Abstract
Purpose
Isolated locoregional recurrences (ILRR) of breast cancer confer a significant risk of developing distant metastasis. Management practices and second-ILRR events in the CALOR trial are investigated.
Methods
162 patients with ILRR were randomly assigned to receive post-operative chemotherapy, or no chemotherapy. Descriptive statistics characterize outcomes according to local therapy and the influence of hormone receptor status on subsequent recurrences. Competing risk regression models, Kaplan-Meier estimates, and Cox proportional hazards models evaluate associations between treatment, site of second recurrence and outcome.
Results
The median follow-up was 4.9 years. Of the 98 patients who received breast-conserving primary surgery (BCS), 89 had an ipsilateral-breast tumor recurrence (IBTR); salvage mastectomy was performed in 73 and repeat lumpectomy in 16. Another 8 had nodal-ILRR and 1 chest wall-ILRR. Among 64 whose primary surgery was mastectomy, 52 had chest wall/skin-ILRR and 12 nodal-ILRR. Fifteen patients developed a second-ILRR at a median time from ILRR of 1.6 years (range: 0.08–4.8). All second-ILRR occurred in patients with PR-negative ILRR. Seven (47%) of 15 patients with second-ILRR, and 19 (51%) of 37 with a distant recurrence have died. On multivariable analysis, chemotherapy for the primary cancer (HR 3.55, 95% CI 1.15–10.9, p=0.03) and time interval (continuous) from primary surgery (HR 0.87 95% CI 0.75–1.00, p=0.05) were significant predictors of survival following either a second-ILRR or distant recurrence.
Conclusions
Second-ILRRs represented about one-third of all recurrence events after ILRR and all were PR negative. These second-ILRRs, as well as distant metastases, portend an unfavorable outcome.
INTRODUCTION
Distant metastases after isolated locoregional recurrences (ILRR) of breast cancer occur in approximately 20 to 80% of women, depending on whether the primary surgical treatment was mastectomy or breast-conserving surgery1–8. Since most first ILRRs are operable, management of the recurrence is aimed at control of local disease via surgical excision and selective use of radiation therapy, depending on prior treatments9–13. Adjuvant therapies have long been demonstrated to decrease local recurrences as well as improve survival. With the reporting of the CALOR (Chemotherapy as Adjuvant for LOcally Recurrent breast cancer) trial, the beneficial role of chemotherapy for ILRR is now clear14.
There is limited information on the incidence of second isolated locoregional recurrence (second-ILRR) events after the treatment of an ILRR and the prognosis of patients who have a second-ILRR. For example, local failure rates following salvage lumpectomy for ipsilateral breast tumor recurrences (IBTR) with or without repeat radiation have been reported exclusively in retrospective institutional series. Reported second-IBTR rates range from 15%–71%15–18. Survival after second-IBTR ranges from a median of 33 months19 to 80.7% at 5 years20. Treatment of post-mastectomy nodal or chest wall recurrences is associated with lasting local control of disease in about 50% of cases, but no series provide outcomes following a second-ILRR6,21.
The CALOR trial prospectively collected all relapse events occurring at any time, including second-ILRR. This report describes the rate of second-ILRR, taking into consideration the management of the primary cancer and the management of the first ILRR, and examines outcomes after second-ILRR according to the hormone receptor status of the ILRR.
METHODS
The CALOR study was an international multicenter trial conducted from 2003 to 2010 by the International Breast Cancer Study Group (IBCSG), the Breast International Group (BIG) and the National Surgical Adjuvant Breast and Bowel Project (NSABP)14. A total of 162 patients who met the eligibility criteria were randomly assigned to receive chemotherapy or no chemotherapy after resection of ILRR. Eligibility criteria have been previously described14. Patients were stratified by hormone receptor status of ILRR, location of recurrence, and prior chemotherapy. Hormone receptor status was evaluated and defined per participating institution guidelines. Supraclavicular node recurrences were excluded. Details regarding the extent of treatment for the primary cancer were collected. Patients were not excluded from participating in this trial based on the characteristics of primary tumor therapy such as type of breast surgery, margin status, use of radiation therapy or nodal staging procedures.
CALOR allowed investigators to choose chemotherapy agents while recommending the use of two or more drugs for 3 to 6 months. Endocrine therapy was required for ER-positive and/or PR-positive ILRR, and anti-HER2 therapy was recommended. Radiation therapy was recommended except after salvage mastectomy, and modifications were allowed for patients with previous irradiation. Following treatment of the ILRR, sites of subsequent recurrence were recorded as local or regional (second-ILRR), distant recurrence, second (non-breast) malignancy, and contralateral breast cancer.
Statistical Analysis
The primary endpoint of the trial was disease free-survival, defined as time from randomization to first occurrence of invasive breast cancer event, second (non-breast) primary, or death. Descriptive statistics are used to characterize outcomes (site of first subsequent recurrence as locoregional versus distant) according to local therapy and the relation to hormone receptor status of the ILRR. Competing risk regression models22 were used to account for the competing risks of second-ILRR or distant relapse as first site of subsequent recurrence. Kaplan-Meier estimates were used to evaluate survival after subsequent recurrence. Univariable Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association of the overall survival time following a subsequent recurrence with site of the ILRR, the ER and PR status of the ILRR, receipt of adjuvant chemotherapy for the primary, interval from primary surgery to ILRR (continuous) randomized treatment for ILRR (chemotherapy vs. no-chemotherapy) and site of first subsequent recurrence (locoregional vs distant) for patients who had a second recurrence. A multivariable Cox model was fit for significant univariable factors and the site of first subsequent recurrence. Analyses are retrospective and hypothesis generating. SAS 9.2 (SAS Institute, Cary, NC) and R version 3 (The R Foundation for Statistical Computing, Vienna, Austria) were used. Participating institutions’ ethics committees or institutional review boards approved the trial according to local laws and regulations. All patients gave written informed consent, and the trial was done in compliance with the Helsinki Declaration.
RESULTS
Characteristics of ILRR
As previously published, the distribution of site of ILRRs was similar across treatment arms14. The median time interval between primary surgery and ILRR was 5.5 years. For the 89 patients with IBTRs, the median time to recurrence was 5.7 years (range 0.6–21.8), and was 5.4 years (0.3–31.6) for the 73 patients with ILRR recurrences outside the ipsilateral breast (chest wall (N=53) and nodal regional (N=20)). The predominant histology of the ILRR was ductal (N=133) followed by lobular (N=17), and mucinous (N=3). Of the 123 recurrent cancers with known grade, 22 were grade 1, 50 grade 2 and 51 grade 3. ILRR was ≤2 cm for 108 patients (73% of 148 known). Microscopic margins were positive in 14 cases (9% of 162).
Overall, 68% (110/162) of ILRR were ER-positive and/or PR-positive, although PR status was not reported for 4 recurrences.. ILRRs occurred in 34 women while on endocrine therapy or within 6 months of completing adjuvant treatment for the primary cancer. ER status of the ILRR was discordant with the primary cancer in 21 (15%) cases. ER status changed from ER-negative to ER-positive in 6 (4%), and 15 (9%) ER-positive primary cancers were classified as ER-negative ILRRs. PR expression was discordant in 35 (26%) of 137 primary cancers with known PR status. Among these, 8 (6%) had a PR-negative primary and a PR-positive recurrence while 27 (20%) had a PR-positive primary and a PR-negative recurrence. 93 patients were reported to have had HER2 testing, but positive or negative results were not recorded. However, use of anti-HER2 treatments for the ILRR was recorded, and 10 patients received traztuzumab (4 in the no chemotherapy group and 6 in the chemotherapy group). Of the 15 patients with second- ILRR, two received trastuzumab for their second-ILRR, both in the chemotherapy group.
Local-Regional Treatment of ILRRs
Figure 1 presents a summary of the trial participants in the CALOR trial with regard to their primary and ILRR treatments, starting with all 162 patients enrolled and ending with the 15 who experienced a second-ILRR. Among the 98 women who had breast-conserving surgery, 90 (92%) had undergone nodal staging as part of their initial therapy as had 61 (95%) of the 64 mastectomy-treated patients (Supplementary Table 1). Breast irradiation had been administered in 92 (94%) patients with breast conserving surgery (BCS), including a boost to the tumor bed in 41 (45%). The ipsilateral breast was the site of the initial ILRR in 89 of these 98 (91%) patients. The location of the IBTR in relation to the site of primary cancer was not collected in CALOR. Salvage mastectomy was performed for 73 (82%) of the 89 IBTRs and the remaining 16 had repeat breast-conserving surgery. Microscopically negative margins were achieved in all salvage mastectomy operations. Post-operative chest wall irradiation was used in only two of the salvage mastectomy-treated patients and breast irradiation on three of the 16 repeat breast-conserving cases, one of whom had not received breast irradiation before.
Figure 1.
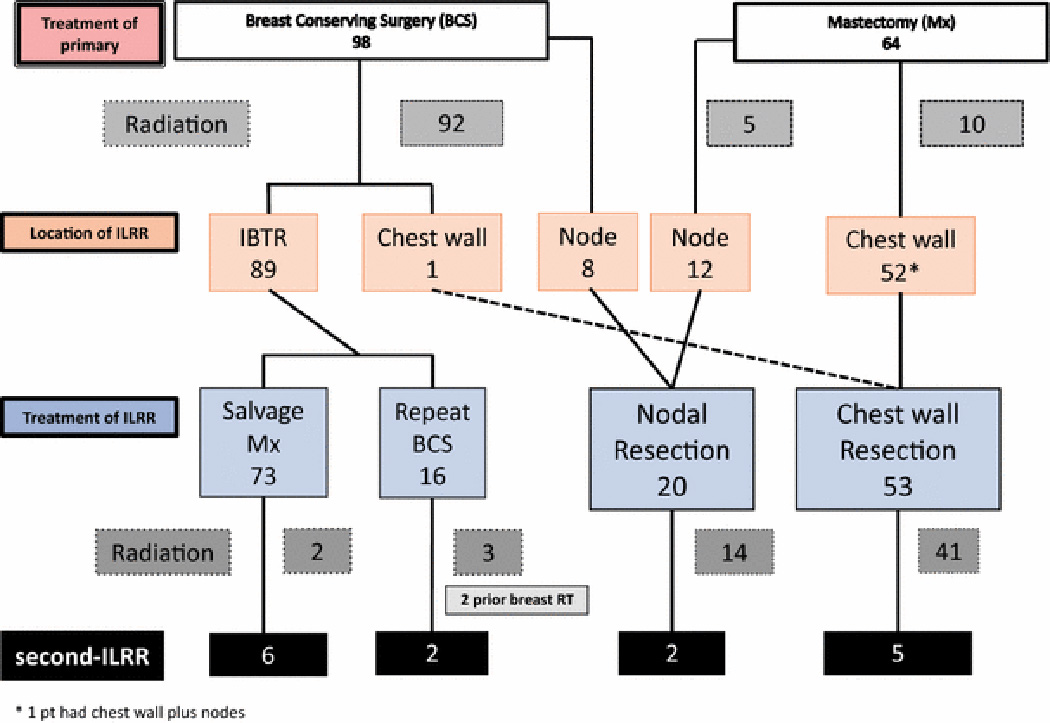
Flowchart showing the treatment of the primary, and sites and treatment of isolated locoregional recurrence (ILRR), starting with all patients enrolled and ending with the 15 patients who experienced a second-ILRR in the CALOR trial.
There were 52 (81%) mastectomy scar/chest wall-ILRRs among the 64 mastectomy-treated primary cancers and 12 (19%) nodal recurrences. Among the 52 with mastectomy scar/chest wall-ILRR, 10 had received prior post-mastectomy irradiation. Resection of the ILRR achieved clear surgical margins in 50 (96%) patients, and 41 (79%) received radiotherapy following resection of ILRR, as prescribed by the protocol.
Second Locoregional Recurrences
Fifteen patients (9% of the study population), developed a second-ILRR as site of first subsequent recurrence following treatment of ILRR (Fig. 2a), 9 of 77 (12%) in the no-chemotherapy arm and 6 of 85 (7%) in the chemotherapy arm.. The median time interval from surgery for ILRR to surgery for second-ILRR for the 15 patients who developed second-ILRR was 1.6 yrs (range: 0.08–4.8). Thirty-seven patients (23%) developed a distant recurrence as site of first subsequent recurrence following treatment of ILRR (four with synchronous locoregional events, three local and one regional; not included among the 15 patients with second-ILRR) (Fig. 2a); median time interval to distant recurrence for these 37 patients was 1.1 years (range 0.1 to 6.8).
Figure 2.
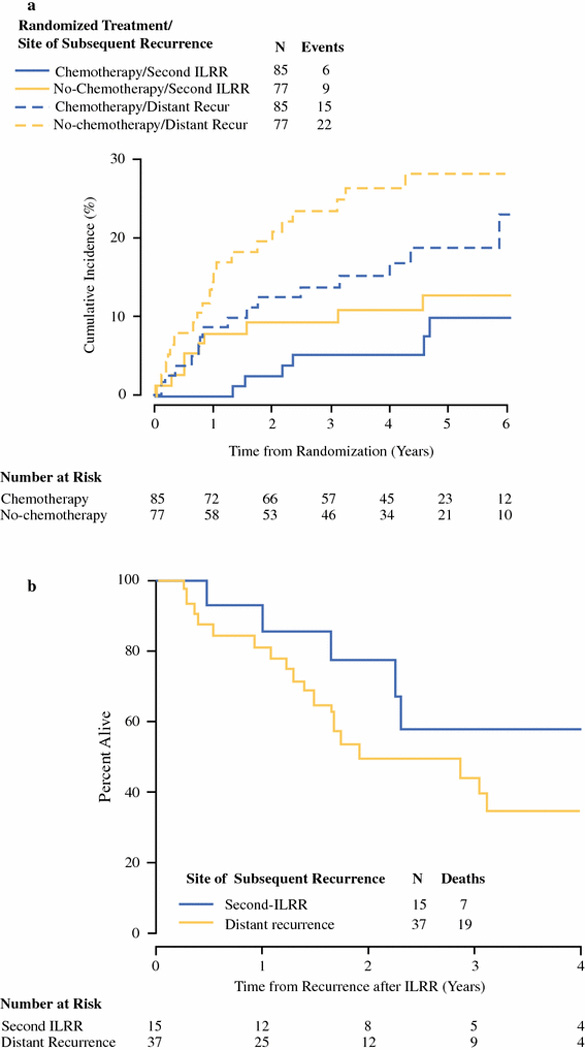
(a) Cumulative incidence of subsequent recurrence from time of randomization to CALOR, according to randomized treatment group and site of first subsequent recurrence (second-ILRR or distant). (b) Overall Survival from time of subsequent recurrence after ILRR according to site, distant or second-ILRR, for the 52 patients who have had a subsequent locoregional or distant breast cancer recurrence on the CALOR trial.
Abbreviations: ILRR: Isolated loco-regional recurrence
The sites of the 15 second-ILRRs according to surgical treatment of ILRR are shown in Supplementary Table 2. The incidence of second-ILRR events after salvage mastectomy and after chest wall resection were similar, 8.2% and 9.4%, respectively. Likewise, 12.5% and 10.0% second-ILRR occurred after repeat breast-conserving surgery and nodal resection, respectively.
Receptor Status and second-ILRR
Both ER and PR were reported for 158 of the 162 ILRRs and for all 15 second-ILRRs. None of the 79 patients with PR-positive ILRR had a second-ILRR (73 ER-positive/PR-positive and 6 ER-negative/PR-positive), and thus all 15 with second-ILRR were PR-negative (6 ERpositive/ PR-negative and 9 ER-negative/PR-negative) (Table 1).
Table 1.
Distribution of Second-ILRR by Sites and Hormone Receptor Status of ILRR
| ER/PR Status of First ILRR | |||||
|---|---|---|---|---|---|
| Site of ILRR | N | ER+/PR+ | ER+/PR− | ER−/PR+ | ER−/PR− |
| Breast-ILRR | 88 | 36 | 10 | 5 | 37 |
| second-ILRR | 8 | 0 | 2 (20%) | 0 | 6 (16%) |
| Chest wall-ILRR | 50 | 27 | 13 | 0 | 10 |
| second-ILRR | 5 | 0 | 3 (23%) | 0 | 2 (20%) |
| Nodes-ILRR | 20 | 10 | 5 | 1 | 4 |
| second-ILRR | 2 | 0 | 1 (20%) | 0 | 1 (25%) |
| Total ILRR | 158* | 73 | 28 | 6 | 51 |
| Total second-ILRR | 15 | 0 | 6 (21%) | 0 | 9 (18%) |
PR status of ILRR was not available for 4 patients enrolled, but was available for all 15 second-ILRR.
Abbreviations: ILRR: isolated locoregional recurrence, ER: estrogen receptor, PR: progesterone receptor
Table 2 shows the impact of the randomized treatment group on subsequent DFS events according to ER and PR status of the ILRR. In the ER-negative/PR-negative subgroup, 3 of 27 patients (11%) in the chemotherapy arm had a second-ILRR, while 6 of 24 (25%) in the no-chemotherapy arm had a second-ILRR. By contrast, in the ER-positive/PR-negaive subgroup, second-ILRRs occurred in 3 of 12 patients (25%) in the chemotherapy arm, and 3 of 16 patients (19%) in the no-chemotherapy arm. Overall the proportion of all subsequent DFS events following an ILRR was higher in the ER-positive/PR-negative (15 of 28; 54%) than in the ER-positive/ PR-positive (15 of 73; 21%) cohort (Table 2). In contrast, 43% of ER-negative/PR-negative ILRRs (22 of 51) experienced a subsequent DFS event. This exploratory analysis of ILRRs based on small subgroups suggests that ER-positive/PR-negative and ER-negative/PR-negative ILRRs have a poor prognosis.
Table 2.
Distributions of DFS Events and of Second-ILRR by Treatment and Hormone Receptor Status* of ILRR
| Hormone Receptor Status* of ILRR | |||||
|---|---|---|---|---|---|
| Treatment of ILRR | N | ER+/PR+ | ER+/PR− | ER−/PR+ | ER−/PR− |
| No Chemotherapy | 75 | 31 | 16 | 4 | 24 |
| Any DFS event** | 32 | 6 (19%) | 9 (56%) | 3 (75%) | 14 (58%) |
| Second-ILRR | 9 | 0 | 3 (19%) | 0 | 6 (25%) |
| Distant recurrence | 20 | 4 (13%) | 5 (31%) | 3 (75%) | 8 (33%) |
| Chemotherapy | 83 | 42 | 12 | 2 | 27 |
| Any DFS event** | 23 | 9 (21%) | 6 (50%) | 0 | 8 (30%) |
| Second-ILRR | 6 | 0 | 3 (25%) | 0 | 3 (11%) |
| Distant recurrence | 15 | 7 (17%) | 3 (25%) | 0 | 5 (19%) |
| Total | 158 | 73 | 28 | 6 | 51 |
| Any DFS event** | 55 | 15 (21%) | 15 (54%) | 3 (50%) | 22 (43%) |
| Second-ILRR | 15 | 0 | 6 (21%) | 0 | 9 (18%) |
| Distant recurrence | 35 | 11 (15%) | 8 (29%) | 3 (50%) | 13 (25%) |
PR status of ILRR was not available for 4 patients enrolled, 2 of whom experienced distant recurrence and 1 experienced an other DFS event (not second-ILRR nor distant).
Includes second-ILRR, distant recurrence, and other DFS events
Abbreviations: ILRR: isolated locoregional recurrence, ER: estrogen receptor, PR: progesterone receptor
Deaths After Locoregional or Distant Subsequent Recurrences
With a median follow-up after subsequent breast cancer recurrence of 3.67 years, seven of 15 women (47%) with a second-ILRR have died (one from a non-breast event, CVA), compared with 19 of 37 women (51%) experiencing distant recurrence events after ILRR (Fig. 2b) The difference in survival from time of first subsequent recurrence after ILRR by site (distant versus second-ILRR) was not statistically significant (multivariable HR=1.97 95% CI 0.73–5.29); p=0.18) (Table 3, Fig 2b). Chemotherapy for primary cancer and time interval from primary surgery to the ILRR were significant factors for survival following a subsequent recurrence after ILRR (Table 3).
Table 3.
Univariable and Multivariable* Analyses of Overall Survival from Time of First Subsequent Recurrence after ILRR (n=52)
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Covariate | HR | 95% CIs | p-value | HR | 95% CIs | p-value |
| Estrogen-receptor of ILRR (pos v neg) | 0.61 | 0.27, 1.36 | 0.23 | |||
| Progesterone-receptor of ILRR (pos v neg)** |
0.73 | 0.27, 1.98 | 0.54 | |||
| Site of ILRR | ||||||
| Breast-ILRR (reference) | - | - | ||||
| Chest wall-ILRR | 1.44 | 0.60, 3.43 | 0.41 | |||
| Nodes-ILRR | 1.04 | 0.30, 3.65 | 0.95 | |||
| Adjuvant chemotherapy (for primary) (Yes v No) |
4.53 | 1.51, 13.56 | 0.007 | 3.55 | 1.15, 10.9 | 0.03 |
| Interval from primary surgery in years (continuous) |
0.87 | 0.76, 0.99 | 0.04 | 0.87 | 0.75, 1.00 | 0.05 |
| Randomized Treatment for ILRR (Chemotherapy v No Chemotherapy) |
0.94 | 0.39, 2.32 | 0.90 | |||
| Site of subsequent recurrence (distant v second-ILRR) |
1.84 | 0.73, 4.68 | 0.20 | 1.97 | 0.73, 5.29 | 0.18 |
Two covariates found to be significant in univariable analyses plus the site of subsequent recurrence were included in the multivariable analysis based on 52 patients and 26 deaths
Based on 50 patients, excluding 2 with PR of ILRR unknown
Abbreviations: ILRR: isolated locoregional recurrence, ER: estrogen receptor, PR: progesterone receptor
DISCUSSION
CALOR tested the efficacy of systemic chemotherapy as an adjuvant treatment for patients with operable, resectable ILRR14. This trial also provides an opportunity to evaluate the incidence, location and prognosis of recurrences subsequent to the ILRR. Distant recurrences as site of first subsequent recurrence after treatment for an ILRR were more common than second-ILRR events as site of first subsequent recurrence. Overall, the incidence of second-ILRR events was similar whether the relapse occurred after chest wall resection, salvage mastectomy or repeat breast lumpectomy.
All second-ILRR occurred among patients with PR-negative ILRR with or without ER expression. Admittedly, the number of patients is quite small, but on this relatively short followup of 5 years, the grouping of these recurrence in the PR-negative subgroup was striking. When it occurred, the time to second-ILRR was very short, underscoring the aggressive biology associated with these events that foreshadow poor outcomes. Our findings suggest that a subsequent recurrence occurring in a population receiving multimodality therapy for an ILRR is associated with poor outcomes. Specifically, after 3.7 years of median follow up, the mortality after either a second-ILRR or a distant metastasis was approximately 50%, indicating a subgroup of patients with biologically aggressive disease. Thus, second-ILRR events represent probably persistent subclinical locoregional disease or de novo neoplastic transformation in residual breast tissue.
This study provides a perspective on local management of operable ILRR. As has been shown in the adjuvant and neoadjuvant settings, systemic chemohormonal regimens improve local control of disease23,24. Trial entry was predicated on the complete gross excision of the recurrent tumor. While radiation therapy was recommended for all cases, only a handful received this treatment post-salvage mastectomy or repeat breast-conserving surgery.
Salvage mastectomy was the most common operation used in women experiencing an IBTR. The remaining 18% were treated by repeat breast conservation, with 19% undergoing re-radiation. Notably, the majority of patient achieved very good local control after treatment of ILRR, with comparable rates of second-ILRR; 8.2% of the salvage mastectomy population and 12.5% in the repeat breast conserving surgery group. In-breast recurrences after repeat breast-conserving surgery ranges between 7% to 38% (36 to 120 month follow-up)11, 18. Gentilini et al found a higher second local failure rate than in our trial; 27% of 161 IBTR cases treated by repeat breast conservation18. Re-radiation of the breast was reported in a minority of patients in our study (3 of 16). Publications of non-randomized series report its feasibility and higher local control rates with additional radiation25–27, but even with restricted volumes such as brachytherapy or intraoperative radiation therapy (IORT), greater tissue/skin toxicity is reported28. Forthcoming data on the prospective phase 2 trial involving repeat lumpectomy with 3D-conformal partial breast re-radiation to 45Gy, will provide additional information on the efficacy of this approach (RTOG trial 1014). LRR events are significantly associated with a higher risk of developing distant metastasis3, 4. Tanis et al analyzed late ILRRs across EORTC trials29. Even 10 years after breast conserving treatment for a primary cancer occurrence, an ILRR was a highly unfavorable, independent prognostic indicator, associated with distant metastases. In two retrospective analyses of lumpectomy-treated patients in 5 node-positive and 5 node-negative randomized trials conducted by the NSABP, women who experienced other-LRR had worse DFS and OS than those with IBTR5, 7. These differences were not apparent in the CALOR trial, where patient entry was influenced by investigator and prospective participant considerations, on the potential benefits or harms of chemotherapy.
The debate on the biological significance of ILRR continues. Clearly, the size of recurrence is not a significant determinant on subsequent prognosis. In our study, 67% of ILRRs were 2 cm or less, and in the absence of adjuvant chemotherapy, the 5-year DFS for ER+ tumors was 69% and for ER- tumors 35%14. One perspective is centered on optimizing local therapy as a means of decreasing distant metastases30. Lowering LRR with the use of adjuvant radiotherapy, demonstrates improvements in 15-year DFS and survival in prospective randomized trials31. However, for many patients, local failures are important indicators of poorer prognosis and denote biologically resistant disease for those that received optimal first line adjuvant treatments.
Few clinical trials have addressed the questions of persistent locoregional control after the treatment of an ILRR. At a median follow up of 4.9 years, the overall results of the CALOR trial showed chemotherapy significantly prolonged DFS, HR 0.59 [CI (0.35,0.99); p= 0.046] and OS, 0.41 [CI (0.19, 0.89); p=0.02]14. Most dramatic was the effect seen in the subgroup of ER-negative/PR-negative patients wherein the risk of distant disease was reduced by 68% and death by 57%14. The current analysis also suggests that the administration of adjuvant chemotherapy at the time of ILRR may reduce second-ILRR as well, and that subsequent recurrences, whether local or distant, occur early and portend a high likelihood of death. This poor outcome after second-ILRR should be taken into account when treating such recurrences.
Supplementary Material
Synopsis.
Distant metastases and second-isolated locoregional recurrences (second-ILRR) of breast cancer are the most common events following treatment of a first-isolated locoregional recurrence. Prevalence of second-ILRR is similar whether breast-ILRR, node-ILRR or chest wall-ILRR preceded. Second-ILRR portend poor prognosis.
Acknowledgments
Support
The CALOR trial was supported in part by Public Service Grants U10-CA-180868, -189867, -180822, 75362 from the National Cancer Institute, Department of Health and Human Services. The International Breast Cancer Study Group is supported in part by the Swiss Group for Clinical Cancer Research (SAKK), Frontier Science and Technology Research Foundation, Australia and New Zealand Breast Cancer Trials Group, Swedish Cancer Society, Cancer Research Switzerland/Oncosuisse, Cancer Association of South Africa, Foundation for Clinical Research of Eastern Switzerland (OSKK). Spanish participation was funded by Grupo Español de Investigación en Cáncer de Mama (GEICAM), and Dutch participation by BOOG, the Dutch Breast Cancer Trialists' Group.
Footnotes
Disclosure: None of the authors have any conflicts of interest to report.
Clinical Trial Registration: clinicaltrials.gov NCT00074152
Contributor Information
Irene L. Wapnir, Department of Surgery, Stanford University School of Medicine, Stanford, CA, USA.
Shari Gelber, IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, and Frontier Science and Technology Research Foundation, Boston, MA, USA
Stewart J. Anderson, Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Eleftherios P. Mamounas, University of Florida Health Cancer Center at Orlando Health, Orlando, FL
André Robidoux, Centre Hospitalier de l'Universite de Montreal, Montreal, QC, Canada
Miguel Martín, GEICAM, Instituto de Investigacion SanitariaGregorio Marañon, Universidad Complutense, Madrid, Spain
Johan W.R. Nortier, BOOG, Dutch Breast Cancer Trialists' Group, Leids Universitair Medisch Centrum, Leiden, Netherlands
Charles E. Geyer, Jr, Virginia Commonwealth University Massey Cancer Center, Richmond, VA, USA
Alexander H.G. Paterson, Tom Baker Cancer Centre, Calgary, Alberta, Canada
István Láng, IBCSG and National Institute of Oncology, Budapest, Hungary
Karen N. Price, IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, and Frontier Science and Technology Research Foundation, Boston, MA, USA
Alan S. Coates, IBCSG, Bern, Switzerland and University of Sydney, Sydney, Australia
Richard D. Gelber, IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard TH Chan School of Public Health, Harvard Medical School, and Frontier Science and Technology Research Foundation, Boston, MA, USA
Priya Rastogi, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA
Meredith M. Regan, IBCSG Statistical Center, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Norman Wolmark, Allegheny Health Network Cancer Institute, Pittsburgh, PA, USA
Stefan Aebi, IBCSG, Luzerner Kantonsspital, Lucerne and University of Berne, Switzerland and Swiss Group for Clinical Cancer Research (SAKK), Bern, Switzerland.
REFERENCES
- 1.Halverson KJ, Perez CA, Kuske RR, et al. Survival following locoregional recurrence of breast cancer: univariate and multivariate analysis. Int J Radiat Oncol Biol Phys. 1992;23:285–291. doi: 10.1016/0360-3016(92)90743-2. [DOI] [PubMed] [Google Scholar]
- 2.Fowble B, Solin LJ, Schultz DJ, et al. Breast recurrence following conservative surgery and radiation: patterns of failure, prognosis, and pathologic findings from mastectomy specimens with implications for treatment. Int J Radiat Oncol Biol Phys. 1990;19:833–842. doi: 10.1016/0360-3016(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Anderson S, Fisher ER, et al. Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet. 1991;338:327–331. doi: 10.1016/0140-6736(91)90475-5. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Marubini E, Del Vecchio M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 5.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HM, Overgaard M, Grau C, et al. Loco-regional recurrence after mastectomy in high-risk breast cancer--risk and prognosis. An analysis of patients from the DBCG 82 b&c randomization trials. Radiother Oncol. 2006;79:147–155. doi: 10.1016/j.radonc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botteri E, Rotmensz N, Sangalli C, et al. Unavoidable mastectomy for ipsilateral breast tumour recurrence after conservative surgery: patient outcome. Ann Oncol. 2009;20:1008–1012. doi: 10.1093/annonc/mdn732. [DOI] [PubMed] [Google Scholar]
- 9.Kuerer HM, Arthur DW, Haffty BG. Repeat breast-conserving surgery for in-breast local breast carcinoma recurrence: the potential role of partial breast irradiation. Cancer. 2004;100:2269–2280. doi: 10.1002/cncr.20257. [DOI] [PubMed] [Google Scholar]
- 10.Schuck A, Könemann S, Matthees B, et al. Radiotherapy in the treatment of locoregional relapses of breast cancer. Br J Radiol. 2002;75:663–669. doi: 10.1259/bjr.75.896.750663. [DOI] [PubMed] [Google Scholar]
- 11.Siglin J, Champ CE, Vakhnenko Y, et al. Radiation therapy for locally recurrent breast cancer. Int J Breast Cancer. 2012;2012:571946. doi: 10.1155/2012/571946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannoun-Levi J-M, Ihrai T, Courdi A. Local treatment options for ipsilateral breast tumour recurrence. Cancer Treat Rev. 2013;39:737–741. doi: 10.1016/j.ctrv.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Maaskant-Braat AJG, Voogd AC, Roumen RMH, et al. Repeat sentinel node biopsy in patients with locally recurrent breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2013;138:13–20. doi: 10.1007/s10549-013-2409-1. [DOI] [PubMed] [Google Scholar]
- 14.Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15:156–163. doi: 10.1016/S1470-2045(13)70589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurtz JM, Jacquemier J, Amalric R, et al. Is breast conservation after local recurrence feasible? Eur J Cancer. 1991;27:240–244. doi: 10.1016/0277-5379(91)90505-8. [DOI] [PubMed] [Google Scholar]
- 16.Salvadori B, Marubini E, Miceli R, et al. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg. 1999;86:84–87. doi: 10.1046/j.1365-2168.1999.00961.x. [DOI] [PubMed] [Google Scholar]
- 17.Ishitobi M, Komoike Y, Nakahara S, et al. Repeat lumpectomy for ipsilateral breast tumor recurrence after breast-conserving treatment. Oncology. 2011;81:381–386. doi: 10.1159/000335265. [DOI] [PubMed] [Google Scholar]
- 18.Gentilini O, Botteri E, Veronesi P, et al. Repeating conservative surgery after ipsilateral breast tumor reappearance: criteria for selecting the best candidates. Ann Surg Oncol. 2012;19:3771–3776. doi: 10.1245/s10434-012-2404-5. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz JM. Factors influencing the risk of local recurrence in the breast. Eur J Cancer. 1991;28:660–666. doi: 10.1016/s0959-8049(05)80121-2. [DOI] [PubMed] [Google Scholar]
- 20.Ishitobi M, Okumura Y, Nishimura R, et al. Repeat lumpectomy for ipsilateral breast tumor recurrence (IBTR) after breast-conserving surgery: the impact of radiotherapy on second IBTR. Breast Cancer. 2014;21:754–760. doi: 10.1007/s12282-013-0454-6. [DOI] [PubMed] [Google Scholar]
- 21.van der Pol CC, van Geel AN, Menke-Pluymers MBE, et al. Prognostic factors in 77 curative chest wall resections for isolated breast cancer recurrence. Ann Surg Oncol. 2009;16:3414–3421. doi: 10.1245/s10434-009-0662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 2012;94:496–509. [Google Scholar]
- 23.Mamounas EP, Tang G, Liu Q. The importance of systemic therapy in minimizing local recurrence after breast-conserving surgery: the NSABP experience. J Surg Oncol. 2014;110:45–50. doi: 10.1002/jso.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;.32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannoun-Levi J-M, Houvenaeghel G, Ellis S, et al. Partial breast irradiation as second conservative treatment for local breast cancer recurrence. Int J Radiat Oncol Biol Phys. 2004;60:1385–1392. doi: 10.1016/j.ijrobp.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Sedlmayer F, Zehentmayr F, Fastner G. Partial breast re-irradiation for local recurrence of breast carcinoma: Benefit and long term side effects. Breast. 2013;2(22 Suppl):S141–S146. doi: 10.1016/j.breast.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Hannoun-Levi J-M, Resch A, Gal J, et al. Accelerated partial breast irradiation with interstitial brachytherapy as second conservative treatment for ipsilateral breast tumour recurrence: multicentric study of the GEC-ESTRO Breast Cancer Working Group. Radiother Oncol. 2013;108:226–231. doi: 10.1016/j.radonc.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Trombetta M, Hall M, Julian TB. Long-term followup of breast preservation by re-excision and balloon brachytherapy after ipsilateral breast tumor recurrence. Brachytherapy. 2014;13:488–492. doi: 10.1016/j.brachy.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Tanis E, van de Velde CJH, Bartelink H, et al. Locoregional recurrence after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer. 2012;48:1751–1756. doi: 10.1016/j.ejca.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 30.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


