindole has displayed a moderate antifungal activity in vitro [173]. Hamacanthine A and B [174] and melinonine F [175] display good antifungal activity and compounds such as β-cyanoglutamic acid [176], benzothiazole derivatives [177], bgugaine, a pyrrolidine alkaloid from Arisarum vulgare, pyrrololinosalicyclic acid derivatives and melithiazole A have all been shown to have antifungal activities [1]. Lipopeptides such as cyclopeptolide [178] and jasplankinolide [179] (Fig. 23) are known to display broad antifungal activities. Disorazoles as represented by disorazole A1 [180], xanthone isoquinoline derivatives such as SCH54445 [181], LL-Z1271 alpha [182], sonomolide B [183], coniochaetone A, 1-dehydroxyarthrinone [184, 185], pseudolaric acid B [186], soraphen A1α [187], and clabistrin A [188], all display antifungal activities, several of which are comparable to amphotericin B. Fusarielin A displays antifungal activity against A. fumigatus [189]. The structures of several key compounds are shown in Fig. 23.
Fig. (23).
Agents Exhibiting Antifungal Activity, with Unknown Mechanism of Action.
FUTURE APPROACHES FOR IDENTIFICATION OF NOVEL DRUG TARGETS
Significant effort has been expended on optimizing the specificity of current drugs in an attempt to make more selective anti-fungal agents which are less toxic to the patient [190]. However, chemical modifications of drugs have inherent limitations which may compromise their effectiveness. In addition, the development of drug resistance as a result of mutations in the microorganism considerably limits the useable lifetime of drugs aimed at inhibiting a specific target. For these reasons, the need to identify novel, fungal-specific targets is currently a major undertaking. Ideal targets require two major features. First they need to be essential to cell viability, and second, they need to be unique to the fungal organism.
Annotated genome databases can serve as the main sources of information to identify genes with the potential to be therapeutic targets. These databases gather information on gene structure and function, which is compiled from published works and curated for accuracy. Currently, the most complete database is the Saccharomyces Genome Database (SGD) [191]. The SGD genome inventory, as of June 29, 2015, indicates that budding yeast contain 6,604 Open Reading Frames or ORFs (gene sequences which are predicted to encode for a protein) and 78% have been verified. Of these, 1,284 genes are essential indicating that their inactivation leads to cellular inviability [192]. Further analysis can indicate which of these essential genes are unique to the organism and absent in humans.
Similarly, databases for pathogenic fungi are available and are updated with genomic information as it becomes available. For example, the Candida Genome Database [193] collects information for C. albicans, C. glabrata, C. parapsilosis and C. dubliniensis. At this time, C. albicans has 6,218 ORFs but only 1,548 ORFs (24.9%) have been verified. As sequences become available and the ORF function including viability of the null alleles is determined, potential therapeutic targets can be identified. The Aspergillus Genome Database [194] collects genomic information for A. nidulans, A. fumigatus, A. niger and A. oryzae. A genome snapshot of A. nidulans indicates it has 10,678 ORFs, of which only 1,198 (11.2%) have been verified so far [194].
The growing number of organisms subjected to genomic sequencing is generating large amounts of data and this has required the consolidation of the findings in the Fungal and Oomycete Genomics Resources Database, known as FungiDB [195]. However, since the most investigated organism is the budding yeast S. cerevisiae, the SGD provides a tool that restricts BLAST searches to fungal genomes [196] which can serve as the starting point to identify potentially essential genes in other fungi.
An alternative approach to increasing the effectiveness of current anti-fungal drugs involves the identification of genes which when inactivated render the organism hypersensitive to the agent [197]. These genes are identified through genome-wide screens which are performed using libraries of deletion strains arranged in an array, usually in a 96-well format. The library is replicated onto both media containing the drug and media without the drug (as a control for the different growth rates of each strain). Those strains that do not grow in the plate containing the drug are classified as sensitive, and the identity of the deleted gene in the strain is determined by their position in the array or by PCR. These genes are potential targets of therapeutic value since their inactivation results in hypersensitivity to the anti-fungal agent tested.
Currently, the majority of genome-wide screens are performed in S. cerevisiae for which deletion strain libraries are available. The information derived from these screens can prove useful when other fungi contain the same genes identified in the screen, or at least provide additional information regarding the mechanism of action of the anti-fungal agent and the cellular response required to protect the cell from its toxicity [197].
CONCLUSION
Opportunistic fungal infections are frequently observed in immunocompromised patients and present serious life-threatening risks. Significant advances in our understanding of fungal biology have provided numerous potential targets that may yield more effective antifungal agents of potential use in the clinic. In parallel with laboratory evaluation of safety and efficacy, it is hoped that the availability of new knowledge of the mechanisms of action of the existing antifungal agents will spur the discovery of new and effective antifungal agents. The advent of genomics in the investigation of pathogenic fungi is expected to reveal new targets that are unique to fungi and essential for their survival, and hopefully lead to the development of novel antifungal agents.
Acknowledgments
This work would not have been possible without the continuing financial support of the RCMI grant number G12 RR 03020. The work was also supported in part by the Pharmaceutical Research Center NIH/NCRR 1C06-RR12512-01 Grant.
Biography

S.Y. Ablordeppey
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Ablordeppey SY, Fan P, Ablordeppey JH, Mardenborough L. Systemic antifungal agents against AIDS-related opportunistic infections: Current status and emerging drugs in development. Curr. Med. Chem. 1999;6:1151–1195. [PubMed] [Google Scholar]
- 2.a) Marui J, Yoshimi A, Hagiwara D, Fujii-Watanabe Y, Oda K, Koike H, Tamano K, Ishii T, Sano M, Machida M, Abe K. Use of the Aspergillus oryzae actin gene promoter in a novel reporter system for exploring antifungal compounds and their target genes. Appl. Microbiol. Biotechnol. 2010;87:1829–1840. doi: 10.1007/s00253-010-2627-y. [DOI] [PubMed] [Google Scholar]; b) Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med. Mycol. 2014;52:2–9. doi: 10.3109/13693786.2013.819592. [DOI] [PubMed] [Google Scholar]
- 3.Gracia-Rodas R, Cordero RJB, Trevijano-Contador N, Janbon G, Moyrand F, Casadevall A, Zaragoza O. Capsule growth in Cryptococcus neoformans is coordinated with cell cycle progression. mBio. 2014;5:e00945-14. doi: 10.1128/mBio.00945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KT, Byun HJ, Jung KW, Hong J, Cheong E, Bahn YS. Distinct and redundant roles of protein tyrosine phosphatases Ptp1 and Ptp2 in governing the differentiation and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell. 2014;13:796–812. doi: 10.1128/EC.00069-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magliani W, Conti S, Giovati L, Zanello PP, Sperindè M, Ciociola T, Polonelli L. Antibody peptide based antifungal immunotherapy. Front. Microbiol. 2012;3:190–197. doi: 10.3389/fmicb.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriengkauykiat J, Ito JI, Dadwal SS. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 2011;3:175–191. doi: 10.2147/CLEP.S12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enoch DA, Ludlam HA, Brown NM. Invasive fungal infections: a review of epidemiology and management options. J. Med. Microbiol. 2006;55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- 8.Martins IM, Cortés JC, Muñoz J, Moreno MB, Ramos M, Clemente-Ramos JA, Durán A, Ribas JC. Differential activities of three families of specific β(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J. Biol. Chem. 2011;286:3484–3496. doi: 10.1074/jbc.M110.174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutcher JD. The Discovery and development of amphotericin B. Dis. Chest. 1968;54:296–298. doi: 10.1378/chest.54.supplement_1.296. [DOI] [PubMed] [Google Scholar]
- 10.Grunberg E, Titsworth E, Bennett M. Chemotherapeutic activity of 5-fluorocytosine. In: Sylvester JC, editor. Antimicrobial Agents and Chemotherapy— 1963; Proceedings of the Third Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington DC. Ann Arbor, Michigan: American Society for Microbiology; 1964. pp. 566–568. [Google Scholar]
- 11.Godefroi EF, Heeres J, Van Cutsem J, Janssen PA. The preparation and antimycotic properties of derivatives of 1-phenethylimidazole. J. Med. Chem. 1969;12:784–791. doi: 10.1021/jm00305a014. [DOI] [PubMed] [Google Scholar]
- 12.Barrera A, Alastruey-Izquierdo A, Martin MJ, Cuesta I, Vizcaino JA. Analysis of the protein domain and domain architecture content in fungi and its application in the search of new antifungal targets. PLoS Comput. Biol. 2014;10:e1003733. doi: 10.1371/journal.pcbi.1003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knechtle P, Diefenbacher M, Greve KB, Brianza F, Folly C, Heider H, Lone MA, Long L, Meyer JP, Roussel P, Ghannoum MA, Schneiter R, Sorensen AS. The natural diyne-furan fatty acid EV-086 is an inhibitor of fungal delta-9 fatty acid desaturation with efficacy in a model of skin dermatophytosis. Antimicrob. Agents Chemother. 2014;58:455–466. doi: 10.1128/AAC.01443-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck-Sagué CM, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J. Infect. Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 15.Vandeputte P, Ferrarri S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012;2012:713687. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhamgaye S, Devaux F, Manoharlal R, Vandeputte P, Shah AH, Singh A, Blugeon C, Sanglard D, Prasad R. In vitro effect of malachite green on Candida albicans involves multiple pathways and transcriptional regulators UPC2 and STP2. Antimicrob. Agents Chemther. 2012;56:495–506. doi: 10.1128/AAC.00574-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nivoix Y, Ubeaud-Sequier G, Engel P, Levêque D, Herbrecht R. Drug-drug interactions of triazole antifungal agents in multimorbid patients and implications for patient care. Curr. Drug Metab. 2009;10:395–409. doi: 10.2174/138920009788499012. [DOI] [PubMed] [Google Scholar]
- 18.Vande Velde G, Kuchariková S, Van Dijck P, Himmelreich U. Bioluminescence imaging of fungal biofilm development in live animals. Methods Mol. Biol. 2014;1098:153–167. doi: 10.1007/978-1-62703-718-1_13. [DOI] [PubMed] [Google Scholar]
- 19.Bellmann R. Pharmacodynamics and pharmacokinetics of antifungals for treatment of invasive aspergillosis. Curr. Pharm. Des. 2013;19:3629–3647. doi: 10.2174/13816128113199990332. [DOI] [PubMed] [Google Scholar]
- 20.Francis P, Lee JW, Hoffman A, Peter J, Francesconi A, Bacher J, Shelhamer J, Pizzo P, Walsh TJ. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 1994;169:356–368. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 21.Cheah HL, Lim V, Sandai D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS One. 2014;9(4):e95951. doi: 10.1371/journal.pone.0095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev. Infect. Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 23.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 24.Villa NY, Moussatche P, Chamberlin SG, Kumar A, Lyons TJ. Phylogenetic and preliminary phenotypic analysis of yeast PAQR receptors: potential antifungal targets. J. Mol. Evol. 2011;73:134–152. doi: 10.1007/s00239-011-9462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brajtburg J, Powderly WG, Kobayashi GS, Medoff G. Amphotericin B: current understanding of the mechanisms of action. Antimicrob. Agents Chemother. 1990;34:183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnock DW. Amphotericin B: an introduction. J. Antimicrob. Chemother. 1991;28:27–38. doi: 10.1093/jac/28.suppl_b.27. [DOI] [PubMed] [Google Scholar]
- 27.Adler-Moore JP, Proffitt RT. Development, characterization, efficacy and mode of action of Ambisome, a unilamellar liposomal formulation of amphotericin B. J. Liposome Res. 1993;3:429–450. [Google Scholar]
- 28.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–392. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Meunier F. New methods for delivery of antifungal agents. Rev. Infect. Dis. 1989;11:S1605–S1609. doi: 10.1093/clinids/11.supplement_7.s1605. [DOI] [PubMed] [Google Scholar]
- 30.Hanson LH, Stevens DA. Comparison of the antifungal activity of amphotericin B deoxycholate suspension with that of amphotericin cholesteryl sulfate colloidal dispersion. Antimicrob. Agents Chemother. 1992;36:486–488. doi: 10.1128/aac.36.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alder-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemoth. 2002;49:21–30. doi: 10.1093/jac/49.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 32.Lewis RE. Current Concepts in Antifungal Pharmacology. Mayo Clin. Proc. 2011;86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. The biology and chemistry of antifungal agents: a review. Bioorg. Med. Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 34.Onyewu C, Blankenship JR, Del Poeta M, Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003;47:956–964. doi: 10.1128/AAC.47.3.956-964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fromtling RA. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988;1:187–217. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hitchcock CA. Cytochrome P-450-dependent 14 alpha-sterol demethylase of Candida albicans and its interaction with azole antifungals. Biochem. Soc. Trans. 1991;19:782–787. doi: 10.1042/bst0190782. [DOI] [PubMed] [Google Scholar]
- 37.Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 2005;28:1805–1808. doi: 10.1248/bpb.28.1805. [DOI] [PubMed] [Google Scholar]
- 38.Parks LW, Lorenz RT, Casey WM. Functions for sterols in yeast membranes. In: Sutcliffe J, Georgopapadakou NH, editors. Emerging Targets in Antibacterial and Antifungal Chemotherapy. New York: Chapman & Hall; 1992. pp. 393–409. [Google Scholar]
- 39.Barrett-Bee K, Newboult L, Pinder P. Biochemical changes associated with the antifungal action of the triazole ICI 153,066 on Candida albicans and Trichophyton quinckeanum. FEMS Microbiol. Lett. 1991;79:127–132. doi: 10.1016/0378-1097(91)90074-k. [DOI] [PubMed] [Google Scholar]
- 40.Nes WD, Janssen GG, Crumley FG, Kalinowska M, Akihisa T. The structural requirements of sterols for membrane function in Saccharomyces cerevisiae. Arch. Biochem. Biophys. 1993;300:724–733. doi: 10.1006/abbi.1993.1100. [DOI] [PubMed] [Google Scholar]
- 41.Georgopapadakou NH, Dix BA, Smith SA, Freudenberger J, Funke PT. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob. Agents Chemother. 1987;31:46–51. doi: 10.1128/aac.31.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki Y, Yoshihara F, Kondoh M, Nakamura Y, Nakayama N, Arisawa M. Ro 09-1470 is a selective inhibitor of P-450 lanosterol C-14 demethylase of fungi. Antimicrob. Agents Chemother. 1993;37:2662–2667. doi: 10.1128/aac.37.12.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cacciapuoti A, Loebenberg D, Corcoran E, Menzel F, Jr, Moss EL, Jr, Norris C, Michalski M, Raynor K, Halpern J, Mendrick C, Arnold B, Antonacci B, Parmegiani R, Yarosh-Tomaine T, Miller GH, Hare R. S. In vitro and in vivo activities of SCH 56592 (posaconazole), a new triazole antifungal agent, against Aspergillus and Candida. Antimicrob. Agents Chemother. 2000;44:2017–2022. doi: 10.1128/aac.44.8.2017-2022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hata K, Kimura J, Miki H, Toyosawa T, Nakamura T, Katsu K. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob. Agents Chemother. 1996;40:2237–2242. doi: 10.1128/aac.40.10.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yotsuji A, Shimizu K, Araki H, Fujimaki K, Nishida N, Hori R, Annen N, Yamamoto S, Hayakawa H, Imaizumi H, Watanbe Y, Narita H. T-8581, a new orally and parenterally active triazole antifungal agent: in vitro and in vivo evaluations. Antimicrob. Agents Chemother. 1997;41:30–34. doi: 10.1128/aac.41.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petranyi G, Ryder NS, Stütz A. Allylamine derivatives: a new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984;224:1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- 47.Georgopapadakou NH, Bertasso A. Effects of squalene epoxidase inhibitors on Candida albicans. Antimicrob. Agents Chemother. 1992;36:1779–1781. doi: 10.1128/aac.36.8.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryder NS. Mechanism of action and biochemical selectivity of allylamine antimycotic agents. AnnNY. Acad. Sci. 1988;544:208–220. doi: 10.1111/j.1749-6632.1988.tb40405.x. [DOI] [PubMed] [Google Scholar]
- 49.Iwatani W, Arika T, Yamaguchi H. Two mechanisms of butenafine action in Candida albicans. Antimicrob. Agents Chemother. 1993;37:785–788. doi: 10.1128/aac.37.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baloch RI, Mercer EI. Inhibition of sterol D8-D7 isomerase and D14 reductase by fenpropimorph, tridemorph and fenpropidin in cell-free systems from Saccharomyces cerevisiae. Phytochemistry. 1987;26:663–668. [Google Scholar]
- 51.Polak A. Mode of action studies. In: Ryley JF, editor. Handbook of Experimental Pharmacology, Chemotherapy of Fungal Disease. Vol. 96. Heidelberg: Springer-Verlag; 1990. pp. 153–182. [Google Scholar]
- 52.Kelly DE, Rose ME, Kelly SL. Investigation of the role of sterol D8-7-isomerase in the sensitivity of Saccharomyces cerevisiae to fenpropimorph. FEMS Microbiol. Lett. 1994;122:223–226. doi: 10.1111/j.1574-6968.1994.tb07171.x. [DOI] [PubMed] [Google Scholar]
- 53.Marcireau CM, Guyonnet D, Karst F. Construction and growth properties of a yeast strain defective in sterol 14-reductase. Curr. Genet. 1992;22:267–272. doi: 10.1007/BF00317919. [DOI] [PubMed] [Google Scholar]
- 54.Ashman WH, Barbuch RJ, Ulbright CE, Jarrett HW, Bard M. Cloning and disruption of yeast C-8 sterol isomerase gene. Lipids. 1991;26:628–632. doi: 10.1007/BF02536427. [DOI] [PubMed] [Google Scholar]
- 55.Corio-Costet MF, Gerst N, Benveniste P, Schuber F. Inhibition by the fungicide fenpropimorph of cholesterol biosynthesis in 3T3 fibroblasts. Biochem. J. 1988;256:829–834. doi: 10.1042/bj2560829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patton JL, Lester RL. The phosphoinositol sphingolipids of Saccharomyces cerevisiae are highly localized in the plasma membrane. J. Bacteriol. 1991;173:3101–3108. doi: 10.1128/jb.173.10.3101-3108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nimrichter L, Rodrigues ML. Fungal glucosylceramides: from structural components to biologically active targets of new antimicrobials. [Accessed May 8, 2015];Front. Microbiol. 2011 doi: 10.3389/fmicb.2011.00212. URL= http://www.frontiersin.org/Journal/Abstract.aspx?s=727&name=fungi_and_their_interactions&ART_DOI=10.3389/fmicb.2011.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagiec MM, Nagiec EE, Baltisberger JA, Wells GB, Lester RL, Dickson RC. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 1997;272:9809–9817. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 59.Monk BC, Perlin DS. Fungal plasma membrane protein pumps as promising new antifungal targets. Crit. Rev. Microbiol. 1994;20:209–223. doi: 10.3109/10408419409114555. [DOI] [PubMed] [Google Scholar]
- 60.Muroi M, Takasu A, Yamasaki M, Takatsuki A. Folimycin (concanamycin A), an inhibitor of V-type H(+)-ATPase, blocks cell surface expression of virus-envelope glycoproteins. Biochem. Biophys. Res. Commun. 1993;193:999–1005. doi: 10.1006/bbrc.1993.1724. [DOI] [PubMed] [Google Scholar]
- 61.Soteropoulos P, Vaz T, Santangelo R, Paderu P, Huang DY, Tamás MJ, Perlin DS. Molecular characterization of the plasma membrane H(+)-ATPase, an antifungal target in Cryptococcus neoformans. Antimicrob. Agents Chemother. 2000;44:2349–2355. doi: 10.1128/aac.44.9.2349-2355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manavathu EK, Dimmock JR, Vashishtha SC, Chandrasekar PH. Inhibition of H+-ATPase-mediated proton pumping in Cryptococcus neoformans by a novel conjugated styryl ketone. J. Antimicrob. Chemother. 2001;47:491–494. doi: 10.1093/jac/47.4.491. [DOI] [PubMed] [Google Scholar]
- 63.Monk BC, Mason AB, Abramochkin G, Haber JE, Seto-Young D, Perlin DS. The yeast plasma membrane proton pumping ATPase is a viable antifungal target. I. Effects of the cysteine-modifying reagent omeprazole. Biochim. Biophys. Acta. 1995;1239(1):81–90. doi: 10.1016/0005-2736(95)00133-n. [DOI] [PubMed] [Google Scholar]
- 64.Del Palacio-Hernanz A, Guarro-Artigas J, Figueras-Salvat MJ, Esteban-Moreno J, Lopez-Gomez S. Changes in fungal ultrastructure after short-course ciclopiroxolamine therapy in pityriasis versicolor. Clin. Exp. Dermatol. 1990;15:95–100. doi: 10.1111/j.1365-2230.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- 65.Abu-Elteen KH, Hamad M. Antifungal Agents for Use in Human Therapy. In: Kavanagh K, editor. Fungi: Biology and Applications. Chichester, UK: John Wiley & Sons, Ltd.; 2005. pp. 208–209. [Google Scholar]
- 66.Segre A, Bachmann RC, Ballio A, Bossa F, Grgurina I, Iacobellis NS, Marino G, Pucci P, Simmaco M, Takemoto JY. The structure of syringomycins A1, E, and G. FEBS Lett. 1989;255:27–31. doi: 10.1016/0014-5793(89)81054-3. [DOI] [PubMed] [Google Scholar]
- 67.Takemoto JY, Yu Y, Stock SD, Miyakawa T. Yeast genes involved in growth inhibition by Pseudomonas syringae pv. syringae syringomycin family lipodepsipeptides. FEMS Microbiol. Lett. 1993;114:339–342. doi: 10.1111/j.1574-6968.1993.tb06595.x. [DOI] [PubMed] [Google Scholar]
- 68.Sorensen KN, Kim KH, Takemoto J. Y. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob. Agents Chemother. 1996;40:2710–2713. doi: 10.1128/aac.40.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsukuda T, Shiratori Y, Watanabe M, Ontsuka H, Hattori K, Shirai M, Shimma N. Modeling, synthesis and biological activity of novel antifungal agents. Bioorg. Med. Chem. Lett. 1998;8:1819–1824. doi: 10.1016/s0960-894x(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 70.Bierer DE, Gerber RE, Jolad SD, Ubillas RP, Randle J, Nauka E, Latour J, Dener JM, Fort DM. Isolation, structure elucidation, and synthesis of irlbacholine, 1,22-Bis[[[2-(trimethylammonium)ethoxy]phosphinyl]oxy]docosane: A novel antifungal plant metabolite from Irlbachia alata and Anthocleista djalonensis. J. Org. Chem. 1995;60:7022–7026. [Google Scholar]
- 71.Balliano G, Milla P, Ceruti M, Carrano L, Viola F, Brusa P, Cattel L. Inhibition of sterol biosynthesis in Saccharomyces cerevisiae and Candida albicans by 22,23-epoxy-2-aza-2,3-dihydrosqualene and the corresponding N-oxide. Antimicrob. Agents Chemother. 1994;38:1904–1908. doi: 10.1128/aac.38.9.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tesfaye B, Acton JJ, Berger GD, Bergstrom JD, Dufresne C, Kurtz MM, Marquis RW, Parsons WH, Rew DR, Wilson KE. Selective protection and relative importance of the carboxylic acid groups of zaragozic acid A for squalene synthase inhibition. J. Med. Chem. 1994;37:421–424. doi: 10.1021/jm00029a015. [DOI] [PubMed] [Google Scholar]
- 73.Zámocká J, Lacko I, Devínsky F. Synthesis, physico-chemical and antimicrobial activities of 1-alkyl-2-(pyridyl)pyridinium bromides. Pharmazie. 1994;49:66–67. [PubMed] [Google Scholar]
- 74.Georgopapadakou NH, Tkacz JS. The fungal cell wall as a drug target. Trends Microbiol. 1995;3:98–104. doi: 10.1016/s0966-842x(00)88890-3. [DOI] [PubMed] [Google Scholar]
- 75.Schachtschabel D, Arentshorst M, Lagendijk EL, Ram AF. Vacuolar H(+)-ATPase plays a key role in cell wall biosynthesis of Aspergillus niger. Fungal Genet. Biol. 2012;49:284–293. doi: 10.1016/j.fgb.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Cabib E, Roberts RL, Bowers B. Synthesis of the yeast cell wall and its regulation. Annu. Rev. Biochem. 1982;51:763–793. doi: 10.1146/annurev.bi.51.070182.003555. [DOI] [PubMed] [Google Scholar]
- 77.a) Cabib E, Bowers B, Sburlati A, Silverman SJ. Fungal cell wall synthesis: the construction of a biological structure. Microbiol. Sci. 1988;5:370–375. [PubMed] [Google Scholar]; b) Calderone RA, Braun PC. Adherence and receptor relationships of Candida albicans. Microbiol. Rev. 1991;55:1–20. doi: 10.1128/mr.55.1.1-20.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.a) Georgepapadakou NH, Walsh TJ. Antifungal agents: Chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 1996;40:279–291. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fostel JM, Lartey PA. Emerging novel antifungal agents. Drug Discov. Today. 2000;5:25–32. doi: 10.1016/s1359-6446(99)01430-0. [DOI] [PubMed] [Google Scholar]
- 79.Cabib E, Kang MS. Fungal 1,3-β-glucan synthase. Methods Enzymol. 1987;138:637–642. doi: 10.1016/0076-6879(87)38057-7. [DOI] [PubMed] [Google Scholar]
- 80.Hartland RP, Emerson GW, Sullivan PA. A secreted β-glucan-branching enzyme from Candida albicans. Proc. R. Soc. London Ser. B Biol Sci. 1991;246:155–160. doi: 10.1098/rspb.1991.0138. [DOI] [PubMed] [Google Scholar]
- 81.a) Chen SC, Slavin MA, Sorrell TC. Echinocandin antifungal drugs in fungal infections: a comparison. Drugs. 2011;71:11–41. doi: 10.2165/11585270-000000000-00000. [DOI] [PubMed] [Google Scholar]; b) Carter NJ, Keating GM. Micafungin: a review of its use in the prophylaxis and treatment of invasive Candida infections in pediatric patients. Paediatr. Drugs. 2009;11:271–291. doi: 10.2165/00148581-200911040-00006. [DOI] [PubMed] [Google Scholar]
- 82.Perlin DS. Current perspectives on echinocandin class drugs. Future Microbiol. 2011;6:441–457. doi: 10.2217/fmb.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.a) Tkacz JS. Glucan biosynthesis in fungi and its inhibition. In: Sutcliffe J, Georgopapadakou NH, editors. Emerging targets in antibacterial and antifungal chemotherapy. New York: Chapman & Hall; 1992. pp. 495–523. [Google Scholar]; b) Baguley BC, Rommele G, Gruner J, Wehrli W, Papulacandin B. an inhibitor of glucan synthesis in yeast spheroplasts. Eur. J. Biochem. 1979;97:345–351. doi: 10.1111/j.1432-1033.1979.tb13120.x. [DOI] [PubMed] [Google Scholar]
- 84.Bartizal K, Scott T, Abruzzo GK, Gill CJ, Pacholok C, Lynch L, Kropp H. In vitro evaluation of the pneumocandin antifungal agent L-733560, a new water-soluble hybrid of L-705589 and L-731373. Antimicrob. Agents Chemother. 1995;39:1070–1076. doi: 10.1128/aac.39.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukhopadhyay T, Roy K, Bhat RG, Sawant SN, Blumbach J, Ganguli BN, Fehlhaber HW, Kogler H. Deoxymulundocandin- a new echinocandin type antifungal antibiotic. J. Antibiot. 1992;45:618–623. doi: 10.7164/antibiotics.45.618. [DOI] [PubMed] [Google Scholar]
- 86.Iwamoto T, Fujie A, Nitta K, Hashimoto S, Okuhara M, Kohsaka M. WF11899A, B, and C, novel antifungal lipopeptides. II. Biological properties. J. Antibiot (Tokyo) 1994;47:1092–1097. doi: 10.7164/antibiotics.47.1092. [DOI] [PubMed] [Google Scholar]
- 87.Gunawardana G, Rasmussen RR, Scherr M, Frost D, Brandt KD, Choi W, Jackson M, Karwowski JP, Sunga G, Malmberg LH, West P, Chen RH, Kadam S, Clement JJ, McAlpine JB. Corynecandin: a novel antifungal glycolipid from Coryneum modonium. J. Antibiot. 1997;50:884–886. doi: 10.7164/antibiotics.50.884. [DOI] [PubMed] [Google Scholar]
- 88.Kaneto R, Chiba H, Agematu H, Shibamoto N, Yoshioka T, Nishida H, Okamoto R. Mer-WF3010, a new member of the papulacandin family. 1. Fermentation, isolation and characterization. J. Antibiot (Tokyo) 1993;46:247–250. doi: 10.7164/antibiotics.46.247. [DOI] [PubMed] [Google Scholar]
- 89.Jackson M, Frost DJ, Karwowski JP, Humphrey PE, Dahod SK, Choi WS, Brandt K, Malmberg LH, Rasmussen RR, Scherr MH, Flamm RK, Kadam S, McAlpine JB. Fusacandins A and B; novel antifungal antibiotics of the papulacandin class from Fusarium sambucinum. I. Identity of the producing organism, fermentation and biological activity. J. Antibiot (Tokyo) 1995;48:608–613. doi: 10.7164/antibiotics.48.608. [DOI] [PubMed] [Google Scholar]
- 90.Yeung CM, Klein LL, Lartey PA. Preparation and antifungal activity of fusacandin analogs: C-6′ sidechain esters. Bioorg. Med. Chem. Lett. 1996;6:819–822. [Google Scholar]
- 91.Zehavi U, Polacheck I. Saponins as antimycotic agents: glycosides of medicagenic acid. Adv. Exp. Med. Biol. 1996;404:535–546. doi: 10.1007/978-1-4899-1367-8_44. [DOI] [PubMed] [Google Scholar]
- 92.Bulawa CE. Genetics and molecular biology of chitin synthesis in fungi. Annu. Rev. Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- 93.Au-Young J, Robbins PW. Isolation of a chitin synthase gene (CHS1) from Candida albicans by expression in Saccharomyces cerevisiae. Mol. Microbiol. 1990;4:197–207. doi: 10.1111/j.1365-2958.1990.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 94.a) Cabib E, Silverman SJ, Shaw JA. Chitinase and chitin synthase 1: counterbalancing activities in cell separation of Saccharomyces cerevisiae. J. Gen. Microbiol. 1992;138:97–102. doi: 10.1099/00221287-138-1-97. [DOI] [PubMed] [Google Scholar]; b) Shaw JA, Mol PC, Bowers B, Silverman SJ, Valdivieso MH, Duran A, Cabib E. The function of chitin synthases 2 and 3 in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 1991;114:111–123. doi: 10.1083/jcb.114.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaudhary PM, Tupe SG, Deshpande MV. Chitin synthase inhibitors as antifungal agents. Mini Rev. Med. Chem. 2013;13:222–236. doi: 10.2174/138955713804805256. [DOI] [PubMed] [Google Scholar]
- 96.Ruiz-Herrera J, San-Blas G. Chitin synthesis as a target for antifungal drugs. Curr. Drug Targets-Infect. Disord. 2003;3:77–91. doi: 10.2174/1568005033342064. [DOI] [PubMed] [Google Scholar]
- 97.Vijayakumar EK, Roy K, Chatterjee S, Deshmukh SK, Ganguli BN, Fehlhaber HW, Kogler H. Arthrichitin. A new cell wall active metabolite form Arthrinium phaeospermum. J. Org. Chem. 1996;61:6591–6593. doi: 10.1021/jo960769n. [DOI] [PubMed] [Google Scholar]
- 98.Albaugh D, Albert G, Bradford P, Cotter V, Froyd J, Gaughran J, Kirsch DR, Lai M, Rehnig A, Sieverding E, Silverman S. Cell wall active antifungal compound produced by the marine fungus Hypoxylon oceanicum LL-15G256. III. Biological properties of 15G256γ. J. Antibiot. 1998;51:317–322. doi: 10.7164/antibiotics.51.317. [DOI] [PubMed] [Google Scholar]
- 99.Iwamoto T, Fujiie A, Tsurumi Y, Nitta K, Hashimoto S, Okuhara M. FR900403, a new antifungal antibiotic produced by a Kernia sp. J. Antibiot. (Tokyo) 1990;43:1183–1185. doi: 10.7164/antibiotics.43.1183. [DOI] [PubMed] [Google Scholar]
- 100.Takesako K, Ikai K, Haruna F, Endo M, Shimanaka K, Sono E, Nakamura T, Kato I, Yamaguchi H. Aureobasidins, new antifungal antibiotics. Taxonomy, fermentation, isolation, and properties. J. Antibiot. (Tokyo) 1991;44:919–924. doi: 10.7164/antibiotics.44.919. [DOI] [PubMed] [Google Scholar]
- 101.Schüttelkopf AW, Gros L, Blair DE, Frearson JA, van Aalten DM, Gilbert IH. Acetazolamide-based fungal chitinase inhibitors. Bioorg. Med. Chem. 2010;18:8334–8340. doi: 10.1016/j.bmc.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lockhart DE, Schuettelkopf A, Blair DE, van Aalten DM. Screening-based discovery of Aspergillus fumigatus plant-type chitinase inhibitors. FEBS Lett. 2014;588:3282–3290. doi: 10.1016/j.febslet.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vermese A, Guchelaar H-J, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J. Antimicrob. Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- 104.a) Shen LL, Fostel JM. DNA topoisomerase inhibitors as antifungal agents. Adv. Pharmacol. 1994;29B:227–244. doi: 10.1016/s1054-3589(08)61140-0. [DOI] [PubMed] [Google Scholar]; b) Hsieh TS. DNA topoisomerases. Curr. Opin. Cell Biol. 1992;4:396–400. doi: 10.1016/0955-0674(92)90004-v. [DOI] [PubMed] [Google Scholar]
- 105.Liu LF. DNA topoisomerase poisons as antitumor drugs. Annu. Rev. Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- 106.Watt PM, Hickson ID. Structure and function of type II topoisomerases. Biochem. J. 1994;303:681–695. doi: 10.1042/bj3030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.a) Fostel JM, Montgomery DA, Shen LL. Characterization of DNA topoisomerase I from Candida albicans as a target for drug discovery. Antimicrob. Agents Chemother. 1992;36:2131–2138. doi: 10.1128/aac.36.10.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fostel J, Montgomery D. Identification of the aminocatechol A-3253 as an in vitro poison of DNA topoisomerase I from Candida albicans. Antimicrob. Agents Chemother. 1995;39:586–592. doi: 10.1128/AAC.39.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moldave K. Eukaryotic protein synthesis. Annu. Rev. Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- 109.Kamath A, Chakraburtty K. Role of yeast elongation factor 3 in the elongation cycle. J. Biol. Chem. 1989;264:15423–15428. [PubMed] [Google Scholar]
- 110.Uritani M, Miyazaki M. Characterization of the ATPase and GTPase activities of elongation factor 3 (EF-3) purified from yeasts. J. Biochem. 1988;103:522–530. doi: 10.1093/oxfordjournals.jbchem.a122302. [DOI] [PubMed] [Google Scholar]
- 111.Colthurst DR, Santos M, Grant CM, Tuite MF. Candida albicans and three other Candida species contain an elongation factor structurally and functionally analogous to elongation factor 3. FEMS Microbiol. Lett. 1991;64:45–50. doi: 10.1016/0378-1097(91)90207-q. [DOI] [PubMed] [Google Scholar]
- 112.Gooday GW. The potential of novel antifungal drugs for the treatment of disease in immunocompromised host. Exp. Opin. Invest. Drugs. 1995;4:679–691. [Google Scholar]
- 113.Dominguez JM, Kelly VA, Kinsman OS, Marriott MS, Gómez de las Heras F, Martín JJ. Sordarins: a new class of antifungals with selective inhibition of the protein synthesis elongation cycle in yeasts. Antimicrob. Agents Chemother. 1998;42:2274–2278. doi: 10.1128/aac.42.9.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herreros E, Martinez CM, Almela MJ, Marriott MS, Gomez De Las Heras F, Gargallo-Viola D. Sordarins: in vitro activities of new antifungal derivatives against pathogenic yeasts, Pneumocystis carinii, and filamentous fungi. Antimicrob. Agents Chemother. 1998;42:2863–2869. doi: 10.1128/aac.42.11.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vallim MA, Fernandes L, Alspaugh JA. The RAM1 gene encoding a protein-farnesyltransferase β-subunit homologue is essential in Cryptococcus neoformans. Microbiology. 2004;150:1925–1935. doi: 10.1099/mic.0.27030-0. [DOI] [PubMed] [Google Scholar]
- 116.Johnson DR, Bhatnagar RS, Knoll LJ, Gordon JI. Genetic and biochemical studies of protein N-myristoylation. Annu. Rev. Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- 117.Lodge JK, Jackson-Machelski E, Toffaletti DL, Perfect JR, Gordon JI. Targeted gene replacement demonstrates that myristoyl CoA:protein N-myristoyltransferase is essential for viability of Cryptococcus neoformans. Proc. Natl. Acad. Sci. USA. 1994;91:12008–12012. doi: 10.1073/pnas.91.25.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duronio RJ, Towler DA, Heuckeroth RO, Gordon JI. Disruption of the yeast N-myristoyltransferase gene causes recessive lethality. Science. 1989;243:796–800. doi: 10.1126/science.2644694. [DOI] [PubMed] [Google Scholar]
- 119.a) Yamamoto K, Shiinoki Y, Nishio M, Matsuda Y, Inouye Y, Nakamura S. A new group of antibiotics, hydroxamic acid antimycotic antibiotics. III. Isolation and characterization of enactin congeners. J. Antibiot. (Tokyo) 1990;43:1012–1017. doi: 10.7164/antibiotics.43.1012. [DOI] [PubMed] [Google Scholar]; b) Okada H, Yamamoto K, Tsutano S, Inouye Y, Nakamura S, Furukawa J. A new group of antibiotics, hydroxamic acid antimycotic antibiotics. II. The structure of neoenactins NL1 and NL2 and structure-activity relationship. J. Antibiot. (Tokyo) 1989;42:276–282. doi: 10.7164/antibiotics.42.276. [DOI] [PubMed] [Google Scholar]; c) Roy SK, Inouye Y, Nakamura S, Furukawa J, Okuda S. Isolation, structural elucidation and biological properties of neoenactins B1, B2, M1 and M2, neoenactin congeners. J. Antibiot. (Tokyo) 1987;40:266–274. doi: 10.7164/antibiotics.40.266. [DOI] [PubMed] [Google Scholar]; d) Roy SK, Nakamura S, Furukawa J, Okuda S. The structure of neoenactin A, a new antifungal antibiotic potentiating polyene antifungal antibiotics. J. Antibiot. (Tokyo) 1986;39:717–720. doi: 10.7164/antibiotics.39.717. [DOI] [PubMed] [Google Scholar]
- 120.Carballeira NM, Ortiz D, Parang K, Sardari S. Total synthesis and in vitro-antifungal activity of (±)-2-methoxytetradecanoic acid. Arch. Pharm (Weinheim) 2004;337:152–155. doi: 10.1002/ardp.200300824. [DOI] [PubMed] [Google Scholar]
- 121.Okatani T, Koyama J, Suzuta Y, Tagahara K. The effects of solvent and temperature on the orientation of cycloaddition reaction of 1,2,3-triazine enamines: Its application to the synthesis of alkaloids, onychine and 6-methoxyonychine. Heterocycles. 1988;27:2213–2217. [Google Scholar]
- 122.Bracher F. Polycyclic aromatic alkaloids, 10: Annonaceous alkaloids with antimycotic activity. Arch. Pharm. (Weinheim) 1994;327:371–375. doi: 10.1002/ardp.19943270605. [DOI] [PubMed] [Google Scholar]
- 123.Konishi M, Nishio M, Saitoh K, Miyaki T, Oki T, Kawaguchi H. Cispentacin, a new antifungal antibiotic. I. Production, isolation, physico-chemical properties, and structure. J. Antibiot. (Tokyo) 1989;42:1749–1755. doi: 10.7164/antibiotics.42.1749. [DOI] [PubMed] [Google Scholar]
- 124.Oki T, Hirano M, Tomatsu K, Numata K-I, Kamei H. Cispentacin, a new antifungal antibiotic. II. In vitro and in vivo antifungal activities. J. Antibiot. (Tokyo) 1989;42:1756–1762. doi: 10.7164/antibiotics.42.1756. [DOI] [PubMed] [Google Scholar]
- 125.Iwamoto T, Tsujii E, Ezaki M, Fujie A, Hashimoto S, Okuhara M, Kohsaka M, Imanaka H, Kawabata K, Inamoto Y, Sakane K. FR 109615, a new antifungal antibiotic from Streptomyces setonii Taxonomy, fermentation, isolation, physicochemical properties and biological activity. J. Antibiot. 1990;43:1–7. doi: 10.7164/antibiotics.43.1. [DOI] [PubMed] [Google Scholar]
- 126.Yamaki H, Yamaguchi M, Imamura H, Suzuki H, Nishimura T, Saito H, Yamaguchi H. The mechanism of antifungal action of (s)-2-amino-4-oxo-5-hydroxypentanoic acid, RI-331: the inhibition of homoserine dehydrogenase in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1990;168:837–843. doi: 10.1016/0006-291x(90)92397-i. [DOI] [PubMed] [Google Scholar]
- 127.Ohwada J, Umeda I, Ontsuka H, Aoki Y, Shimma N. Synthesis and structure-activity relationships of a novel antifungal agent azoxybacillin. Chem. Pharm. Bull. (Tokyo) 1994;42:1703–1705. doi: 10.1248/cpb.42.1703. [DOI] [PubMed] [Google Scholar]
- 128.Tabor CW, Tabor H. Polyamines. Annu. Rev. Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 129.Pfaller MA, Riley J, Gerarden T. Polyamine deprivation and growth inhibition of Cryptococcus neoformans by alpha-difluoromethylornithine and cyclohexylamine. Mycopathologia. 1990;112:27–32. doi: 10.1007/BF01795176. [DOI] [PubMed] [Google Scholar]
- 130.Saric M, Clarkson AB., Jr Ornithine decarboxylase in Pneumocystis carinii and implications for therapy. Antimicrob. Agents Chemother. 1994;38:2545–2552. doi: 10.1128/aac.38.11.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chan JH, Hong JS, Kuyper LF, Jones ML, Baccanari DP, Tansik RL, Boytos CM, Rudolph SK, Brown AD. Synthesis of 1,3-diamino-7,8,9,10-tetrahydropyrido[3,2-f]-quinazolines. Inhibitors of Candida albicans dihydrofolate reductase as potential antifungal agents. J. Heterocycl. Chem. 1997;34:145–151. [Google Scholar]
- 132.Cleveland DW, Sullivan KF. Molecular biology and genetics of tubulin. Annu. Rev. Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- 133.Kilmartin JV. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981;20:3629–3633. doi: 10.1021/bi00515a050. [DOI] [PubMed] [Google Scholar]
- 134.Cardenas ME, Sanfridson A, Cutler NS, Heitman J. Signal-transduction cascades as targets for therapeutic intervention by natural products. Trends Biotechnol. 1998;16:427–433. doi: 10.1016/s0167-7799(98)01239-6. [DOI] [PubMed] [Google Scholar]; b) Blankenship JR, Steinbach WJ, Perfect JR, Heitman J. Teaching old drugs new tricks: reincarnating immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs. 2003;4:192–199. [PubMed] [Google Scholar]
- 135.Fernandes L, Araújo MA, Amaral A, Reis VC, Martins NF, Felipe MS. Cell signaling pathways in Paracoccidioides brasiliensis - inferred from comparisons with other fungi. Genet. Mol. Res. 2005;4:216–231. [PubMed] [Google Scholar]
- 136.a) Bastida RJ, Reedy JL, Morales-Johansson H, Heitman J, Cardenas ME. Signaling cascades as drug targets in model and pathogenic fungi. Curr. Opin. Investig. Drugs. 2008;9:856–864. [PMC free article] [PubMed] [Google Scholar]; b) Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.a) Bandhakavi S, Xie H, O'Callaghan B, Sakurai H, Kim DH, Griffin TJ. Hsf1 activation inhibits rapamycin resistance and TOR signaling in yeast revealed by combined proteomic and genetic analysis. PLoS One. 2008;3:e1598. doi: 10.1371/journal.pone.0001598. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hartmann T, Sasse C, Schedler A, Hasenberg M, Gunzer M, Krappmann S. Shaping the fungal adaptome-Stress responses of Aspergillus fumigatus. Int. J. Med. Microbiol. 2011;301:408–416. doi: 10.1016/j.ijmm.2011.04.008. [DOI] [PubMed] [Google Scholar]; c) Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J. Antimicrob. Chemother. 2003;51:313–316. doi: 10.1093/jac/dkg090. [DOI] [PubMed] [Google Scholar]
- 138.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem. Sci. 1995;20:303–307. doi: 10.1016/s0968-0004(00)89056-8. [DOI] [PubMed] [Google Scholar]
- 139.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.a) Ueki M, Abe K, Hanafi M, Shibata K, Tanaka T, Taniguchi M. UK-2A, B, C and D, novel antifungal antibiotics from Streptomyces sp. 517-02. I. Fermentation, isolation, and biological properties. J. Antibiot. (Tokyo) 1996;49:639–643. doi: 10.7164/antibiotics.49.639. [DOI] [PubMed] [Google Scholar]; b) Ueki M, Taniguchi M. The mode of action of UK-2A and UK-3A, novel antifungal antibiotics from Streptomyces sp. 517-02. J. Antibiot. (Tokyo) 1997;50:52–57. doi: 10.7164/antibiotics.50.1052. [DOI] [PubMed] [Google Scholar]
- 141.Huang CB, Alimova Y, Myers TM, Ebersole JL. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011;56:650–654. doi: 10.1016/j.archoralbio.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hazan R, Levine A, Abeliovich H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2004;70:4449–4457. doi: 10.1128/AEM.70.8.4449-4457.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.a) Goettsch RW, Danti AG, Cronk DH. Synthesis of some derivatives of sorbic acid and their antifungal properties. J. Pharm. Sci. 1962;51:728–730. doi: 10.1002/jps.2600510805. [DOI] [PubMed] [Google Scholar]; b) Stratford M, Plumridge A, Nebe-von-Caron G, Archer DB. Inhibition of spoilage mould conidia by acetic acid and sorbic acid involves different modes of action, requiring modification of the classical weak-acid theory . Int. J. Food Microbiol. 2009;136:37–43. doi: 10.1016/j.ijfoodmicro.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 144.Woong PS, Klotzsche M, Wilson DJ, Boshoff HI, Eoh H, Manjunatha U, Blumenthal A, Rhee K, Barry CE, III, Aldrich CC, Ehrt S, Schnappinger D. Evaluating the sensitivity of Mycobacterium tuberculosis to biotin deprivation using regulated gene expression. PLoS Pathog. 2011;7:e1002264. doi: 10.1371/journal.ppat.1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soares da Costa TP, Tieu W, Yap MY, Zvarec O, Bell JM, Turnidge JD, Wallace JC, Booker GW, Wilce MC, Abell AD, Polyak SW. Biotin analogues with antibacterial activity are potent inhibitors of biotin protein ligase. ACS Med. Chem. Lett. 2012;3:509–514. doi: 10.1021/ml300106p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.a) Harrison EF, Zygmunt WA. Haloprogin: mode of action studies in Candida albicans. Can. J. Microbiol. 1974;20:1241–1245. doi: 10.1139/m74-191. [DOI] [PubMed] [Google Scholar]; b) [Accessed May 7, 2015]; www.scribd.com/doc/13287186/Fungi. [Google Scholar]
- 147.a) Patya M, Zahalka MA, Vanichkin A, Rabinkov A, Miron T, Mirelman D, Wilchek M, Lander HM, Novogrodsky A. Allicin stimulates lymphocytes and elicits an antitumor effect: a possible role of p21ras. Int. Immunol. 2004;16:275–281. doi: 10.1093/intimm/dxh038. [DOI] [PubMed] [Google Scholar]; b) Davis SR. An overview of the antifungal properties of allicin and its breakdown products-the possibility of a safe and effective antifungal prophylactic. Mycoses. 2005;48:95–100. doi: 10.1111/j.1439-0507.2004.01076.x. [DOI] [PubMed] [Google Scholar]
- 148.Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004;53:1081–1085. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 149.a) Zoric N, Horvat I, Kopjar N, Vucemilovic A, Kremer D, Tomic S, Kosalec I. Hydroxytyrosol expresses antifungal activity in vitro. Curr. Drug Targets. 2013;14:992–998. doi: 10.2174/13894501113149990167. [DOI] [PubMed] [Google Scholar]; b) Amin A, Khan MA, Shah S, Ahmad M, Zafar M, Hameed A. Inhibitory effects of Olea ferruginea crude leaves extract against some bacterial and fungal pathogen. Pak. J. Pharm. Sci. 2013;26:251–254. [PubMed] [Google Scholar]
- 150.Chandler CJ, Segel IH. Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis. Antimicrob. Agents Chemother. 1978;14:60–68. doi: 10.1128/aac.14.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pergament I, Carmelli S. Schizotrin A: novel antimicrobial cyclic peptide from a cyanobacterium. Tetrahedron Lett. 1994;35:8473–8476. [Google Scholar]
- 152.Lee C-H, Kim S, Hyun B, Suh J-W, Yon C, Kim C, Lim Y, Kim C. Cepacidine A, a novel antifungal antibiotic produced by Pseudomonas cepacia. I. Taxonomy, production, isolation, and biological activity. J. Antibiot. (Tokyo) 1994;47:1402–1405. doi: 10.7164/antibiotics.47.1402. [DOI] [PubMed] [Google Scholar]
- 153.Arai T, Mikami Y, Fukushima K, Utsumi T, Yazawa K. A new antibiotic, leucinostatin, derived from Penicillium lilacinum. J. Antibiot. (Tokyo) 1973;26:1606–1612. doi: 10.7164/antibiotics.26.157. [DOI] [PubMed] [Google Scholar]
- 154.a) Fukushima K, Arai T, Mori Y, Tsuboi M, Suzuki M. Studies on peptide antibiotics, leucinostatins. I. Separation, physico-chemical properties and biological activities of leucinostatins A and B. J. Antibiot. (Tokyo) 1983;36:1606–1612. doi: 10.7164/antibiotics.36.1606. [DOI] [PubMed] [Google Scholar]; b) Fukushima K, Arai T, Mori Y, Tsuboi M, Suzuki M. Studies on peptide antibiotics, leucinostatins. II. The structures of leucinostatins A and B. J. Antibiot. (Tokyo) 1983;36:1613–1630. doi: 10.7164/antibiotics.36.1613. [DOI] [PubMed] [Google Scholar]; c) Mikami Y, Fukushima K, Arai T, Abe F, Shibuya H, Ommura Y. Leucinostatins, peptide mycotoxins produced by Paecilomyces lilacinus and their possible roles in fungal infection. Zentralbl. Bakteriol. Mikrobiol. Hyg. A. 1984;257:275–283. [PubMed] [Google Scholar]; d) Mori Y, Suzuki M, Fukushima K, Arai T. Structure of leucinostatin B, an uncoupler on mitochondria. J. Antibiot. (Tokyo) 1983;36:1084–1086. doi: 10.7164/antibiotics.36.1084. [DOI] [PubMed] [Google Scholar]
- 155.Fuji K, Fujita E, Takaishi Y, Fujita T, Arita I, Komatsu M, Hirasuka N. New antibiotics, Trichopolyns A and B: isolation and biological activity. Experientia. 1978;34:237–239. doi: 10.1007/BF01944702. [DOI] [PubMed] [Google Scholar]
- 156.Gräfe U, Ihn W, Ritzau M, Schade W, Stengel C, Schlegel B, Fleck WF, Künkel W, Härtl A, Gutsche W. Helioferins: novel antifungal lipopeptides from Mycogone rosea: screening, isolation, structures, and biological properties. J. Antibiot. (Tokyo) 1995;48:126–133. doi: 10.7164/antibiotics.48.126. [DOI] [PubMed] [Google Scholar]
- 157.a) Puri S, Edgerton M. How does it kill?: understanding the candidacidal mechanism of salivary histatin 5. Eukaryot. Cell. 2014;13:958–964. doi: 10.1128/EC.00095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Petruzzelli R, Clementi ME, Marini S, Coletta M, Di Stasio E, Giardina B, Misiti F. Respiratory inhibition of isolated mammalian mitochondria by salivary antifungal peptide histatin-5. Biochem. Biophys. Res. Commun. 2003;311:1034–1040. doi: 10.1016/j.bbrc.2003.10.104. [DOI] [PubMed] [Google Scholar]; c) Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008;4:e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Towers GHN, Bruening RC, Balza F, Abramowski ZA, Lopez-Bazzochi I. Thiarubrine Antifungal Agents. 5, 202,348. U.S. Patent. 1993 Apr 13;
- 159.Litaudon M, Trigalo F, Martin MT, Frappier F, Guyot M. Lissoclinotoxins: Antibiotic polysulfur derivatives from the tunicate Lissoclinum perforatum. Revised structure of lissoclinotoxin A. Tetrahedron. 1994;50:5323–5334. [Google Scholar]
- 160.Odebiyi OO, Sofowora EA. Antimicrobial alkaloids from a Nigerian chewing stick - Fagara zanthoxyloides. Planta Medica. 1979;36:204–207. doi: 10.1055/s-0028-1097271. [DOI] [PubMed] [Google Scholar]
- 161.Mardenborough LG, Fan PC, Ablordeppey SY, Nimrod A, Clark AM. Identification of a novel antifungal agent based on a pharmacophoric group in cryptolepine. Med. Chem. Res. 1999;9:118–132. [Google Scholar]
- 162.Mazu TK, Etukala JR, Zhu XY, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Identification of 3-phenylaminoquinolinium and 3-phenylaminopyridinium salts as new agents against opportunistic fungal pathogens. Bioorg. Med. Chem. 2011;19:524–533. doi: 10.1016/j.bmc.2010.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mazu TK, Etukala JR, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. δ-Carbolines and their ring-opened analogs: Synthesis and evaluation against fungal and bacterial opportunistic pathogens. Eur. J. Med. Chem. 2011;46:2378–2385. doi: 10.1016/j.ejmech.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bolden S, Boateng CA, Zhu XY, Etukala JR, Eyunni SK, Jacob MR, Khan SI, Ablordeppey SY. CoMFA studies and in vitro evaluation of some 3-substituted benzylthio quinolinium salts as anticryptococcal agents. Bioorg. Med. Chem. 2013;21:7194–7201. doi: 10.1016/j.bmc.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bolden S, Jr, Zhu XY, Etukala JR, Boateng C, Mazu T, Flores-Rozas H, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Structure-activity relationship (SAR) and preliminary mode of action studies of 3-substituted benzylthioquinolinium iodide as anti-opportunistic infection agents. Eur. J. Med. Chem. 2013;70:130–142. doi: 10.1016/j.ejmech.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boateng CA, Zhu XY, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Optimization of 3-(phenylthio)quinolinium compounds against opportunistic fungal pathogens. Eur. J. Med. Chem. 2011;46:1789–1797. doi: 10.1016/j.ejmech.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boateng CA, Eyunni SVK, Zhu XY, Etukala JR, Bricker BA, Ashfaq MK, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Benzothieno[3,2-b] quinolinium and 3- (phenylthio)quinolinium compounds: Synthesis and evaluation against opportunistic fungal pathogens. Bioorg. Med. Chem. 2011;19:458–470. doi: 10.1016/j.bmc.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kumar EV, Etukala JR, Ablordeppey SY. Indolo[3,2-b]quinolines; synthesis, biological evaluation and structure activity-relationships. Mini Rev. Med. Chem. 2008;8:538–554. doi: 10.2174/138955708784534418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhu XY, Mardenborough LG, Li S, Khan A, Zhang W, Fan P, Jacob M, Khan S, Walker L, Ablordeppey SY. Synthesis and evaluation of isosteres of N-methyl indolo[3,2-b]-quinoline (cryptolepine) as new antiinfective agents. Bioorg. Med. Chem. 2007;5:686–695. doi: 10.1016/j.bmc.2006.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mardenborough LG, Zhu XY, Fan P, Jacob MR, Khan SI, Walker LA, Ablordeppey SY. Identification of bisquindolines as new antiinfective agents. Bioorg. Med. Chem. 2005;13:3955–3963. doi: 10.1016/j.bmc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 171.Ablordeppey SY, Fan P, Li S, Clark AM, Hufford CD. Substituted indoloquinolines as new antifungal agents. Bioorg. Med. Chem. 2002;10:1337–1346. doi: 10.1016/s0968-0896(01)00401-1. [DOI] [PubMed] [Google Scholar]
- 172.Ablordeppey SY, Fan P, Clark AM, Nimrod A. Probing the N-5 region of the indoloquinoline alkaloid, cryptolepine for anticryptococcal activity. Bioorg. Med. Chem. 1999;7:343–349. doi: 10.1016/s0968-0896(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 173.Adeoye AO, Oguntimein BO, Clark AM, Hufford CD. 3-Dimethylallylindole: an antibacterial and antifungal metabolite from Monodora tenuifolia. J. Nat. Prod. 1986;49:534–537. doi: 10.1021/np50045a031. [DOI] [PubMed] [Google Scholar]
- 174.Gunasekera SP, McCarthy PJ, Kelly-Borges M. Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha sp. J. Nat. Prod. 1994;57:1437–1441. doi: 10.1021/np50112a014. [DOI] [PubMed] [Google Scholar]
- 175.Quetin-Leclercq J, Favel A, Balansard G, Regli P, Angenot L. Screening for in vitro antifungal activities of some indole alkaloids. Planta Med. 1995;61:475–477. doi: 10.1055/s-2006-958141. [DOI] [PubMed] [Google Scholar]
- 176.Naruse N, Yamamoto S, Yamamoto H, Kondo S, Masuyoshi S, Numata K, Fukagawa Y, Oki T. β-cyanoglutamic acid, a new antifungal amino acid from a streptomycete. J. Antibiot. (Tokyo) 1993;46:685–686. doi: 10.7164/antibiotics.46.685. [DOI] [PubMed] [Google Scholar]
- 177.Bujdáková H, Kuchta T, Sidóová E, Gvozdjaková A. Anti-Candida activity of four antifungal benzothiazoles. FEMS. Microbiol. Lett. 1993;112:329–333. doi: 10.1111/j.1574-6968.1993.tb06471.x. [DOI] [PubMed] [Google Scholar]
- 178.Emmer G, Grassberger MA, Meingassner JG, Schulz G, Schaude M. Derivatives of a novel cyclopeptolide. 1. Synthesis, antifungal activity, and structure-activity relationships. J. Med. Chem. 1994;37:1908–1917. doi: 10.1021/jm00039a002. [DOI] [PubMed] [Google Scholar]
- 179.Scott VR, Boehme R, Matthews TR. New class of antifungal agents: jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob. Agents Chemother. 1988;32:1154–1157. doi: 10.1128/aac.32.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Irschik H, Jansen R, Gerth K, Höfle G, Reichenbach H. Disorazol A, an efficient inhibitor of eukaryotic organisms isolated from myxobacteria. J. Antibiot. 1994;48:31–35. doi: 10.7164/antibiotics.48.31. [DOI] [PubMed] [Google Scholar]
- 181.Chu M, Truumees I, Mierzwa R, Terracciano J, Patel M, Loebenberg D, Kaminski JJ, Das P, Puar MS. Sch 54445: A new polycyclic xanthone with highly potent antifungal activity produced by Actinoplanes sp. J. Nat. Prod. 1997;60:525–528. doi: 10.1021/np960737v. [DOI] [PubMed] [Google Scholar]
- 182.Barrero AF, Sánchez JF, Elmerabet J. An efficient synthesis of the antifungal dilactone LL-Z1271α and of other biologically active compounds. Tetrahedron Lett. 1995;36:5251–5254. [Google Scholar]
- 183.Morris SA, Curotto JE, Zink DL, Dreikom S, Jenkins R, Bills GF, Thompson JR, Vicente F, Basilio A, Liesch JM, Schwartz RE. Sonomolides A and B, new broad spectrum antifungal agents isolated from a Coprophilous fungus. Tetrahedron Lett. 1995;36:9101–9104. [Google Scholar]
- 184.Wang H-J, Gloer JB, Scott JA, Malloch D. Coniochaetones A and B: New antifungal benzopyranones from the coprophilous fungus Coniochaeta Saccardoi. Tetrahedron Lett. 1995;36:5847–5850. [Google Scholar]
- 185.Whyte AC, Gloer KB, Gloer JB, Koster B, Malloch D. New antifungal metabolites from the coprophilous fungus Cercophora sordarioides. Can. J. Chem. 1997;75:768–772. [Google Scholar]
- 186.Li E, Clark AM, Hufford CD. Antifungal evaluation of pseudolaric acid B, a major constituent of Pseudolarix kaempferi. J. Nat. Prod. 1995;58:57–67. doi: 10.1021/np50115a007. [DOI] [PubMed] [Google Scholar]
- 187.Kiffe M, Schummer D, Höfle G. Antibiotics from Gliding Bacteria, LXXIX. - Chemical modification of the antifungal macrolide soraphen A1α: Deoxygenation in the south-east ring segment. Liebigs Ann. /Recl. 1997;1997:245–252. [Google Scholar]
- 188.Brill GM, Chen RH, Rasmussen RR, Whittern DN, McAlpine JB. Calbistrins, novel antifungal agents produced by Penicillium restrictum. II. Isolation and elucidation of structure. J. Antibiot. (Tokyo) 1993;46:39–47. doi: 10.7164/antibiotics.46.39. [DOI] [PubMed] [Google Scholar]
- 189.Kobayashi H, Sunaga R, Furihata K, Morisaki N, Iwasaki S. Isolation and structures of an antifungal antibiotic, fusarielin A, and related compounds produced by a Fusarium sp. J. Antibiot. 1994;48:42–52. doi: 10.7164/antibiotics.48.42. [DOI] [PubMed] [Google Scholar]
- 190.Chapman SW, Sullivan DC, Cleary JD. In search of the holy grail of antifungal therapy. Trans. Am. Clin. Climatol. Assoc. 2008;119:197–215. [PMC free article] [PubMed] [Google Scholar]
- 191.Saccharomyces Genome Database. [Accessed 06/29/2015]; http://www.yeastgenome.org/ [Google Scholar]
- 192.Saccharomyces Genome Database (SGD) [Accessed 06/29/2015];Observable: inviable. Gene list. http://www.yeastgenome.org/observable/inviable/overview. [Google Scholar]
- 193.Candida Genome Database. [Accessed 06/29/2015]; http://www.candidagenome.org/ [Google Scholar]
- 194.Aspergillus Genome Database. [Accessed 06/29/ 2015]; http://www.aspergillusgenome.org/ [Google Scholar]
- 195.Fungal and Oomycete Genomics Resources Database. (FungiDB) [Accessed 06/29/2015]; http://fungidb.org/fungidb/ [Google Scholar]
- 196.Saccharomyces Genome Database. [Accessed 06/29/2015];BLAST searches to fungal genomes. http://yeastgenome.org/blast-fungal. [Google Scholar]
- 197.Agarwal AK, Tripathi SK, Xu T, Jacob MR, Li X-C, Clark AM. Exploring the molecular basis of antifungal synergies using genome-wide approaches. Front. Microbiol. 2012;3:115. doi: 10.3389/fmicb.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]