Abstract
There are a number of reports demonstrating a relationship between the alterations in DFF40 expression and development of some cancers. Here, increased DFF40 expression in T-47D cells in the presence of doxorubicin was envisaged for therapeutic usage. The T-47D cells were transfected with an eukaryotic expression vector encoding the DFF40 cDNA. Following incubation with doxorubicin, propidium iodide (PI) staining was used for cell cycle distribution analysis. The rates of apoptosis were determined by annexin V/PI staining. Apoptosis was also evaluated using the DNA laddering analysis. The viability of DFF40-transfected cells incubated with doxorubicin was significantly decreased compared with control cells. However, there were no substantial changes in the cell cycle distribution of pIRES2-DFF40 cells incubated with doxorubicin compared to control cells. The expression of DFF40, without doxorubicin incubation, had also no significant effect on the cell cycle distribution. There was no DNA laddering in cells transfected with the empty pIRES2 vector when incubated with doxorubicin. In contrast, DNA laddering was observed in DFF40 transfected cells in the presence of doxorubicin after 48 h. Also, the expression of DFF40 and DFF45 was increased in DFF40 transfected cells in the presence of doxorubicin enhancing cell death. Collectively our results indicated that co-treatment of DFF40-transfected cells with doxorubicin can enhance the killing of these tumor cells via apoptosis. Thus, modulation of DFF40 level may be a beneficial strategy for treatment of chemo-resistant cancers.
Keywords: DFF40, doxorubicin, apoptosis, cancer
Introduction
Defects in DFF (DNA fragmentation factor) expression are associated with several different cancers in humans. In addition, an important role for DFF in maintaining genomic stability and suppressing tumor development is proposed (Hsieh et al. 2003, 2005; Yamaguchi et al. 2004; Yan et al. 2006). DFF40 is a protein that acts in the terminal stages of apoptosis. In normal cells, the DFF40 chaperon (DFF45) associates with DFF40 and successively acts as an inhibitor blocking its homo-oligomerization that is essential for nuclease activation (Otomo et al. 2000). In apoptosis induction, DFF45 is cleaved by caspase-3 and dissociates from DFF40. Cleavage of DFF45 results in homo-oligomerization of DFF40, followed by its effective binding to DNA. DFF40 creates 50–300 kbp pieces of DNA in initial stages of apoptotic chromosomal DNA fragmentation (Oberhammer et al. 1993) that is thought to precede internucleosomal DNA cleavage (Sakahira et al. 1998).
Doxorubicin or adriamycin is one of the most important anticancer drugs and is categorized in anthracycline family. It is used in the treatment of several cancers, including breast, prostate, esophageal carcinomas, osteosarcoma, Kaposi’s sarcoma, and Hodgkin’s and non-Hodgkin’s lymphomas (Hortobágyi 1997). Despite the vast clinical usage of doxorubicin, its main mechanism of action has not been fully ascertained. The cytotoxic effects of doxorubicin are attributed to the free radical formation and DNA damage induced by inhibition of topoisomerase II (Gewirtz 1999). It is also reported that doxorubicin induces apoptosis via the activation of caspases (Gamen et al. 2000). Other reports have demonstrated that treatment with a low dose of doxorubicin results in a senescence-like phenotype (Chang et al. 1999; Wang et al. 1999). Nevertheless, despite the desirable anti-tumor activity of doxorubicin, its clinical utility is limited due to its systemic side effects including acute nausea, myelosuppression, vomiting, alopecia, and cumulative cardiotoxicity.
Our previous work indicated that the combination of DFF40 overexpression along with another drug treatment, such as acetazolamide or sulfabenzamide, resulted in enhanced death of cancer cells by apoptosis (Bagheri et al. 2014). Thus, the usefulness of this overexpression along with doxorubicin co-treatment was envisaged here to show its possible advantage for cancer treatment.
Materials and methods
Cell treatment with doxorubicin
The details of pIRES2-EGFP-DFF40 preparation was previously described by us (Bagheri et al. 2014). Cells transfected with empty vector (pIRES2) or pIRES2-DFF40 were seeded 24 h before treatment and incubated with freshly prepared doxorubicin at 50% confluence. The cell viability, cell cycle distribution, and rates of apoptosis were evaluated at desired time points.
Cell viability assay
MTT assay was utilized for determination of viability of cells transfected with empty pIRES2 or pIRES2-DFF40 vectors in the presence of doxorubicin. Succinctly, 24, 48, and 72 h after incubation with doxorubicin (0.17, 0.22, and 0.33 μmol/L), the fresh medium containing MTT solution (5 mg/mL in PBS) was added in a 4:1 ratio. After incubation at 37 °C for 2 h, the medium was removed and the precipitate was dissolved in dimethyl sulfoxide (DMSO). Absorbance was measured at the wavelength of 570 nm and cell viability was calculated as the percent value relative to the untreated group.
Real-time RT-PCR analysis
The changes in the levels of DFF40, DFF45, and caspase-3 mRNA in the presence of doxorubicin in two groups were determined by real-time RT-PCR analysis. In brief, total RNA was prepared from cultured cells using TRIzol reagent. The cDNA was generated and amplified by specific DFF40 (forward: 5′-TTGGAGTCCCGATTTCAGAG-3′, reverse: 5′-CTGTCGAAGTAGCTGCCATTG-3′), DFF45 (forward: 5′-TTCTGTGTCTACCTTCCAATACTA-3′, reverse: 5′-CTGTCTGTTTCATCTACATCAAAG-3′) and caspase-3 (forward: 5′-CAAACTTTTTCAGAGGGGATCG-3′, reverse: 5′-GCATACTGTTTCAGCATGGCAC-3′) primers. Power SYBR® Green PCR Master Mix was used in an ABI device (Applied Biosystems) to determine the relative amounts of DFF40, DFF45, and caspase-3 mRNA using GAPDH as the reference gene. To confirm PCR specificity, the PCR products were subjected to a melting-curve analysis.
Cell cycle analysis by fluorescence-activated cell sorting (FACS)
After the incubation period (48 h in 0.33 μmol/L doxorubicin), cells were collected, centrifuged, and washed with PBS. The cells were then fixed with ice-cold 70% ethanol for 2 h. After centrifugation, the cell pellet was resuspended in 1 mL of water containing PI (10 μg/mL) and RNase A (100 μg/mL) at room temperature for 30 min. In flow cytometry studies, 10 000 events were acquired, and analysis was performed using a FACS Caliber flow cytometer (BD Biosciences).
Detection of apoptotic and necrotic cells
Apoptotic and necrotic cells were detected using Annexin-V-FLUOS staining kit. The cells transfected with empty or DFF40 vector were incubated with 0.33 μmol/L doxorubicin for 48 h. The annexin V-FITC/PI staining was used to determine early and late apoptotic and necrotic cells. The apoptosis incidence in each group was assayed 3 times.
DNA laddering
The transfected cells with empty pIRES2 or pIRES2-DFF40 vectors were cultured in 25 cm2 flask and incubated with 0.33 μmol/L doxorubicin for 24 and 48 h. After incubation the cells were lysed in lysis buffer containing 6 mol/L guanidine-HCl, 10 mmol/L urea, 10 mmol/L Tris-HCl, and 20% Triton X-100. After the 10 min incubation, isopropanol (100 μL) was added to the samples and the positive control and transferred into filter tubes. After centrifugation (1 min at 6000g), the bound DNA separated from the filter tube by pre-warmed elution buffer. The amount of DNA was quantified using a spectrophotometer at 260 nm. DNA (3 μg) was loaded on 1% agarose gel, and electrophoresis was carried out at 75 V for 90 min. DNA bands were visualized with UV light after ethidium bromide staining.
Scratch wound assay
To assess the effects of DFF40 overexpression on migration of T-47D cells, we used a scratch wound assay. In brief, cells seeded into a 6-well plate to create a confluent monolayer. The cell monolayer was then scraped in a straight line with a sterile 10 μL pipette tip. The cell debris was removed by washing the cells once with 1 mL of the growth medium and fed with 2 mL of growth medium. The width of the wound area was monitored with an inverted microscope at the first hour and after incubation for 24 and 48 h.
Statistical analysis
Data were expressed as the mean ± SD. Differences between any 2 groups were determined by analysis of variance (ANOVA) using SPSS version 16 package and Excel 2007 software. P < 0.05 was considered statistically significant.
Results
Overexpression of DFF40 resulted in an additional decrease in cell viability in the presence of doxorubicin
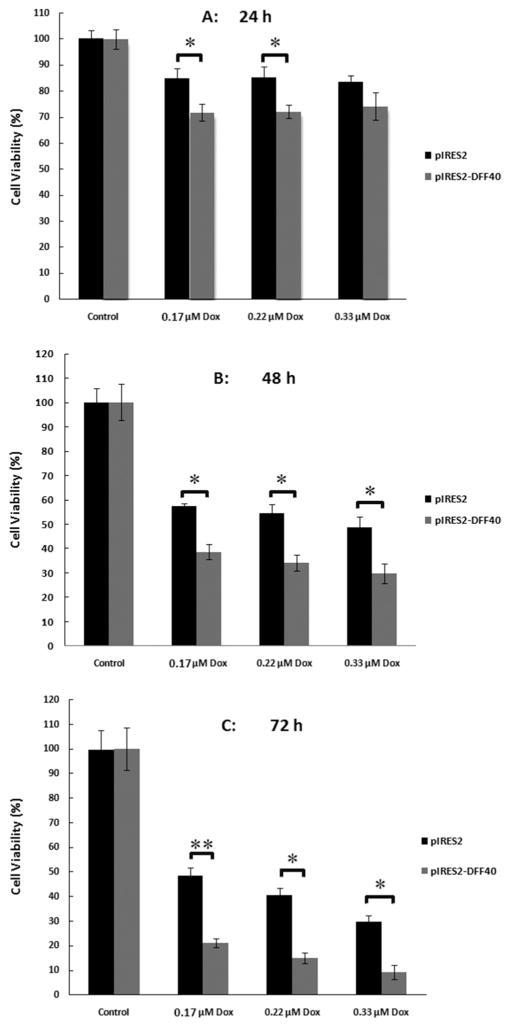
The percentage of live cells in pIRES2 or pIRES2-DFF40 transfected cells was evaluated using MTT assay in the presence of doxorubicin (0.17, 0.22, and 0.33 μmol/L) for 24, 48, and 72 h (Fig. 1). Interestingly, 48 h co-treatment of pIRES2 transfected cells with doxorubicin (black columns in Fig. 1B) diminished the percentage of live cells by up to 50% at 0.33 μmol/L doxorubicin; that is equal to what is registered for doxorubicin alone at its IC50 concentration. Thus, pIRES2 vector (empty vector) had no additional cytotoxic effect by itself and did not decrease cell viability greater than doxorubicin alone. However, overexpression of DFF40 in pIRES2-DFF40 transfected cells amplified the cytotoxic effects of doxorubicin by approximately 50% compared with pIRES2 group after 48 and 72 h of treatment (compare black and shaded columns in Figs. 1A–1C).
Fig. 1.
Impact of DFF40 expression and doxorubicin treatments on cell viability. Cell viability was assessed by the MTT assay and was calculated as percentage value relative to the blank (untreated) group. Concurrent treatment of doxorubicin (0.17, 0.22, and 0.33 μmol/L) with pIRES2 empty vector and pIRES2-DFF40 transfected cells (A: 24 h; B: 48 h; C: 72 h treatment) showed that DFF40-transfected cells exhibit a significant decrease in cell viability compared to the empty vector transfected cells, illustrating the augmented cytotoxicity of doxorubicin in its individual state. Data are shown as mean ± SD from 3 independent experiments. *P < 0.05 and **P < 0.001 indicate statistically significant difference between two columns, illustrated using the column linking “
 ” symbol.
” symbol.
DFF40 overexpression resulted in increased levels of DFF40 and DFF45 following doxorubicin treatment
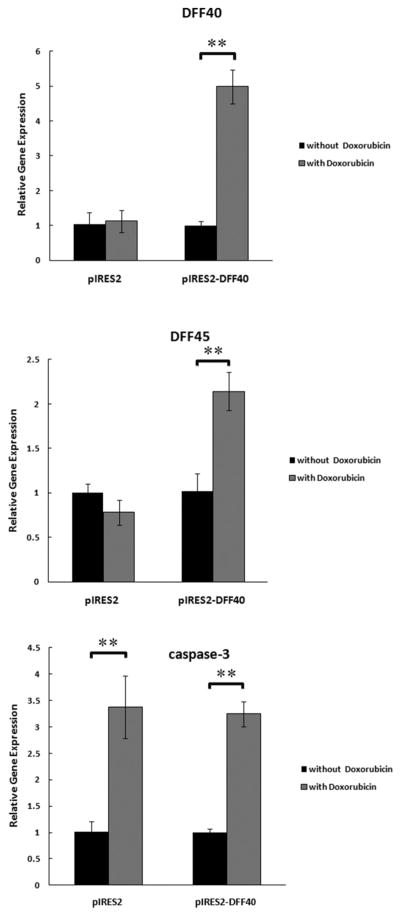
We have previously shown that transfection of T-47D cells with DFF40 results increased levels of DFF40 in these cells (Bagheri et al. 2014). Here we determined whether doxorubicin treatment of cells transfected with empty vector or DFF40 affects the levels of DFF40, DFF45, and caspase-3 (Fig. 2). The results were normalized to the Dox-untreated cells in each group (black columns are equal to unity). The real-time RT-PCR results demonstrated that DFF40 overexpression did not have any effect on the expression of DFF45 and caspase-3 in Dox-untreated cells (not shown), whereas, doxorubicin-treatment of DFF40 transfected cells resulted in increased expression of DFF40, DFF45, and caspase-3 (compare black and shaded columns at the right side of Fig. 2). In the control (pIRES2 empty vector group) after incubation with doxorubicin, the expression level of DFF40 and DFF45 did not change, but the expression level of caspase-3 was increased (left-side duplicated black and shaded columns in Fig. 2).
Fig. 2.
The effect of DFF40 overexpression on DFF40, DFF45, and caspase-3 expression after incubation with doxorubicin was evaluated by real-time RT-PCR. There are 2 sets of column duplets in each diagram, one for pIRES2 empty vector and the other for pIRES2-DFF40 group. In each set of column duplet, the expression level of the genes (DFF40, DFF45, and caspase-3) was analyzed independently using the expression level of each gene in the absence of doxorubicin as the control. This notion resulted in the height of the black columns in pIRES2 empty vector or pIRES2-DFF40 vector being equal to unity. The expression of all genes was increased in DFF40 transfected cells after treatment with doxorubicin. Only caspase-3 expression level was increased in the both groups after doxorubicin treatment. *P < 0.05 and **P < 0.001 indicate statistically significant difference between two columns, illustrated using the column linking “
 ”symbol.
”symbol.
DFF40 overexpression did not affect cell cycle distribution
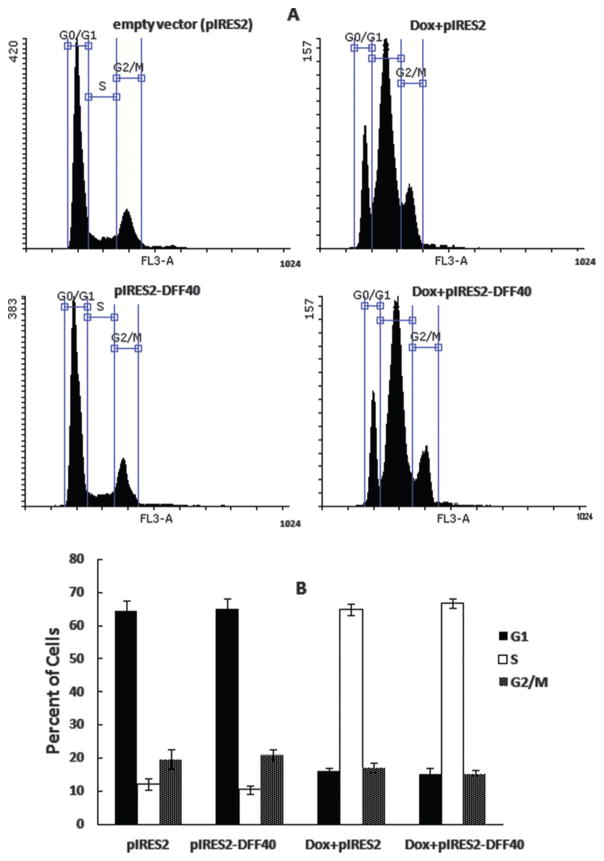
Although doxorubicin is one of the most widely used anticancer agents, its mechanism of action is not fully understood. Doxorubicin induces single and double strand breaks in DNA mediated by topoisomerase II (Tewey et al. 1984). Furthermore, other mechanisms of action have been suggested as modes of doxorubicin-induced cell death, including inhibition of macromolecular biosynthesis (such as DNA and RNA) and production of free radicals. Others have shown that doxorubicin arrests cells in S and G2/M phases of the cell cycle (Feinstein et al. 1993; Momparler et al. 1976; Munger et al. 1988). We evaluated the cell cycle distribution of DFF40-transfected T-47D cells and incubated with or without doxorubicin. The overexpression of DFF40 in pIRES2-DFF40 group did not affect the cell cycle distribution patterns compared to pIRES2 control group. Thus, overexpression of DFF40 in T-47D cells had no effect on their cell cycle distribution (Fig. 3). In addition, incubation of pIRES2-DFF40 transfected cells with doxorubicin also had no additional effect on the cell cycle patterns relative to vector control cells (Fig. 3).
Fig. 3.
Cell cycle analysis was performed at IC50 concentration of doxorubicin after 48 h. (A) Cell cycle dissipation for empty pIRES2 and pIRES2-DFF40 transfected cells (left column) and doxorubicin treatments for pIRES2 and pIRES2-DFF40 controls (right column). (B) Histograms for the cell cycle distribution. Similar heights for G1, G2/M, and S columns in Dox+pIRES2 and Dox+pIRES2-DFF40 show that there is no distinct effect of DFF40 overexpression on the cell cycle distribution. Data representative at least 3 independent experiments.
DFF40 overexpression increased doxorubicin-triggered apoptosis
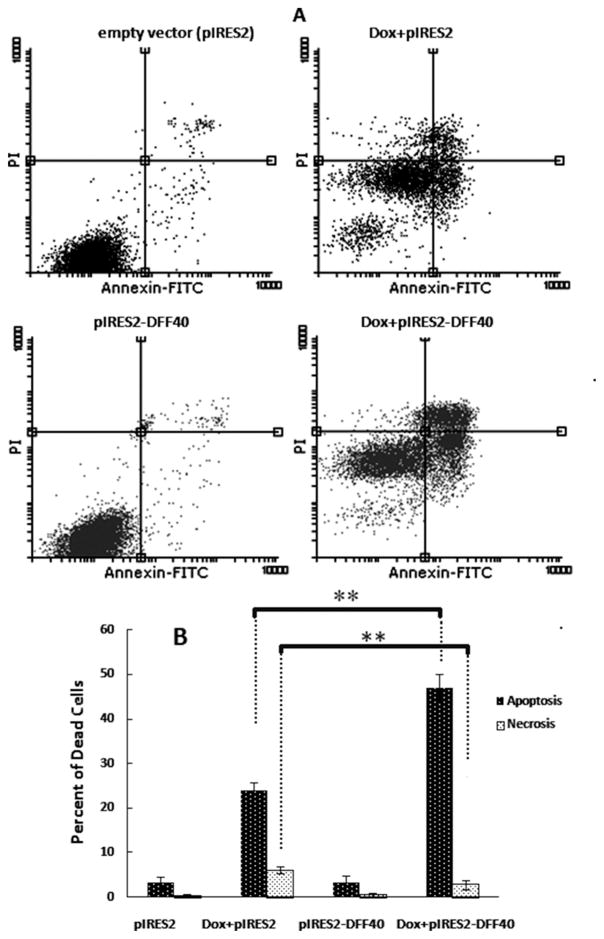
Our results using annexin V/PI staining and FACS analysis showed that the possible mechanism of action for doxorubicin in diminishing cell viability was through apoptosis. The amount of apoptotic cells in Dox+pIRES2 group was 24%, and this was increased to 50% in Dox+pIRES2-DFF40 group, indicating the enhanced apoptotic effects of DFF40 compared with doxorubicin alone (Fig. 4).
Fig. 4.
Doxorubicin induced apoptosis in DFF40 transfected cells. The cells transfected with empty pIRES2 or pIRES2-DFF40 vector were incubated with doxorubicin at IC50 concentration (0.33 μmol/L) for 48 h. The apoptosis evaluated by annexin V/PI staining and FACS analysis. In (A), two-dimensional plots of pIRES2 and pIRES2-DFF40 controls are shown in the left column. The plots for doxorubicin treatments in pIRES2 and pIRES-DFF40 transfected cells are shown in the right column. In the lower part (B), the histogram related to the upper part is depicted. The percentage of dead cells is shown as mean ± SD (n = 3). *P < 0.05 and **P < 0.001 indicate statistically significant difference between 2 columns, illustrated using the column linking “
 ” symbol.
” symbol.
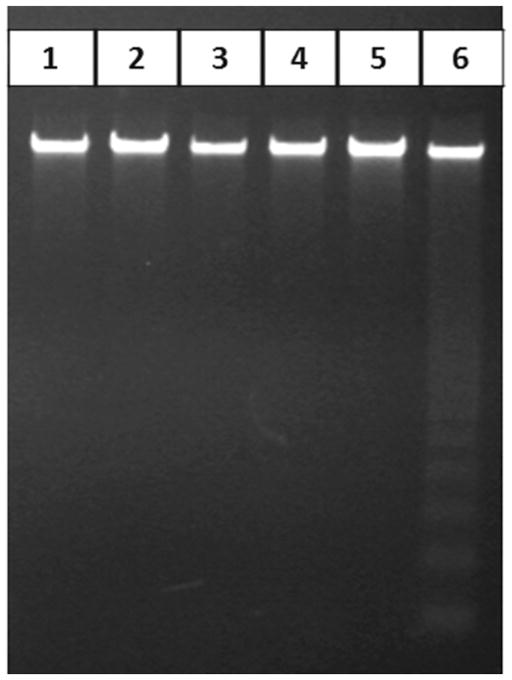
The appearance of DNA laddering in DFF40 overexpressing cells
DNA fragmentation is the typical biochemical sign of cells undergoing apoptosis. DFF40 cleaves DNA in internucleosomal linker regions, as shown in our study (Fig. 5). Here, doxorubicin treatment of pIRES2 (empty vector) transfected cells did not result in detectable DNA fragmentation after 24 and 48 h, while the DNA isolated from Dox+pIRES2-DFF40 group showed the DNA ladder of apoptosis in a time-dependent manner after 48 h (Fig. 5).
Fig. 5.
DNA gel electrophoresis. Cells transfected with empty pIRES2 or pIRES2-DFF40 vectors were incubated with doxorubicin. After 24 or 48 h, genomic DNA was extracted and subjected to gel electrophoresis. DNA ladder formation was observed in pIRES2-DFF40 transfected cells incubated with doxorubicin after 48 h. Lane 1: pIRES2 (−DFF40); lane 2: pIRES2-DFF40 (+DFF40); lane 3: Dox+pIRES2 (24 h treatment); lane 4: Dox+pIRES2-DFF40 (24 h treatment); lane 5: Dox+pIRES2 (48 h treatment); lane 6: Dox+pIRES2-DFF40 (48 h treatment).
DFF40 overexpression had no effect on the migration of T-47D cells
The tendency for cell migration was investigated by the scratch wound assay in the pIRES2 and pIRES2-DFF40 groups in the presence of doxorubicin (0.33 μmol/L) (Fig. 6). Cells exhibited a flattened morphology with distinct borders and good appearance at time zero in pIRES2 and pIRES2-DFF40 groups in the presence or absence of doxorubicin. After 24 and 48 h, there was no distinct tendency for the cells to migrate and fill the wound. Only in the Dox-pIRES2+DFF40 group, some dead cells were present in the furrow, especially after 48 h of treatment. Thus, overexpression of DFF40 minimally affected the migration of T-47D cells.
Fig. 6.
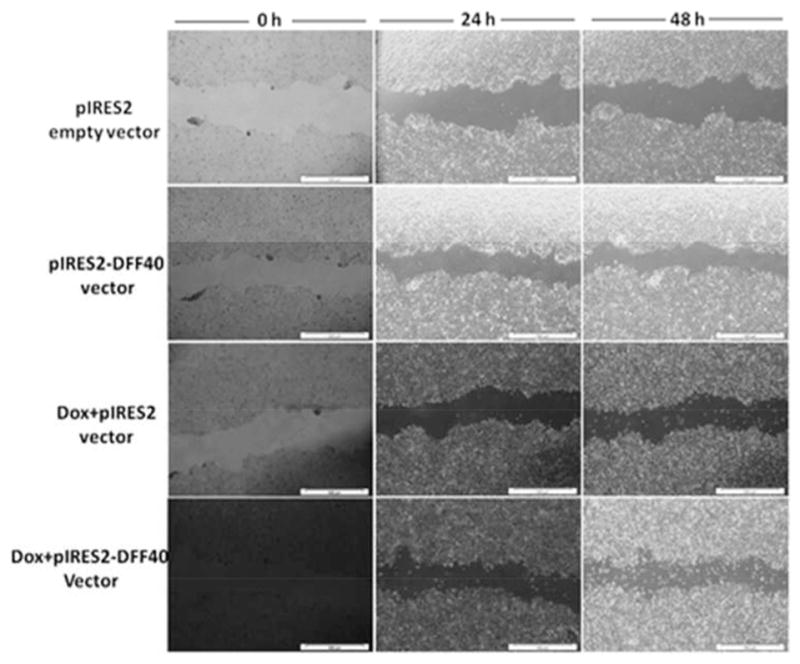
Scratch wound assay for determination of the effect of DFF40 overexpression on cell migration at time zero, 24 and 48 h with and without doxorubicin. There was no distinct effect of DFF40 overexpression on cell migration.
Discussion
Numerous studies suggest that alterations in apoptosis regulatory pathways may result in cancer development. Recent studies have shown that some cancer cells have defective apoptosis and encounter less apoptosis compared with their normal cell equivalent. In addition, the main factor in response of cancer cells to chemotherapeutic drugs is the activation of the apoptotic pathways. For this reason, activation of the suppressed apoptotic pathways in the cancer cells can prevent cancer progression and improve response to chemotherapy. DFF40 is a protein involved in late stages of apoptotic pathways, which creates DNA double strand breaks. This event is the final step in irreversibly driving cancer cell death.
Doxorubicin is routinely used for treatment of diverse types of cancer. However, in spite of its efficacy in treatment of various cancers, it exhibits systemic side effects with the most important one being cardiotoxicity. The exact mechanism of doxorubicin in cancer treatment is not obvious, but different mechanisms have been suggested (Feinstein et al. 1993; Momparler et al. 1976; Munger et al. 1988; Tewey et al. 1984). Doxorubicin can display its cytotoxic effects through blocking of topoisomerase II activity causing cell cycle arrest. It may also activate caspase-3, the most important enzyme in apoptosis and cell death.
In this study, enhancement of the anticancer potency of doxorubicin was our main priority and was carried out via amplification of cellular tendency towards apoptosis. Augmented tendency for apoptosis was achieved by overexpression of DFF40, the main DNA nuclease in the apoptosis pathway and cell death. We stably overexpressed DFF40, and unlike the Yuichi Kimura et al. study that showed that transient expression of DFF40 induced cell death (Kimura et al. 2004), we observed no additional cell death in the absence of drug treatment (Fig. 4). This can be attributed to the DFF40 need for DFF45 for correct folding; however, DFF45 can also inhibit it (Liu et al. 1997, 1999; Zhou et al. 2001). In fact, both the existence of DFF45 and also its elimination via apoptosis induction are compulsory for DFF40 to fragment DNA. Our real-time RT-PCR results showed that increased DFF40 expression was concomitant with that of DFF45 expression after doxorubicin treatment (Fig. 2), and activation of the excess levels of DFF40 could not be achieved until the inhibiting effect of DFF45 is removed by caspase activity. Interestingly, our results showed that the expression level of caspase-3 was also increased in parallel with DFF40 and DFF45 in Dox+pIRES2-DFF40 group (Fig. 2). These findings support the exposing of DFF40 activity for induction of apoptosis.
Our results indicated that DFF40-overexpressing cells exhibited enhanced apoptosis in response to doxorubicin. The net enhancements in apoptosis were 25% (Fig. 4). This supported the MTT results where the viability of DFF40-overexpressing cells was significantly decreased, from 50% in 0.33 μmol/L Dox+pIRES2 group to 30% in 0.33 μmol/L Dox+pIRES2-DFF40 group (a net decrease of 20%) after 48 h of treatment (Fig. 1). The lower viability of the cells in Dox+pIRES2-DFF40 group was also observed at different concentrations of doxorubicin after 24, 48, and 72 h of incubation (Fig. 1). The enhanced decrease in viability and the increased apoptosis in Dox+pIRES2-DFF40 group indicated that the cause for additional cell death in DFF40-overexpressed cells is apoptosis. These results support our hypothesis regarding augmentation of the potency of drugs for apoptosis in DFF40-overexpressing cells. Thus, enhanced DFF40 expression is able to increase cell sensitivity to apoptosis and could be envisaged as a therapeutic mean for cancer treatment.
We studied the cell cycle arrest induction in T-47D cells transfected with the empty pIRES2 or pIRES-DFF40 vector (Fig. 3). There was no change in the cell cycle distribution with DFF40 overexpression alone (Fig. 3A). The doxorubicin treatment of the cells transfected with pIRES2 vector exhibited significant arrest in S phase, which is known to arrest cells in S or G2/M phase (Fig. 3B). Doxorubicin treatment of DFF40transfected cells (Dox+pIRES2-DFF40 group) did not change the pattern of cell cycle distribution compared to Dox+pIRES2 group, illustrating no additional effect of DFF40 overexpression on the doxorubicin-mediated cell cycle arrest (Fig. 3B). Therefore, our data illustrated that cell cycle arrest was not the main mechanism contributing to the increased anticancer effects of doxorubicin in DFF40-overexpressing cells (Fig. 1).
DNA gel electrophoresis is an additional tool for detection of cells undergoing apoptosis. As shown in Fig. 5, DNA fragmentation was only observed in Dox+pIRES2-DFF40 group after 48 h, indicating that the enhanced apoptosis in DFF40-overexpressing cells is the main cause for DNA laddering with doxorubicin treatment. The possible effect of DFF40 overexpression on cell migration was also studied to show that the adopted strategy for gene therapy does not have any effects on the migratory properties of cancer cells (Fig. 6).
In summary, the present results indicated that increased expression of DFF40 was effective for amplification of apoptosis, particularly for those drugs that have the potential for induction of apoptosis. Thus, modulation of DFF40 levels in the presence of doxorubicin or other apoptosis-inducing drugs might be targeted as a therapeutic treatment for breast and perhaps other cancers.
Acknowledgments
The authors would like to thank Iran National Science Foundation (INSF; grant No. 93002224) and Royan Institute of Stem Cell Biology and Technology for their financial support. The authors express their appreciation to the Research Council of the University of Tehran for their valuable patronages. The authors have no conflicts of interest.
Contributor Information
Fatemeh Bagheri, Department of Cell and Molecular Biology, School of Biology, College of Science, University of Tehran, Tehran, Iran; Department of Stem Cells and Developmental Biology, Cell Sciences Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran; Biotechnology Group, Department of Chemical Engineering, Tarbiat Modares University, Tehran, Iran.
Shahrokh Safarian, Department of Cell and Molecular Biology, School of Biology, College of Science, University of Tehran, Tehran, Iran.
Mohamadreza Baghaban Eslaminejad, Department of Stem Cells and Developmental Biology, Cell Sciences Research Center, Royan Institute for Stem Cell Biology and Technology, ACECR, Tehran, Iran.
Nader Sheibani, Department of Ophthalmology and Visual Sciences and Biomedical Engineering, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA.
References
- Bagheri F, Safarian S, Eslaminejad MB, Sheibani N. Stable overexpression of DNA fragmentation factor in T-47D cells: sensitization of breast cancer cells to apoptosis in response to acetazolamide and sulfabenzamide. Mol Biol Rep. 2014;41(11):7387–7394. doi: 10.1007/s11033-014-3626-3. doi:10.1007/s11033-014-3626-3. PMID:25086620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J, Roninson IB. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 1999;18(34):4808–4818. doi: 10.1038/sj.onc.1203078. doi:10.1038/sj.onc.1203078. PMID:10490814. [DOI] [PubMed] [Google Scholar]
- Feinstein E, Canaani E, Weiner LM. Dependence of nucleic acid degradation on in situ free-radical production by adriamycin. Biochemistry. 1993;32(48):13156–13161. doi: 10.1021/bi00211a026. doi:10.1021/bi00211a026. PMID:7694653. [DOI] [PubMed] [Google Scholar]
- Gamen S, Anel A, Perez-Galan P, Lasierra P, Johnson D, Pineiro A, Naval J. Doxorubicin treatment activates a Z-VAD-sensitive caspase, which causes deltapsim loss, caspase-9 activity, and apoptosis in Jurkat cells. Exp Cell Res. 2000;258(1):223–235. doi: 10.1006/excr.2000.4924. doi:10.1006/excr.2000.4924. PMID:10912804. [DOI] [PubMed] [Google Scholar]
- Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57(7):727–741. doi: 10.1016/s0006-2952(98)00307-4. doi:10.1016/S0006-2952(98) 00307-4. PMID:10075079. [DOI] [PubMed] [Google Scholar]
- Hortobágyi GN. Anthracyclines in the treatment of cancer. An overview Drugs. 1997;54(Suppl 4):1–7. doi: 10.2165/00003495-199700544-00003. doi:10.2165/00003495-199700544-00003. PMID:9361955. [DOI] [PubMed] [Google Scholar]
- Hsieh SY, Liaw SF, Lee SN, Hsieh PS, Lin KH, Chu CM, Liaw YF. Aberrant caspase-activated DNase (CAD) transcripts in human hepatoma cells. Br J Cancer. 2003;88(2):210–216. doi: 10.1038/sj.bjc.6600695. doi:10.1038/sj.bjc.6600695. PMID: 12610505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SY, Chen WY, Yeh TS, Sheen IS, Huang SF. High-frequency Alu-mediated genomic recombination/deletion within the caspase-activated DNase gene in human hepatoma. Oncogene. 2005;24(43):6584–6589. doi: 10.1038/sj.onc.1208803. doi:10.1038/sj.onc.1208803. PMID:16007181. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Sugimoto C, Matsukawa S, Sunaga H, Igawa H, Yamamoto H, et al. Combined treatment of cisplatin and overexpression of caspase-activated deoxyribonuclease (CAD) promotes apoptosis in vitro and in vivo. Oral Oncol. 2004;40(4):390–399. doi: 10.1016/j.oraloncology.2003.07.001. doi:10.1016/j.oraloncology.2003.07.001. PMID:14969818. [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89(2):175–184. doi: 10.1016/s0092-8674(00)80197-x. doi:10.1016/S0092-8674(00)80197-X. PMID: 9108473. [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Widlak P, Garrard W, Wang X. Activation of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease). Oligomerization and direct interaction with histone H1. J Biol Chem. 1999;274(20):13836–13840. doi: 10.1074/jbc.274.20.13836. doi:10.1074/jbc.274.20.13836. PMID:10318789. [DOI] [PubMed] [Google Scholar]
- Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36(8):2891–2895. [PubMed] [Google Scholar]
- Munger C, Ellis A, Woods K, Randolph J, Yanovich S, Gewirtz D. Evidence for inhibition of growth related to compromised DNA synthesis in the interaction of daunorubicin with H-35 rat hepatoma. Cancer Res. 1988;48(9):2404–2411. [PubMed] [Google Scholar]
- Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, et al. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otomo T, Sakahira H, Uegaki K, Nagata S, Yamazaki T. Structure of the heterodimeric complex between CAD domains of CAD and ICAD. Nat Struct Biol. 2000;7(8):658–662. doi: 10.1038/77957. doi:10.1038/77957. PMID:10932250. [DOI] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391(6662):96–99. doi: 10.1038/34214. doi:10.1038/34214. PMID:9422513. [DOI] [PubMed] [Google Scholar]
- Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226(4673):466–468. doi: 10.1126/science.6093249. doi:10.1126/science.6093249. PMID:6093249. [DOI] [PubMed] [Google Scholar]
- Wang Y, Blandino G, Givol D. Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene. 1999;18(16):2643–2649. doi: 10.1038/sj.onc.1202632. doi:10.1038/sj.onc.1202632. PMID:10353608. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Uzzo R, Dulin N, Finke JH, Kolenko V. Renal carcinoma cells undergo apoptosis without oligonucleosomal DNA fragmentation. Biochem Biophys Res Commun. 2004;318(3):710–713. doi: 10.1016/j.bbrc.2004.04.086. doi:10.1016/j.bbrc.2004.04.086. PMID:15144896. [DOI] [PubMed] [Google Scholar]
- Yan B, Wang H, Zhuo D, Li F, Kon T, Dewhirst M, Li CY. Apoptotic DNA fragmentation factor maintains chromosome stability in a P53-independent manner. Oncogene. 2006;25(39):5370–5376. doi: 10.1038/sj.onc.1209535. doi:10.1038/sj.onc. 1209535. PMID:16619042. [DOI] [PubMed] [Google Scholar]
- Zhou P, Lugovskoy AA, McCarty JS, Li P, Wagner G. Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45. Proc Natl Acad Sci USA. 2001;98(11):6051–6055. doi: 10.1073/pnas.111145098. doi:10.1073/pnas.111145098. PMID:11371636. [DOI] [PMC free article] [PubMed] [Google Scholar]