Abstract
Heightened reactivity to uncertain threat (U-threat) is an important individual difference factor that may characterize fear-based internalizing psychopathologies (IPs) and distinguish them from distress/misery IPs. To date, however, the majority of existing research examining reactivity to U-threat has been within individuals with panic disorder and major depressive disorder (MDD) and no prior study has directly tested this hypothesis across multiple IPs. The current study therefore explored whether heightened reactivity to U-threat is a psychophysiological indicator of fear-based psychopathology across five groups: current 1) social anxiety disorder (SAD), 2) specific phobia (SP), 3) generalized anxiety disorder (GAD), 4) MDD, and 5) individuals with no history of psychopathology (controls). All 160 adults completed a well-validated threat-of-shock task designed to probe responses to predictable (P-) and U-threat. Startle eyeblink potentiation was recorded as an index of aversive arousal. Results indicated that individuals with SAD and SP evidenced greater startle potentiation to U-threat, but not P-threat, relative to individuals with GAD, MDD and controls (who did not differ). The current findings, along with the prior panic disorder and MDD literature, suggest that heightened reactivity to U-threat is a psychophysiological indicator of fear-based disorders and could represent a neurobiological organizing principle for internalizing psychopathology. The findings also suggest that individuals with fear disorders generally display a hypersensitivity to uncertain aversive events, which could contribute to their psychopathology.
Keywords: uncertain threat, social anxiety disorder, specific phobia, fear, distress
Introduction
Internalizing psychopathologies (IPs), including mood and anxiety disorders, commonly co-occur within the same individual (Kessler et al., 1994, 2005). Among individuals with a current IP, between 35 and 65% have a lifetime diagnosis of a separate, additional IP (Brown, Campbell, Lehman, Grisham, & Mancill, 2001; Kessler et al., 2005). This pattern of comorbidity has called into question the validity of discrete IP diagnoses and our current psychiatric nosology (Insel et al., 2010; Kendell & Jablensky, 2003). It has also stimulated a considerable amount of research on the unique and shared aspects of IPs in an effort to better understand the etiology and pathophysiology of depression and anxiety and potentially refine our diagnostic system based on empirically validated organizing principles.
Towards this end, evidence indicates that all IPs share several factors including high levels of negative affect and neuroticism (Clark & Watson, 1991; Shankman & Klein, 2003; Watson, 2009). Data also suggest that there are important distinctions. Most notably, several large-scale factor analytic and family studies indicate that the IPs cluster into two distinct, broad factors labeled ‘distress/misery’ and ‘fear’ (Hettema, Prescott, Myers, Neale, & Kendler, 2005; Slade & Watson, 2006; Vollebergh et al., 2001; Watson, 2005). Twin studies of comorbidity have also found these two factors, highlighting that this structure occurs both phenotypically and genotypically (Kendler, Prescott, Myers, & Neale, 2003). Major depressive disorder (MDD), dysthymia, and generalized anxiety disorder (GAD) each load onto the ‘distress/misery’ dimension, whereas panic disorder, social anxiety disorder (SAD), and specific phobia load onto ‘fear.’ The findings for post-traumatic stress disorder (PTSD) and obsessive-compulsive disorder (OCD) are mixed as PTSD and OCD include aspects of both distress/misery and fear (Raines, Allan, Oglesby, Short, & Schmidt, 2015; Watson, 2009). Importantly, laboratory studies support the above distinction and have begun to identify unique behavioral and neural correlates of the two factors (Lang, McTeague, & Bradley, 2016; McTeague & Lang, 2012; Shankman et al., 2013; Nelson et al., 2015). This line of work is noteworthy for many reasons as it broadly seeks to link IP diagnoses to core neurobiological systems and mechanisms of dysfunction consistent with the National Institute on Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative (Cuthbert, 2014; Insel et al., 2010; Kozak & Cuthbert, 2016). It is the hope of both RDoC, and the field at large, that by examining basic, transdiagnostic mechanisms underlying psychopathology, the field will uncover neurobiologically-based diagnostic phenotypes that will aid in the development of targeted, mechanistically-driven interventions.
One neurobiological construct that may characterize fear-based IPs, and distinguish them from distress/misery disorders, is heightened reactivity to uncertain threat (U-threat), defined as threat that is unpredictable in its temporality, intensity, frequency or duration. U-threat is a specific form of stress/threat that elicits a generalized feeling of apprehension and hypervigilance that is not associated with a clearly identifiable source, referred to as anticipatory anxiety (Barlow, 2000; Davis, 1998; Jackson, Nelson, & Proudfit, 2015). U-threat is in contrast with predictable threat (P-threat), which is signaled by a discrete cue and elicits a phasic response to an identifiable stimulus that is time-locked to the threat (Barlow, 2000; Davis, Walker, Miles, & Grillon, 2010). U-threat and P-threat produce distinguishable aversive states that are pharmacologically distinct (Grillon et al., 2006) and mediated by overlapping, but separable, neural circuits (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Davis, 2006). More specifically, both fear and anxiety are mediated by initial activation of the basolateral nucleus of the amygdala (BLA) by sensory inputs (Tovote, Fadock, & Lüthi, 2015). From the BLA, however, the two circuits diverge (see Davis et al., 2010 for a review of the neural circuits mediating fear vs. anxiety). With regard to fear, the BLA activates the medial division of the central nucleus of the amygdala, which directs information to the hypothalamus and brainstem to generate ‘fight or flight’ responses. For anxiety, the BLA and the paraventricular nucleus of the hypothalamus together activate the lateral division of the central nucleus of the amygdala, which engages the bed nucleus of the stria terminalis (BNST) – a region essential for sustained apprehension (Walker, Toufexis, & Davis, 2003). It is the BNST that then sends outputs to the same hypothalamic and brainstem targets implicated in fear to produce anticipatory anxiety. Although fear and anxiety, and therefore response to P- and U-threat are clearly related, they are also distinct and accumulating evidence points to the fact that individual differences in reactivity to U-threat (specifically) play a role in the onset and maintenance of multiple forms of psychopathology, especially internalizing disorders (see Grupe & Nitschke, 2013 for a review). Thus, in recent years, reactivity to U-threat has emerged as a key, transdiagnostic clinical construct.
In order to isolate reactivity to U-threat in the laboratory, Grillon and colleagues developed the widely used No-predictable-unpredictable threat (NPU) paradigm (Schmitz & Grillon, 2012). Throughout the task, startle eyeblink responses are recorded as an index of aversive arousal, which is useful as a research tool given that startle is sensitive to changes in both valence and arousal (Lang, Bradley, & Cuthbert, 1990; Lang, 1995) and is relatively easy to record across multiple species (Davis, 1998, 2006). To date, there have been a number of studies that have utilized the NPU paradigm and related tasks that manipulate the predictability of threat. Preliminary findings indicate that there is an important association between reactivity to U-threat and fear-based psychopathology. For instance, across several indices of aversive responding (e.g., startle, neural response, skin conductance) individuals with panic disorder have been shown to exhibit heightened aversive reactivity to U-threat relative to healthy controls (Gorka, Nelson, Phan, & Shankman, 2014; Grillon et al., 2008; Shankman et al., 2013; Richter et al., 2012). Reactivity to U-threat has also been shown to uniquely predict family history of panic disorder, but not MDD, above and beyond an individuals’ own psychopathology (Nelson et al., 2013), suggesting that reactivity to U-threat is a familial risk factor for panic disorder. Although there have been only a handful of studies on U-threat in fear-based disorders other than panic disorder, there is some initial evidence to suggest a similar pattern in individuals with SP (Straube, Mentzel, & Miltner, 2007) and PTSD (Grillon et al., 2008; Simmons et al., 2013).
As for the distress/misery disorders, one prior study showed that individuals with GAD do not display heightened reactivity to U-threat (Grillon et al., 2009). Our laboratory has also demonstrated in several studies that individuals with MDD and healthy controls do not differ in their reactivity to U-threat (Gorka et al., 2014; Shankman et al., 2013), and that reactivity to U-threat is not associated with familial risk for MDD (Nelson et al., 2013). Meanwhile, however, Grillon and colleagues have found the opposite – individuals with MDD display greater reactivity to U-threat compared with healthy controls (Grillon, Franco-Chaves, Mateus, Ionescu, & Zarate, 2013; Robinson et al., 2012). The findings within depression are therefore mixed.
Taken together, converging evidence suggests that heightened reactivity to U-threat is a clinically important individual difference factor that characterizes fear-based IPs and may distinguish them from distress/misery IPs. This hypothesis has been propelled by the panic disorder literature and to date very few studies have investigated whether principal fear disorders besides panic disorder (i.e., SAD and SP) are associated with heightened reactivity to U-threat. Although a few studies have shown that PTSD is associated with reactivity to U-threat, PTSD loads onto the fear and distress/misery dimension making it difficult to conceptualize in the two-factor framework (Forbes et al., 2011). There have also been some mixed findings within the depression literature, calling into question the specificity of the association between reactivity to U-threat and fear-based IPs. Research examining reactivity to U-threat across distress/misery and fear IPs, including SAD and SP, is therefore critically needed to clarify this literature.
The current study was designed to address these gaps and examine whether startle potentiation to U-threat is uniquely associated with fear-based psychopathology. As noted above, our lab has shown in prior papers that panic disorder is related to heightened reactivity to U-threat (Gorka et al., 2014; Nelson et al., 2013; Shankman et al., 2013). Therefore, the current study sought to test whether these panic disorder findings generalized to other forms of fear-based psychopathology. The sample was accordingly comprised of 5 diagnostic groups: those with current 1) SAD, 2) SP, 3) GAD, 3) MDD, and 4) no lifetime history of psychopathology (i.e., controls). All participants completed the NPU-threat paradigm during which startle eyeblink potentiation was recorded. It was hypothesized that individuals with SAD and SP would display heightened startle potentiation to U-threat, but not P-threat, relative to individuals with GAD and MDD and healthy controls who would not differ from each other.
Methods
Participants and Procedure
Participants were drawn from two larger studies designed to examine individual differences in threat responding across internalizing disorders. Both studies were conducted at the University of Illinois at Chicago, used similar recruitment techniques, and had identical laboratory protocols, making them well-suited for combined analyses. All participants were recruited via advertisements posted in the Chicago community, local psychiatric clinics, and nearby college campuses. A variety of advertisements were used to target different populations in an effort to enroll a diverse, internalizing disorder patient sample. Of the 160 volunteers included in the current study, 80 came from Study 1 and 80 from Study 2. Both protocols were approved by the university Institutional Review Board and participants provided written informed consent. In both studies, participants completed a set of laboratory tasks, a battery of questionnaires and a semi-structured clinical interview, and received cash as payment for participation.
Study 1 was designed to recruit individuals with no lifetime history of psychopathology (i.e., controls) and treatment seeking adults with internalizing psychopathology severe enough to warrant randomization to cognitive-behavioral therapy or selective serotonin reuptake inhibitors (SSRIs). Participants were required to be 18–65 years old and able to provide consent. Exclusion criteria included a major active medical or neurological problem, lifetime history of mania or psychosis, any contraindication to receiving SSRIs, being already engaged in psychiatric treatment (including medication), history of traumatic brain injury, left-handedness, and being pregnant. Current and lifetime diagnoses were assessed using the Structured Clinical Interview for DSM-5 (SCID; First, Williams, Karg, & Spitzer, 2015). A consensus panel of at least 3 study staff/trained clinicians determined each subjects’ eligibility for Study 1 and if there were co-occurring current disorders, which was the primary disorder warranting treatment. This decision was based on which IP symptoms were determined to be most severe and impairing at the time of study admission based on clinician interpretation and participant self-report. A distinction between primary vs. secondary IP was made in-order to inform later treatment decisions (e.g., assignment to a particular SSRI, use of a particular cognitive-behavioral therapy manual) and track treatment progress. Given the highly co-occurring nature of internalizing psychopathologies, and recent initiatives to conduct empirical investigations with representative clinical samples (Morris & Cuthbert, 2012), individuals were not excluded for comorbid disorders. Rather, they were classified by their clinician-determined primary diagnosis in order to test whether individuals presenting to treatment with a primary fear-based disorder, in a more naturalistic, real-world setting, would exhibit heightened reactivity to U-threat. Of the 80 individuals from Study 1, 12 were healthy controls, 18 had primary MDD, 21 had primary GAD, and 29 had primary SAD. No participants from Study 1 had a primary SP diagnosis (likely due to the more mild nature of SP relative to the other IPs).
A major aim of Study 2 was to examine threat responding within families and thus, required the enrollment of biological, sibling dyads. Participants were required to be between the ages of 18 and 30, be able to provide consent, and have at least one biological sibling willing and able to participate. Exclusion criteria included a personal or family history of mania or psychosis, a major medical or neurological illness, a history of serious head trauma, and left-handedness. Participants were not required to have DSM diagnoses but current and lifetime psychopathology was assessed via the same SCID interview that was used in Study 1. It is important to highlight that given these differences in aims, Study 1 and Study 2 differed in their recruitment of individuals with psychopathology and the way in which comorbidity was coded and handled. Most notably, within Study 2, there was no initial determination of a primary vs. secondary IP. Therefore, for the current study, participants were only included if they had one current IP and no co-occurring current IPs to ensure that the present IP was primary. Of the 80 individuals from Study 2, 29 were healthy controls, 7 had primary (i.e., current) MDD, 8 had primary GAD, 12 had primary SAD, and 24 had primary SP.
Threat Task
All participants completed the same laboratory procedures and threat task, which has been extensively described by our group (Gorka et al., 2013, 2015; Shankman et al., 2013). In brief, prior to the task, shock electrodes were placed on participants’ left wrist and a shock work-up procedure was completed to identify the level of shock intensity each participant described as “highly annoying but not painful” (between 1–5 mA). Participants also completed a 2-min startle habituation task pre- and post- electrode placement to reduce early, exaggerated startle potentiation. The task itself was modeled after Grillon and colleagues NPU threat task and included no shock (N), predictable shock (P), and unpredictable shock (U) conditions. Text at the bottom of the computer monitor continuously informed participants of the current condition. Each condition lasted 145-s, during which a 4-s visual countdown (CD) was presented six times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 15 to 21-s during which only the text describing the condition was on the screen. No shocks were delivered during the N condition. A shock was delivered every time the CD reached 1 during the P condition. Shocks were delivered at random during the U condition (both during the CD and ISI). Startle probes were administered during both the CD and ISI and probes and shocks were separated by at least 10-s. Each condition was presented two times in a randomized order (counterbalanced). Participants received 24 total electric shocks (12 in P; 12 in U) and 60 total startle probes (20 in N; 20 in P; 20 in U).
Startle Data Collection and Processing
Startle data were acquired using BioSemi Active Two system (BioSemi, Amsterdam, The Netherlands). Stimuli were administered using Presentation (Albany, CA) in Study 1 and PSYLAB (Contact Precision Instruments, London, UK) in Study 2. Electric shocks lasted 400-ms and acoustic startle probes were 40-ms duration, 103-dB bursts of white noise with near-instantaneous rise time presented binaurally through headphones.
Startle responses were recorded from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye. The ground electrode was located at the frontal pole (Fpz) of an electroencephalography cap that participants were wearing as part of the larger studies. One startle electrode was placed 1-cm below the pupil and the other was placed 1-cm lateral of that electrode. Data were collected using a bandpass filter of DC-500-Hz at a sampling rate of 2000-Hz.
Blinks were processed (and scored) according to published guidelines (Blumenthal et al., 2005). These steps included applying a 28 Hz high-pass filer, rectifying, and then smoothing using a 40 Hz low-pass filter. Peak amplitude was defined within 20–150-ms following the probe onset relative to baseline (i.e., average activity for the 50-ms preceding probe onset). Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as non-responses if activity during the post-stimulus time frame did not produce a peak that is visually differentiated from baseline. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency. Blink magnitude values (i.e., condition averages include values of 0 for non-responses) were used in all analyses.
Internalizing Symptoms
Participants in both studies completed the well-validated Inventory for Depression and Anxiety Symptoms-II (IDAS-II; Watson et al., 2012) – a 99-item self-report measure designed to assess symptoms of internalizing psychopathology during the previous two weeks. Participants are asked to respond to each item using a 5-point Likert scale ranging from 1 (not at all) to 5 (extremely) and scores are summed to create subscales that are linked to DSM-IV (APA, 2000) mood and anxiety symptom profiles. The scale yields 19 factor analytically derived symptom scales: depression, dysphoria, lassitude, insomnia, suicidality, appetite gain, appetite loss, ill-temper, well-being, panic, social anxiety, claustrophobia, euphoria, mania, traumatic intrusions, traumatic avoidance, and tendencies for checking, ordering and cleaning. Prior research has demonstrated that the IDAS has excellent psychometric properties including internal consistency, test-retest reliability, and convergent and discriminant validity (Watson et al., 2012). Reliability of the IDAS subscales in the current study ranged from good to excellent (α=0.79 – 0.91). Within the current study, the IDAS was used to characterize the sample. Subscale means by diagnostic group are presented in Table 1.
Table 1.
Demographics and clinical characteristics
| HC (n=41) | MDD (n=25) | GAD (n=29) | SAD (n=41) | SP (n=24) | Significant Group Differences | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 25.6 (12.7) | 26.2 (8.2) | 27.9 (8.1) | 23.0 (5.7) | 22.8 (2.9) | GAD > SAD, SP |
| Sex (% female) | 60.5% | 92.0% | 72.4% | 73.2% | 54.2% | MDD > HC, SP |
| Race/Ethnicity | ||||||
| Caucasian | 31.7% | 44.0% | 65.5% | 56.1% | 37.5% | GAD, SAD > All Others |
| African American | 14.6% | 24.0% | 13.8% | 9.8% | 12.5% | MDD > All Others |
| Hispanic | 24.4% | 4.0% | 3.4% | 26.8% | 25.0% | MDD, GAD < All Others |
| Asian | 26.8% | 8.0% | 10.3% | 4.9% | 16.7% | HC > SP > MDD, GAD > SAD |
| Other/Biracial | 2.4% | 20.0% | 10.3% | 2.4% | 8.3% | MDD > GAD, SP > SAD, HC |
| Comorbid Diagnoses and Meds | ||||||
| Total Num. of Current IPs | 0.0 (0.0) | 1.6 (0.8) | 2.0 (1.1) | 1.4 (0.8) | 1.0 (0.0) | GAD > MDD, SAD > SP > HC |
| Other Current Fear IP | 0.0% | 36.0% | 58.6% | 7.3% | 0.0% | GAD > MDD > SAD > SP, HC |
| Other Current Distress IP | 0.0% | 24.0% | 34.5% | 26.8% | 0.0% | GAD, SAD, MDD > SP, HC |
| Other Lifetime Fear IP | 0.0% | 8.0% | 31.0% | 26.8% | 16.7% | GAD, SAD > SP, MDD > HC |
| Other Lifetime Distress IP | 0.0% | 12.0% | 51.7% | 53.6% | 33.3% | GAD, SAD > SP > MDD > HC |
| Current AUD or SUD | 0.0% | 0.0% | 3.4% | 4.9% | 12.5% | SP > SAD, GAD, MDD, HC |
| Lifetime AUD | 0.0% | 12.0% | 10.3% | 19.5% | 37.5% | SP > SAD, MDD, GAD > HC |
| Lifetime SUD | 0.0% | 4.0% | 17.2% | 19.5% | 29.2% | SP > GAD, SAD > MDD > HC |
| Current Eating Disorder | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | N/A |
| Lifetime Eating Disorder | 0.0% | 4.0% | 0.0% | 2.4% | 0.0% | N/A |
| Taking Psychotropic Meds | 0.0% | 4.0% | 0.0% | 4.9% | 8.3% | N/A |
| IDAS Subscale Scores | ||||||
| Depression | 27.7 (7.1) | 67.5 (7.4) | 56.5 (11.3) | 50.5 (13.1) | 38.0 (9.2) | MDD > GAD, SAD > SP, HC |
| Dysphoria | 12.1 (3.1) | 35.6 (4.1) | 30.0 (6.9) | 24.6 (8.0) | 17.8 (5.8) | MDD > GAD > SAD > SP > HC |
| Lassitude | 8.6 (2.5) | 20.8 (5.1) | 17.3 (5.5) | 15.4 (4.2) | 12.0 (4.3) | MDD > SAD, GAD > SP > HC |
| Insomnia | 8.8 (4.1) | 15.7 (5.3) | 15.7 (5.2) | 13.8 (6.3) | 11.7 (3.9) | HC < All Others |
| Suicidality | 6.1 (0.5) | 10.1 (4.1) | 7.0 (2.3) | 7.1 (1.9) | 6.5 (0.9) | MDD > All Others |
| Appetite Loss | 4.0 (2.2) | 8.8 (2.7) | 6.7 (2.8) | 6.5 (3.3) | 5.0 (2.0) | MDD > All Others |
| Appetite Gain | 3.8 (1.3) | 6.2 (3.4) | 6.9 (3.1) | 5.4 (2.4) | 4.7 (2.5) | HC < All Others |
| Ill-Temper | 5.5 (1.0) | 11.2 (4.4) | 11.5 (4.9) | 9.2 (3.9) | 7.5 (2.9) | |
| Well-Being | 28.0 (7.0) | 12.5 (4.4) | 17.9 (5.2) | 20.1 (5.6) | 23. 7 (7.2) | HC > SP, SAD > MDD, GAD |
| Mania | 5.9 (1.7) | 8.6 (3.2) | 10.6 (4.2) | 8.8 (3.9) | 7.6 (3.8) | HC < SP, MDD, GAD, SAD; SP < GAD |
| Euphoria | 6.7 (1.8) | 6.1 (1.4) | 7.0 (2.2) | 6.7 (2.2) | 7.3 (3.2) | N/A |
| Panic | 8.7 (2.4) | 10.6 (3.3) | 12.9 (5.2) | 14.5 (5.5) | 10.6 (3.3) | HC < All Others |
| Social Anxiety | 6.8 (1.3) | 13.2 (5.6) | 14.4 (5.5) | 15.0 (4.2) | 8.9 (3.8) | HC < SP < MDD < GAD, SAD |
| Claustrophobia | 5.1 (0.5) | 6.8 (3.0) | 6.7 (3.6) | 6.5 (2.5) | 6.1 (2.1) | N/A |
| Traumatic Intrusions | 4.5 (1.4) | 9.0 (3.6) | 8.2 (3.6) | 6.7 (2.4) | 5.8 (1.6) | HC < SP < All Others |
| Traumatic Avoidance | 5.0 (1.9) | 10.2 (3.9) | 8.6 (4.2) | 8.2 (3.7) | 8.2 (4.1) | HC < All Others |
| Checking | 3.9 (1.3) | 5.2 (2.7) | 7.7 (3.9) | 5.6 (2.7) | 5.1 (2.6) | HC < All Others |
| Cleaning | 8.0 (1.4) | 10.2 (5.0) | 9.7 (3.9) | 9.8 (4.1) | 10.7 (4.4) | N/A |
| Ordering | 6.9 (2.4) | 7.5 (2.8) | 10.4 (5.7) | 8.5 (2.8) | 8.6 (3.8) | N/A |
| Startle Magnitude | ||||||
| NCD | 48.4 (48.7) | 52.9 (57.1) | 42.1 (35.4) | 57.9 (90.2) | 55.5 (53.5) | N/A |
| NISI | 38.7 (49.5) | 53.7 (53.7) | 33.6 (29.3) | 52.2 (53.8) | 53.1 (54.5) | SAD > GAD |
| PCD | 62.8 (62.1) | 76.9 (68.4) | 57.2 (49.2) | 86.6 (99.4) | 63.8 (40.9) | SAD > All Others |
| PISI | 41.8 (45.1) | 50.0 (49.9) | 43.1 (30.3) | 62.6 (114.0) | 53.8 (37.3) | SAD > GAD, HC |
| UCD | 61.7 (60.7) | 84.9 (72.5) | 70.5 (58.9) | 127.2 (107.8) | 108.6 (82.8) | SAD, SP > All Others |
| UISI | 54.5 (44.5) | 70.9 (66.9) | 56.8 (39.2) | 108.6 (114.5) | 100.9 (80.6) | SAD, SP > All Others |
Note. Significant group differences were tested using pair-wise comparisons (p < .05, chi-square test for categorical variables and Tukey’s honestly significant difference test for continuous variables). Fear disorders include panic disorder, social anxiety disorder, and specific phobia. Distress disorders include major depressive disorder, generalized anxiety disorder, obsessive-compulsive disorder and dysthymia. AUD = alcohol use disorder; SUD = substance use disorder; IP = internalizing psychopathology. N = no-shock; p = predictable shock; U = unpredictable shock; CD = countdown; ISI = interstimulus interval.
Data Analysis Plan
We first conducted a series of chi-square or one-way analyses of variance (ANOVA) to test whether there were any group differences in demographics or current comorbid diagnoses. Any of the tested variables that were found to vary across groups were subsequently included as covariates in our primary model. Prior to hypothesis testing we also conducted a 3 (Condition: N, P, U) x 2 (Cue: CD, ISI) repeated measures ANOVA to confirm that across all subjects the task elicited startle to P- and U-threat as designed. Consistent with prior startle studies (e.g., Shankman et al., 2013), we also created startle potentiation scores for the P- and U-threat conditions to account for baseline individual differences in startle magnitude. For P-threat we subtracted startle magnitude during NCD from PCD. For U-threat, we subtracted average startle magnitude during NCD and NISI from average startle magnitude during UCD and UISI because the two phases of the unpredictable condition (and neutral condition) have the same meaning during the task.
To test for group differences, we next conducted a repeated measures ANOVA where potentiation to the threat conditions (P vs. U) was specified as a within-subjects variable and group (controls vs. GAD vs. MDD vs. SAD vs. SP) as a between-subjects variable. Initially, no covariates were included in order to establish the pattern of results. A significant threat condition x group interaction was followed-up by testing the effect of group at each level of threat using two ANOVAs – one for P-threat and one for U-threat. At each level of threat, significant group effects were probed using post-hoc Fisher’s least significant difference (LSD) tests. In addition to between-group differences, we also tested within-group differences in startle potentiation to U- and P-threat using a series of within group repeated measures ANOVAs.
The same omnibus repeated measures ANOVA was then re-run with several important covariates to determine whether the pattern of results was better accounted for by other factors. The identification of covariates is described below. In brief, because the diagnostic groups differed on biological sex, race, and total number of current comorbid IPs, these variables were included in the model as covariates. Study (1 or 2) was also added as a covariate given differences in recruitment and enrollment procedures. It is important to note that race was originally collected as a 5-level variable (Caucasian, African American, Asian, Hispanic, and Other/Biracial) but as is presented in Table 1, the 5-level variable was not evenly distributed within, or across, groups. Race was therefore re-coded into a 2-level variable for inclusion in the model. Caucasian race was the majority and therefore specified as the referent group (‘0’) and non-Caucasian race was the comparison (‘1’). A significant threat condition x group interaction was followed-up using procedures identical to the original model. For all ANOVAs, Geisser-Greenhouse p-value adjustments were applied when relevant.
Results
Descriptives and Sample Characteristics
Demographic and clinical characteristics of the sample (Study 1 and Study 2) are presented in Table 1. The groups differed on biological sex (χ2 [4]= 12.76, p< 0.05) and race (χ2 [16]= 12.76, p< 0.05). Within the patient groups there were also differences in the total number of current comorbid IPs (F[3, 118] = 8.28, p< 0.01; LSD post-hoc tests revealed GAD > SAD = MDD > SP). With regard to other Axis I disorders, only alcohol use disorder (AUD), illicit substance use disorders (SUDs), and eating disorders were assessed and the groups did not differ in the prevalence of current, comorbid AUD, SUD or eating disorders (all ps> 0.05). Given these results, sex, race, and total number of current IPs (in addition to study) were included as covariates in subsequent analyses.
Startle Task
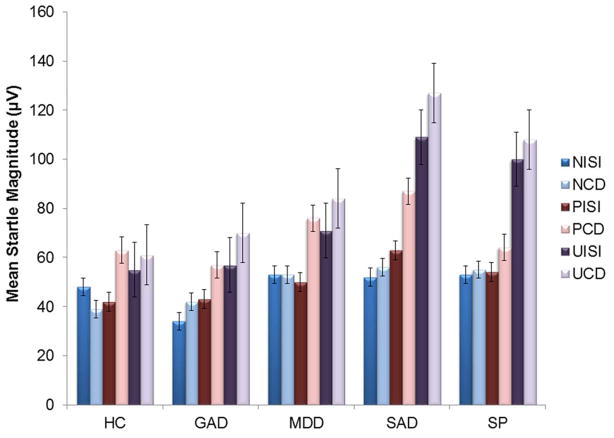
There was a main effect of condition, F(2, 318)= 43.88, p< 0.01, and cue, F(1, 159)=58.89, p< 0.01, and a condition x cue interaction, F(2, 318)= 23.90, p< 0.01. During the CD, startle differed among the conditions, F(2, 318) = 40.34, p< 0.01, such that NCD < PCD < UCD (ps< 0.001). During the ISIs, startle also differed among the conditions, F(2, 318) = 43.56, p< 0.01, such that UISI was greater than PISI and NISI (ps< 0.001) but PISI and NISI did not differ (p= 0.39). The task therefore elicited startle magnitude to threat conditions as designed (Figure 1). There was no significant main effect of study, or any study x condition or cue interactions, indicating the pattern of results was the same across both labs.
Fig. 1.
Mean startle magnitude values during each condition and cue type of the threat task by diagnostic group. N= no-shock; P= predictable shock; U = unpredictable shock; CD = countdowns; ISI = inter-stimulus interval; HC = healthy control; MDD = major depressive disorder; GAD = generalized anxiety disorder; SAD = social anxiety disorder; SP = specific phobia. Bars reflect standard error.
Diagnostic Group Differences
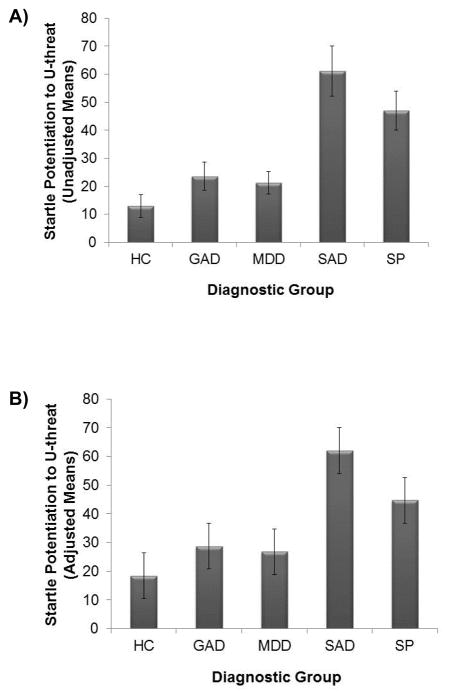
We first ran an omnibus ANOVA without covariates (Model 1). Results of this model are presented in Table 2. There was a significant main effect of group; however, this was qualified by a threat condition x group interaction. Follow-up ANOVAs revealed that the groups differed on startle reactivity to U-threat, F(4, 159)= 8.32, p< 0.001, but not P-threat, F(4, 159)= 1.58, ns. Post-hoc LSD comparisons revealed that individuals with SAD and SP evidenced greater startle potentiation to U-threat relative to healthy controls and individuals with GAD or MDD (all ps< 0.02). Meanwhile, individuals in the healthy control, GAD, and MDD groups did not differ from one another (ps > 0.32), and individuals in the SAD and SP groups did not differ from one another (p= 0.26) (i.e., SAD = SP > controls = GAD = MDD; see Figure 2a).
Table 2.
Results of the ANOVAs testing the effect of group on startle potentiation to P- and U-threat.
| Variable | df | F | p-value |
|---|---|---|---|
| Model 1 – No Covariates | |||
| Threat Condition* | 1, 155 | 12.41 | <0.01 |
| Group* | 4, 153 | 5.27 | <0.01 |
| Threat Condition x Group* | 4, 153 | 2.82 | 0.03 |
| Model 2 – With Covariates | |||
| Threat Condition | 1, 151 | 0.02 | 0.88 |
| Group* | 4, 151 | 4.30 | <0.01 |
| Sex | 1, 151 | <0.01 | 0.95 |
| Race | 1, 151 | 0.06 | 0.80 |
| Num. of Current IPs | 1, 151 | 1.34 | 0.25 |
| Study | 1, 151 | 0.15 | 0.70 |
| Threat Condition x Group* | 4, 151 | 2.69 | 0.03 |
| Threat Condition x Sex | 1, 151 | 0.76 | 0.39 |
| Threat Condition x Race | 1, 151 | 2.38 | 0.13 |
| Threat Condition x Num. of Current IPs | 1, 151 | 0.01 | 0.94 |
| Threat Condition x Study | 1, 151 | 3.42 | 0.08 |
Note.
p < .05. Threat Condition = predictable or unpredictable; Race is coded as binary variable: Caucasian relative to non-Caucasian.
Fig. 2.
A) Mean startle potentiation to uncertain threat across each diagnostic group unadjusted for covariates. B) Mean startle potentiation to uncertain threat across each diagnostic group adjusted for sex, race, number of comorbid internalizing disorders and study. Bars reflect standard error. HC = healthy control; MDD = major depressive disorder; GAD = generalized anxiety disorder; SAD = social anxiety disorder; SP = specific phobia. U-threat = uncertain threat.
With regard to within group comparisons, results indicated that there were no differences in startle potentiation to P- and U-threat within the HC and MDD groups (p > 0.34), and the GAD group though there was a trend-level effect for U-threat greater than P-threat, F(1, 28) = 3.98, p =0.06. Meanwhile, SAD and SP individuals showed greater startle to U-threat compared with P-threat (ps< 0.05).
Model 1 was then re-run with covariates (Model 2). These results are also presented in Table 2 and indicate that the above effects are still observed when statistically controlling for sex, race, number of current comorbid IPs, and study. As anticipated, there was a significant main effect of group that was qualified by a threat condition x group interaction. The groups differed on startle reactivity to U-threat, F(4, 152)= 8.81, p< 0.001, but not P-threat, F(4, 152)= 1.54, ns. Identical to above, individuals with SAD and SP evidenced greater startle to U-threat relative to the other three groups (who did not differ from each other; SAD = SP > controls = GAD = MDD; see Figure 2b). In addition, the SAD and SP groups showed robust startle differentiation between U- and P-threat (ps <0.05), whereas HC, MDD and GAD participants displayed comparable responding across the two threat conditions (ps >0.09).
Discussion
Accumulating evidence suggests that heightened reactivity to U-threat is an important individual difference factor that characterizes fear-based IPs (Grillon et al., 2008; Shankman et al., 2013). However, the majority of existing research on this topic has been centered on panic disorder and it is unclear whether heightened reactivity to U-threat is observed in other fear disorders, particularly SAD and SP. The specificity of reactivity to U-threat to fear-based IPs has also been called into question and no study to date has directly compared reactivity across multiple fear and distress/misery IPs. The current study was therefore designed to address the gaps in the existing literature and test whether heightened reactivity to U-threat is a psychophysiological indicator of fear-based psychopathology. In support of this hypothesis, current results indicate that individuals with SAD and SP evidence greater startle potentiation to U-threat, but not P-threat, relative to individuals with GAD, MDD and healthy controls. Moreover, the findings revealed no differences in reactivity to U-threat between the two principal fear disorders (SAD = SP), and between the distress/misery disorders and healthy controls (GAD = MDD = controls), indicating that, as hypothesized, reactivity to U-threat is elevated in fear-based IPs and not distress/misery IPs. Along with prior data from our lab and others (e.g., Grillon et al., 2008; Shankman et al., 2013), these results suggest that individuals with primary fear-based disorders display an exaggerated sensitivity to uncertain threat and that startle potentiation represents a relatively easy-to-measure (Lang, 1995) neurobiological organizing construct for internalizing psychopathology.
As noted above, the current findings indicate that individuals with fear-based IPs have an exaggerated sensitivity to U-threat, which importantly fits with contemporary theoretical conceptualizations of panic disorder, SAD, and SP. According to both the DSM-5 (APA, 2013) and the broader literature, all traditional fear disorders are characterized by hyperarousal and exaggerated anticipatory anxiety in response to temporally unpredictable or ambiguous feared aversive stimuli. Although the specific type of aversive stimuli/threat varies by disorder (i.e., panic attacks in panic disorder, social situations in SAD, and phobia-related stimuli in SP; see Barlow, 2000), all threats are inherently ambiguous or uncertain in some way which is a key characteristic given that uncertainty diminishes psychophysiological preparedness and drives anticipatory anxiety (Grupe & Nitschke, 2013). For example, an individual with panic disorder and SAD fear different types of threat; however, both experience anticipatory anxiety due to the fact that the timing/onset of their respective threats is often unpredictable and in each instance, the duration and intensity of threat exposure is ambiguous. Uncertainty is therefore a common thread across the fear disorders (see Carleton, 2016) and considering the current findings, aberrant response to such uncertainty may be a core deficit of fear-based IPs that can be measured via startle potentiation.
If individuals with fear-based IPs are characterized by exaggerated psychophysiological responding to U-threat, it is important to consider the neural mechanisms that may underlie this dysfunction. As was briefly mentioned before, research indicates that there is a specific frontolimbic circuit that is engaged by U-threat that includes affect-generating limbic regions such as the amygdala, anterior insula (aINS) and BNST (Davis et al., 2010; Sarinopoulos et al., 2009; Shankman et al., 2014), which project to subcortical structures like the brainstem, but also interact with affect-modulating prefrontal regions such as the dorsolateral, ventrolateral and ventromedial prefrontal cortices, orbitofrontal cortex, and dorsal anterior cingulate cortex (Grupe & Nitschke, 2013; Grupe et al., 2012). Within this circuit, two nodes that may be especially central to psychophysiological responding to U-threat are the aINS and BNST (Avery, Clauss, & Blackford, 2016; Singer, Critchley, & Preuschoff, 2009). The aINS is known to play a critical role in interoceptive awareness and generating anticipatory emotional responses for future events (Craig, 2009), whereas the BNST mediates hypervigilance and sustained arousal (Somerville, Whalen, & Kelley, 2010). Within highly reactive individuals, such as those with fear-based IPs, it is posited that during unpredictable threat aINS hyperactivity drives exaggerated subjective feelings of distress thereby promoting BNST response and anticipatory anxiety (Nitschke, Sarinopoulos, Mackiewicz, Davidson, & Schaefer, 2006). In addition, regulatory prefrontal regions, which typically exert adaptive inhibitory influences on the aINS and BNST to down-regulate anticipatory anxiety, are speculated to fail to respond, ultimately resulting in high levels of unregulated aversive reactivity (Kalisch & Gerlicher, 2014; Shackman et al., 2011). Together, this suggests that dysfunctional frontolimbic circuit functioning mediates heightened startle potentiation to U-threat; though this hypothesis has yet to be empirically tested. If supported, this would suggest that behavioral and brain dysfunction in response to U-threat could reflect a novel phenotype for the fear-based dimension of psychopathology; and perhaps, a valuable prevention and intervention target for panic disorder, SAD and SP.
The results of the current study highlight the potential role of reactivity to U-threat in fear-based IPs. They also help clarify the existing distress/misery literature. Consistent with the current findings, one prior study also found that GAD was not associated with reactivity to U-threat (Grillon et al., 2009). Therefore, although GAD is conceptually related to sensitivity to uncertainty (Carleton, 2012), laboratory startle studies have failed to find this association and response to U-threat in GAD and fear-based IPs seem to qualitatively differ. With regard to MDD, prior evidence has been mixed with some studies finding no association (Gorka et al., 2014; Nelson et al., 2013; Shankman et al., 2013) and others finding a significant positive association between depression and startle potentiation to U-threat (Grillon et al., 2013; Robinson et al., 2012). The present results are consistent with the former set of studies and indicate that individuals with a primary diagnosis of MDD display comparable levels of reactivity to U-threat to healthy controls. Considered together, we argue that although there may be moderators that impact the association between depression and threat responding, which contribute to the mixed findings, heightened reactivity to U-threat is relatively specific to fear-based IPs and does not characterize the distress/misery disorders, including depression. With that said, it is necessary to point out that the majority of studies examining individual differences in U-threat have used electric shock as the aversive stimulus and it is possible that individuals with fear-based IPs may be more sensitive to shock (or other tactile threats) relative to individuals with distress/misery IPs. Relatedly, if the aversive stimulus was non-tactile, such as unpredictable rejection, a different pattern of results could emerge such that individuals with distress/misery, but not fear-based, IPs display an exaggerated sensitivity to U-threat. This question reflects an important next step in this line of work and will be essential in clarifying the role of reactivity to U-threat in internalizing psychopathology.
Additional support for the specificity of the current findings comes from the larger affect-modulated startle potentiation literature. Startle eyeblink potentiation has been used as an index of aversive responding for decades and has been most often employed in studies examining responses to affective pictures (negative and positive) and emotional mental imagery (see Lang & McTeague, 2009). These studies do not manipulate the predictability of aversive stimuli directly and therefore capture defensive responding to negative events rather than anticipation of negative events. Interestingly, despite these methodological differences, the pattern of results across startle studies has been remarkably consistent. For example, in a series of studies using aversive imagery, Lang, McTeague and colleagues have demonstrated that individuals with circumscribed fears or traditional fear-based disorders display exaggerated startle potentiation to threat/negative stimuli relative to healthy controls, whereas individuals with high levels of broad distress and principal distress/misery disorders display attenuated startle potentiation to threat (Lang & McTeague, 2009; Lang, McTeague, & Bradley, 2016; McTeague & Lang, 2012). This further highlights that startle potentiation to threat distinguishes fear-based IPs from distress/misery disorders and that individuals along the fear spectrum display exaggerated startle responding.
The present study was specifically designed to examine differences in startle potentiation across DSM-defined principal IPs given that diagnoses are still the foundation of current psychiatric nosology and for the time being, are heavily relied upon for treatment decision making (APA, 2013). However, DSM disorders are also heterogeneous and it is therefore important to consider the role of transdiagnostic processes and symptoms in neurobiological responding. Interestingly, the startle studies noted above by Lang, McTeague and colleagues (2009, 2016) have found that patterns of startle responding differ not only by DSM diagnosis but also along the broad dimension of self-reported affective distress. More specifically, individuals who have the most circumscribed fear, with little broad affective distress, have been shown to demonstrate the highest level of startle potentiation, whereas individuals with the least circumscribed fear and the highest affective distress, exhibit the lowest level of startle potentiation (Lang & McTeague, 2009; McTeague et al., 2010; McTeague, Lang, Wangelin, Laplante, & Bradley, 2012). Due to differences in the battery of self-report questionnaires that were administered in Study 1 and Study 2, we are unable to combine self-report data in a way that would allow us to identify unique fear and distress/misery dimensions to test how broad IP symptoms map onto startle potentiation to U- and P-threat. This therefore reflects an important next step in this line of RDoC-related work. At the same time, it is also worth mentioning that despite differences in levels of affective distress and varying symptom profiles across disorders (see IDAS subscale means in Table 1) in the present study, a clear pattern emerged based on the DSM distinction between fear IPs and distress/misery IPs. For startle potentiation to U-threat, DSM disorders may therefore reflect a meaningful category/distinction; although, the similarities in responding within the fear-based IPs and the distress/misery IPs are consistent with the notion that internalizing disorders may actually reflect two categories (fear vs. distress) rather than multiple categories (e.g., MDD, GAD, etc.) (Clark & Watson, 2006).
The current study had several strengths including the use of a well-validated threat paradigm and inclusion of multiple diagnostic groups. There are also several limitations that are important to highlight. First, some participants taken from Study 1 had comorbid, current IPs. We should note that we see this as both a strength and a limitation as the study was ultimately designed to inform clinical research and test whether individuals that present to treatment with a primary, principal fear-based IP would display heightened reactivity to U-threat. Our hypothesis was supported which speaks to the potential role of reactivity to U-threat as a real-world clinical target for fear-based disorders and our results were identical whether or not we statistically controlled for current number of comorbid IPs. It is necessary to acknowledge, however, that comorbidity may still have impacted the current results. Second, although the current study included tests of multiple forms of psychopathology, we did not have enough participants to create groups for all internalizing disorders (e.g., panic disorder, OCD, PTSD, bipolar disorder, dysthymia) and therefore the specificity of the current findings to only fear-based disorders is still somewhat unclear and requires further examination.
In sum, the current study indicates that like panic disorder, SAD and SP are associated with heightened reactivity to U-threat, and this response profile distinguishes fear-based from distress/misery IPs. These findings broadly imply that individual differences in reactivity to U-threat may contribute to the pathophysiology of all fear-based IPs and could represent an important prevention and intervention target. They also suggest that startle potentiation to U-threat could be a valuable organizing construct for the IPs that reflects core neurobiological deficits and classifies disorders based on their objective response profile. In other words, rather than distinguishing between the different DSM-defined IPs, an individual may one day be classified by their startle response and treatment decisions could be based on their neurobiological profile. Given increasing interest in both reactivity to uncertainty, and transdiagnostic mechanisms of dysfunction, research should continue to investigate whether heightened reactivity to U-threat holds promise as a neurobiologically-based diagnostic phenotype.
General Scientific Summary.
This study suggests that individuals with current, fear-based internalizing disorders display exaggerated anticipatory anxiety in response to uncertain or ambiguous threat. The findings also suggest that this is not the case for individuals with distress/misery disorders as they were found to display relatively normal responses to uncertain threat. Responding to uncertain threat may be a key neurobiological factor that distinguishes fear-based from distress/misery disorders.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01MH101497 [PI: Phan] and R01MH098093 [PI: Shankman]). Other support for this work was provided by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) award number UL1RR029879 from the National Center for Research Resources.
References
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;1:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41(1):126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55:1247–1263. doi: 10.1037/0003-066X.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110(4):585–599. doi: 10.1037/0021-843X.110.4.585. [DOI] [PubMed] [Google Scholar]
- Carleton RN. The intolerance of uncertainty construct in the context of anxiety disorders: Theoretical and practical perspectives. Expert Review of Neurotherapeutics. 2012;12(8):937–947. doi: 10.1586/ern.12.82. [DOI] [PubMed] [Google Scholar]
- Carleton RN. Fear of the unknown: One fear to rule them all? Journal of Anxiety Disorders. 2016;41:5–21. doi: 10.1016/j.janxdis.2016.03.011. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100(3):316–336. doi: 10.1037/0021-843X.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Distress and fear disorders: an alternative empirically based taxonomy of the ‘mood’ and ‘anxiety’ disorders. The British Journal of Psychiatry. 2006;189(6):481–483. doi: 10.1192/bjp.bp.106.03825. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13(1):28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry. 1998;44:1239–1247. doi: 10.1016/S0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. American Psychologist. 2006;61:741–56. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- Forbes D, Lockwood E, Elhai JD, Creamer M, O’Donnell M, Bryant R, … Silove D. An examination of the structure of posttraumatic stress disorder in relation to the anxiety and depressive disorders. Journal of Affective Disorders. 2011;132(1):165–172. doi: 10.1016/j.jad.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Liu H, Sarapas C, Shankman SA. Time course of threat responding in panic disorder and depression. International Journal of Psychophysiology. 2015;98(1):87– 94. doi: 10.1016/j.ijpsycho.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Phan KL, Shankman SA. Insula response to unpredictable and predictable aversiveness in individuals with panic disorder and comorbid depression. Biology of Mood & Anxiety Disorders. 2014;4(1):1. doi: 10.1186/2045-5380-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug and Alcohol Dependence. 2013;132(1):216–222. doi: 10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses; evidence for enhanced anxious anticipation. PloS One. 2013;8(8):e70969. doi: 10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66(1):47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14(7):488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex. 2012:bhs175. doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62(2):182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research Domain Criteria (RDoC): Developing a valid diagnostic framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, Proudfit GH. In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion. 2015;15(1):12–16. doi: 10.1037/emo0000020. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AM. Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neuroscience & Biobehavioral Reviews. 2014;42:1– 8. doi: 10.1016/j.neubiorev.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Kendell R, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. American Journal of Psychiatry. 2003;160(1):4–12. doi: 10.1176/appi.ajp.160.1.4. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, … Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH research domain criteria initiative: Background, issues, and pragmatics. Psychophysiology. 2016;53(3):286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372– 385. doi: 10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97(3):377–395. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety, Stress, & Coping. 2009;22(1):5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Bradley MM. RDoC, DSM, and the reflex physiology of fear: A biodimensional analysis of the anxiety disorders spectrum. Psychophysiology. 2016;53(3):336–347. doi: 10.1111/psyp.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depression and Anxiety. 2012;29(4):264– 281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Defensive mobilization in specific phobia: fear specificity, negative affectivity, and diagnostic prominence. Biological Psychiatry. 2012;72(1):8–18. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, McGowan SK, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, … Shankman SA. Biomarkers of threat and reward sensitivity demonstrate unique associations with risk for psychopathology. Journal of Abnormal Psychology. 2013;122(3):662–671. doi: 10.1037/a0033982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Hajcak G, Klein DN, Kotov R. Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine. 2015;45(12):2545–2556. doi: 10.1017/S0033291715000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Davidson RJ, Schaefer HS. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Raines AM, Allan NP, Oglesby ME, Short NA, Schmidt NB. Examination of the relations between obsessive–compulsive symptom dimensions and fear and distress disorder symptoms. Journal of Affective Disorders. 2015;183:253–257. doi: 10.1016/j.jad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Richter J, Hamm AO, Pané-Farré CA, Gerlach AL, Gloster AT, Wittchen HU, … Fydrich T. Dynamics of defensive reactivity in patients with panic disorder and agoraphobia: implications for the etiology of panic disorder. Biological Psychiatry. 2012;72(6):512–520. doi: 10.1016/j.biopsych.2012.03.035. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Overstreet C, Letkiewicz A, Grillon C, Allen NB, Trinder J, … Kaplan R. Depressed mood enhances anxiety to unpredictable threat. Psychological Medicine. 2012;42(7):1397. doi: 10.1017/S0033291711002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cerebral Cortex. 2009:bhp155. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nature Protocols. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: an fMRI study. Neuroreport. 2014;25(8):596–600. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN. The relation between depression and anxiety: an evaluation of the tripartite, approach-withdrawal and valence-arousal models. Clinical Psychology Review. 2003;23(4):605–637. doi: 10.1016/S0272-7358(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, … Gorka SM. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. Journal of Abnormal Psychology. 2013;122(2):322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Flagan TM, Wittmann M, Strigo IA, Matthews SC, Donovan H, … Paulus MP. The effects of temporal unpredictability in anticipation of negative events in combat veterans with PTSD. Journal of Affective Disorders. 2013;146(3):426–432. doi: 10.1016/j.jad.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Slade TIM, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychological Medicine. 2006;36(11):1593– 1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry. 2010;68(5):416– 424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16(6):317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Vollebergh WAM, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: The NEMESIS study. Archives of General Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. European Journal of Pharmacology. 2003;463(1):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D. Differentiating the mood and anxiety disorders: A quadripartite model. Annual Review of Clinical Psychology. 2009;5:221–247. doi: 10.1146/annurev.clinpsy.032408.153510. [DOI] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ. Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II) Assessment. 2012;19(4):399–420. doi: 10.1177/1073191112449857. [DOI] [PubMed] [Google Scholar]