Abstract
The past decade has witnessed impressive advances in cancer treatment ushered in by targeted and immunotherapies. However, with significantly prolonged survival, upon recurrence, more patients become inflicted by brain metastasis, which is mostly refractory to all currently available therapeutic regimens. Historically, brain metastasis is an understudied area in cancer research, partly due to the dearth of appropriate experimental models that closely simulate the special biological features of metastasis in the unique brain environment; and to the sophistication of techniques required to perform in-depth studies of the extremely complex and challenging brain metastasis. Yet, with increasing clinical demand for more effective treatment options, brain metastasis research has rapidly advanced in recent years. The present review spotlights the recent major progresses in basic and translational studies of brain metastasis with focuses on new animal models, novel imaging technologies, omics “big data” resources, and some new and exciting biological insights on brain metastasis.
Keywords: Breast cancer, Brain metastasis, Animal models, Genomics, Neuroimaging
I. Introduction
Brain metastasis is the most common central nervous system (CNS) malignant disease, outnumbering primary gliomas by 10:1 [1,2]. Major solid cancers, such as melanoma, lung and breast cancers, produce high incidence of CNS metastasis [3,4]; though sporadic cases of brain metastasis have been reported for a wide variety of malignant cancers [5–8]. Despite dramatic advances in cancer treatment and prolonged patient survival brought upon by targeted and immunotherapies, a growing number of patients manifest brain metastasis in the clinic upon recurrence [9,10], for which the only treatment options remain palliative [11]. For example, the brain metastasis incidence upon recurrence has apparently accelerated since the early 2000s after widespread prescription of trastuzumab in HER2+ breast cancer patients [12,13]. Thus, more effective treatment options for brain metastasis is an emerging unmet need.
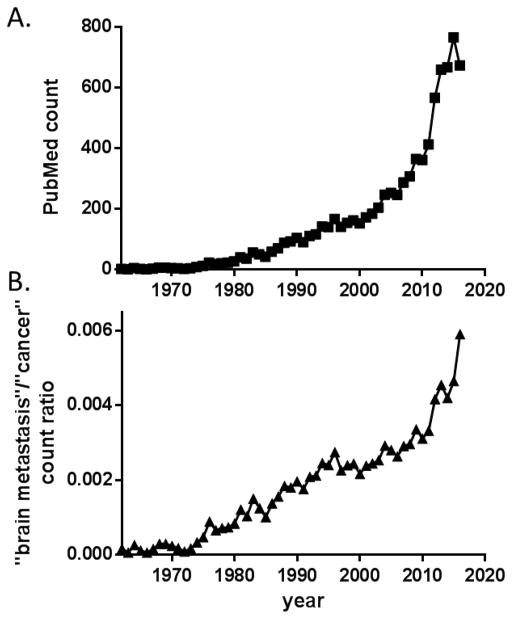
Historically, brain metastasis is an understudied area in cancer research (Fig. 1B), partly due to the dearth of appropriate experimental models and to the sophistication of techniques for in-depth studies. However, history taught us that any revolutionary cancer therapies must come from solid and groundbreaking biological insights of the disease. The research of brain metastasis began to gain attention in recent years. A simple PubMed search for “brain metastasis” or “brain metastases” indicates an exponentially accelerating growth of publication in the topic since around 2010 (Fig. 1). Thus, this review will focus on the progresses that help us gain new insights on the disease; particularly, the new tools for scientific studies of brain metastasis. We will highlight several novel biological discoveries that shed new light on the mechanism of brain metastasis and may translate into outcome-improving clinical practices. For earlier developments of this field, the readers are referred to previous comprehensive reviews [14–17].
Figure 1.
Acceleration of brain metastasis research since 2010. (A) Yearly publication counts using “brain metastasis” or “brain metastases” as search keyword in PubMed. (B) Paper counts featuring “brain metastasis” normalized to publication counts using general search term “cancer”.
II. New models and tools to study brain metastasis
A. New brain metastasis models
Metastasis is a complex biological phenomenon resulting from the successful completion of multiple step of molecular and cellular processes, including leaving the primary tumor, entering the circulation (intravasation), surviving in the circulation, exiting from the circulation (extravasation). Colonizing at the secondary organ site, and finally outgrowing to symptomatic metastatic tumor [18]. Therefore, proper in vivo animal models are indispensable for scientific investigation of brain metastasis [19]. Novel nude rat breast cancer brain metastasis models seem to be good additions in the toolbox for brain metastasis studies [20]. A handful of in vivo brain metastasis models (Table 1) covering distinctive subtypes of breast cancer had been previously reported [21], yet no inflammatory breast cancer (IBC) brain metastasis models has been reported previously. Thus, recent report of new experimental brain metastasis models by tail-vein injection for several inflammatory breast cancer (IBC) lines is a good addition to the repertoire of in vivo breast cancer brain metastasis models [22]. Recently, breast cancer patient-derived xenograft (PDX) models have been successfully used to induce brain metastatic lesions for testing efficacy of dual inhibition of PI3K and mTORC1 in treating HER2+ human breast cancer brain metastasis in mice [23].
Table 1.
In vivo breast cancer brain metastasis models
| Subtype | Human (xenograft) | Rodent (syngeneic) |
|---|---|---|
| TNBC | MDA-MB-231 MDA-MB-435 HCC70 |
4T1 (BALB/c) EO771 (C57BL/6) |
| HER2+ | MDA-MB-361 BT474 HCC1954 DF-BM354 (PDX) DF-BM355 (PDX) |
MMTV-Neu/PTEN−/− (FVB) |
| ER+ | MCF-7 T-47D |
– |
| IBC | SUM149 MDA-IBC3 |
– |
Note: TNBC, triple-negative breast cancer; IBC, inflammatory breast cancer.
Of interesting note, a recent phase 2 clinical trial of pembrolizumab (anti-PD1) immunotherapy in melanoma and non-small-cell lung cancer (NSCLC) patients showed unprecedented durable activity against brain metastasis, suggesting that the host immune system may play critical roles in metastatic cancer outgrowth in the “immune-privileged” CNS microenvironment [24]. This counterintuitive clinical observation certainly warrants further in-depth mechanistic investigations. However, most reported in vivo brain metastasis models have been established using human cancer cells growing in immune-compromised mice. The use of xenograft models of human cancer cell lines or PDXs grown in mice has enabled many important discoveries about brain metastasis. However, xenografts in immunocompromised animals discount the impact of the adaptive immune system on preventing or promoting brain metastasis. To address this issue, the field needs to develop partially humanized animal models to allow for evaluation of human cancer xenografts in an immunocompetent host, which is highly challenging.
To study the impact of immune system on brain metastasis, murine tumor-derived allograft in syngeneic mouse background can be an alternative approach, although mouse tumor models may not perfectly recapitulate human disease. To this end, our group has performed some pilot studies to expand the experimental brain metastasis models into immune-competent mouse strains. In addition to the previously reported brain metastatic 4T1 mammary tumor line [25,26], we have successfully established an experimental brain metastasis model using dissociated MMTV-Neu/PTEN-loss mammary tumor cells in the FVB female mice (unpublished data). Recently, we also showed that EO771, a spontaneous mammary tumor line from the C57BL/6 mice, is able to produce aggressive brain metastasis in the syngeneic host after intracarotid artery injection (unpublished data). Using these brain metastasis models in immuno-competent mice will shed more light on the roles of the host immune system in modulating brain metastasis, and enable development of strategies that potentially enhance the efficacy of powerful immunotherapy treatments against brain metastasis.
B. Novel imaging techniques
Neuroimaging remains the mainstay modality for brain metastasis diagnosis [27,28]. There have been major advances in neuroimaging. For example, technological advances in multiphoton laser scanning microscopy have improved our ability to survey single steps of the CNS metastatic cascade via live imaging through a chronic cranial window in vivo [29]. A modified MRI modality has been applied to evaluate the integrity of the blood-brain barrier in mouse model bearing the MDA-MB-213 breast cancer brain metastasis [30]. Intriguingly, the glial cells are activated and have upregulated translocator protein (TSPO) in response to metastasis; thus, agents targeting the upregulated translocator protein (TSPO) on activated glia have been developed for SPECT/PET imaging [31]. Because glial activation begins at the very early stage of cancer cell metastatic colonization in the CNS, this method could potentially enable detection of brain metastases substantially earlier than the current clinical approach of MRI [31]. In the meantime, radio-immunotherapy has made significant strides forward in treating and monitoring brain metastasis progression using armed antibodies [32]. Confocal imaging of brain slices also prove to be a highly useful technique for the studies of brain metastasis [33]. Another interesting report described a bioluminescence-based imaging approach using the 5HRE-ODD-Luc reporter gene to assess the hypoxia conditions in intracranial lesions [34]. More recently, development of SMART 3D (Spatial filtering-based background removal and Multi-chAnnel forest classifiers-based 3D ReconsTruction), an integrative imaging platform/pipeline for 3D quantitative analysis of heterogeneous metastasis has been reported [35].
From a seemingly unrelated field, new tissue clearing technologies (termed CLARITY, for Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging/Immunostaining/in situ-hybridization-compatible Tissue hYdrogel), developed in the neuroscience field, have dramatically facilitated researcher’s ability to see deep into the tissue, especially the brain [36]; it is reasonable to anticipate that the investigation of brain metastasis can borrow such revolutionary neuroscience technologies to enable detailed delineation of cancer cell behavior deep in the brain. Collectively, it can be envisioned that complementary applications of various new advanced imaging technologies (Table 2) will greatly enhance our ability to visually depict the biological interactions taking place in CNS metastasis at the molecular and cellular levels, which will provide structural insight on functional implications.
Table 2.
Several new research-facilitating imaging technologies for brain metastasis
| Name | Description | Ref |
|---|---|---|
| MPLSM | Multiphoton laser-scanning microscopy through a chronic cranial window to enable long-term in vivo microscopy. | [29] |
| Gd-MRI | Gadolinium-enhanced MRI used to evaluate the blood-brain barrier (BBB) integrity in brain metastasis mouse model. | [30] |
| TSPO-targeted SPECT/PET | Non-invasive SPECT/PET imaging based on induction of translocator protein (TSPO) expression following glial activation. | [31] |
| 5HRE-ODD-luc | HIF-1α reporter construct, 5HRE-ODD-luc, enables non-invasive imaging of hypoxia dynamics in brain metastasis mouse model. | [34] |
| SMART 3D | An integrative imaging platform that enables multiplexed quantitative 3D analysis of metastasis in situ from the molecular level to the whole organ scale. | [35] |
| CLARITY | Transformation of intact tissue into a nanoporous hydrogel-hybridized form for transparent visualization. | [36] |
Note: SMART 3D, Spatial filtering-based background removal and Multi-chAnnel forest classifiers-based 3D ReconsTruction; CLARITY, Clear Lipid-exchanged Acrylamide-hybridized Rigid Imaging/Immunostaining/in situ-hybridization-compatible Tissue hYdrogel.
Omics technologies for deep understanding of brain metastasis
Taking advantage of the rapid progress in technologies enabling comprehensive interrogation of biological systems at the molecular levels, investigators have studied brain metastasis at the genome [37–39], epigenome [40–42]; transcriptome [43], proteome [44–46], and metabolome [47] levels and made interesting findings [48,49]. Several existing datasets (Table 3) have provided great value and insights to the studies of brain metastasis [50,51]. For example, large-scale next-generation sequencing (NGS) was performed for comprehensive genomic profiling of advanced squamous cell lung cancer cells from 79 patients with stage IV disease and revealed PI3K aberrations and clonal heterogeneity in brain metastasis [52]. Built on an 86-patient case series containing multiple tumor types (including lung, breast and renal cell carcinomas), whole-exome sequencing (WES) of matched clinical examples were used to delineate the evolutionary progression from primary cancer to brain metastasis [53]. Intriguingly, the epigenomic landscape of melanoma brain metastasis was also explored for potential new therapeutic targets, which revealed that DNA hypermethylation of the HOX family genes, particularly HOXD9, suppresses its transcriptional activity and promotes brain metastasis. [41]. Interestingly, a 2D-DIGE (Difference in Gel Electrophoresis) proteomic analysis was performed to identify potential regulatory networks in breast cancer brain metastasis [54], and similar integrated genomic profiling of breast cancer brain metastasis sheds important new light by identifying common molecular alterations of the disease, such as enriched overexpression of cell cycle and G2/M transition pathway genes and increased overall methylation [42].
Table 3.
Public datasets related to brain metastasis
| Accession | Description |
|---|---|
| GSE14020 | Affymetrix microarray profiling of distinct organ-specific metastatic tumors (brain, bone, lung and liver) of breast cancer patients. |
| GSE12276 | Affymetrix microarray profiling of primary breast cancer tumors with clinical follow-up of brain or other metastatic relapse and survival. |
| GSE2603 | Affymetrix microarray profiling of MDA-MB-231 sublines of different metastatic tropisms (bone and lung) and primary breast cancer tumors with metastasis follow-up. |
| GSE2034 | Affymetrix microarray profiling of primary human breast cancer tumors with brain metastasis follow-up information. |
| GSE3141 | Affymetrix microarray profiling of primary lung cancer specimens with clinical follow-up data of brain metastasis-free survival time. |
| GSE14690 | Illumina DASL (the cDNA-mediated Annealing, Selection, extension and Ligation) cancer panel profiling of matched and unmatched breast cancer primary tumor and brain metastasis. |
| GSE20016 | NHGRI (National Human Genome Research Institute) cDNA array profiling of laser-captured epithelial cells from resected human brain metastases and unmatched primary breast tumors. |
| GSE38057 | Illumina DASL cancer panel of primary HER2+ breast tumors with or without brain metastasis relapse. |
| GSE19184 | Illumina human and mouse beadchip profiling of 4 different types of cancer (MDA-MB-231Br3, PC14Br4, KM12M, A375SM) experimental brain metastases and orthotopic xenografts in mice. |
Many of these comprehensive profiling studies utilize patient specimens that add significant clinical relevance for the data from such projects; yet there is a general lack of concordance for the conclusions of these studies, because a variety of reasons may complicate the interpretation, such as the heterogeneity nature of source materials. A general consensus framework still waits to be developed for the molecular alternations that promote brain metastasis. Still, the gradual buildup of these “big data” resources is a great leap forward for the field that will undoubtedly facilitate generation of new hypothesis or verification of existing hypothesis with precious patient-derived information.
III. New biological insights on brain metastasis
A. Epigenetic regulations and microRNA in brain metastasis
Epigenetic dysregulation is an integral part of the tumorigenic and metastatic processes, and simultaneous modulation of multiple genetic programs through microRNA is an important epigenetic regulatory mechanism [40]. For example, our team found that PTEN in metastatic breast cancer cells was epigenetically and reversibly down-regulated by astrocyte-released microRNA19-a [50]. MicroRNA profiling that compares the primary and brain metastatic breast cancer specimens identified miR-509 as a critical determinant for brain metastasis [55]. In another study comparing breast cancer patients with or without brain metastasis, up-regulation of microRNA-10b was identified to be strongly associated with the development of brain metastasis [56]. Several microRNAs were able to suppress brain metastasis in breast cancer cells: 1) microRNA-7 down-regulates KLF4 in the cancer stem-like cell (CSC) population of MDA-MB-231 brain-seeking cells [57]; 2) microRNA-146a targets beta-catenin and hnRNPC in the MDA-MB-435 experimental brain metastasis model [58]; 3) microRNA-1258 targets heparanase to suppress brain metastasis in the MDA-MB-231 experimental brain metastasis model [59]. In contrast, miR-141 was shown to promote brain metastasis in several breast cancer cell lines [22].
Similar studies in lung cancer also identified miR-145-5p [60], microRNA-95-3p [61], and microRNA-378 [62] as important determinants in brain metastasis. In lung-to-brain metastasis initiating cells, STAT3 was found to regulate target miR-21 that promotes the brain metastasis phenotype [63].
Notably, because of the multi-target nature of microRNA regulation, they may serve as better classifier for brain metastasis prediction than gene expressions. To that end, studies have aimed to identify microRNA signatures as predictive biomarker for the development of brain metastasis in melanoma [64] and lung cancer [65]. In addition to microRNA, long noncoding RNA (lncRNA) is also an important epigenetic regulatory mechanism [66]. Indeed, it was demonstrated that lncRNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer [67]. The exploration of epigenetic alterations in brain metastasis may lead to novel and alternative targets for therapeutic intervention.
B. Interactions between metastatic cancer cells and CNS microenvironment
An essential feature of metastatic cancer cells is its intimate interaction with the stromal cells in the host organ sites, which could provide a permissible and even supportive microenvironment for metastasis to thrive. Having a highly heterogeneous cell populations and complex cellular structure, the brain stromal cells interact intimately with the invading metastatic cancer cells; however, the mechanistic insights remain elusive. In the past several years, major progresses have been made in this realm with several landmark publications. Our group characterized an intricate relationship among breast cancer cells, astrocytes and microglial cells where astrocyte-released exosomes transfer PTEN-targeting microRNA into cancer cells to mediate PTEN down-regulation in the cancer cells, resulting in CCL2 up-regulation and recruitment of brain metastasis-promoting microglia cells [50]. Interestingly, a reciprocal interaction of mutual promotion of cancer cell proliferation and astrocyte cell survival was also found to be an early event of prostate cancer brain metastasis [68]. Faithful to its essential role in mediating CNS homeostasis, astrocytes were found to facilitate melanoma brain metastasis via secretion of IL-23 and reciprocal up-regulation of MMP2 and metastatic invasion of the cancer cells [69]. Intriguingly, there is another new-found reciprocal interaction where cancer cells promote the assembly of carcinoma-astrocyte gap junctions and utilize these communication complexes to up-regulate secretion of inflammatory cytokines from astrocytes, which ultimately supports tumor growth and survival of cancer cells via the STAT1 and NF-kappaB pathways [51]. Additionally, the CNS stroma-derived plasmin and the plasminogen activator (PA) inhibitory serpins from cancer cells represent another interaction that enhances metastatic outgrowth by promoting cancer cell survival and vascular co-option [70]. All these in-depth mechanistic discoveries are exciting and much hope has been placed on translating these novel findings into groundbreaking therapeutic strategies against brain metastasis.
C. Blood-brain barrier and brain metastasis
Blood-brain barrier (BBB) refers to specialized blood vessel structures in the CNS that are lined by endothelial cells fortified with tight junction complexes, which effectively shield the brain parenchyma from cells and macromolecules in the general circulation; the brain-facing side of the BBB is surrounded by a thick basement membrane, which is supported by pericytes; and then astrocytic end-feet make the outer layer of the BBB. BBB provides a sanctuary organ environment for neuronal functions [71,72]. Apparently, BBB plays crucial roles in 1) affecting metastatic cell colonization in the brain, and 2) modulating treatment efficacy of brain metastasis by serving as a formidable barrier for the majority of therapeutic agents [73–77]. To establish a metastatic lesion, cancer cells have to first pass the BBB for successful extravasation into the CNS parenchyma [77,78]. Many systemically efficacious anti-cancer therapeutics cannot penetrate the BBB and become ineffective as brain metastasis treatment regimen. Therefore, there have been extensive efforts recently aiming to understand the biology of tumor-BBB interaction and to find novel ways that facilitate drug delivery through the BBB. To this end, the permeability of blood-brain barrier is highly heterogeneous at sites of metastasis [79,80,30]. Furthermore, pericytes were shown to have significant contribution to the permeability of blood-tumor barrier in multiple experimental breast cancer brain metastasis models [81]. Src kinase was found to promote the breast cancer cell extravasation through the BBB [82]. Ex vivo assessment of the blood brain barrier with Evans blue also proved to be a useful technique in studies of breast cancer brain metastasis models [82,83]. And new efforts that take advantage of nanotechnology to overcome the BBB demonstrated promising results [84,85]. Intriguingly, focused ultrasound was shown to disrupt the BBB and thus facilitated antibody delivery to the brain metastasis lesions resulting in growth inhibition [86]. Overall, there have overwhelming evidence to support the notion that BBB is highly heterogeneous and selective in the cases of brain metastasis; and new delivery technology such as nanomedicine may finally bring into reality that BBB be disrupted to allow efficient delivery of therapeutics into the brain for brain metastasis treatment.
IV. Conclusion
Scientific and clinical investigations of brain metastasis have boomed in the past decade. Ongoing efforts are aimed for deeper mechanistic understanding and better treatment options of brain metastasis. The right approach is to tackle the challenging issues of brain metastasis from multiple new angles, such as epigenetic alterations in the cancer cells, reciprocal communications between the CNS stromal cells and metastatic cancer cells, different ways to modulate the BBB to improve treatment efficacy, and others. At the same time, a continuous development of new and better experimental technologies (better animal models, higher resolution of tissue imaging, comprehensive and thorough molecular characterization of disease specimens, nanotechnology-enhanced delivery, etc.) certainly expedite the speed of mechanistic and functional discoveries. It is hopeful that such concentrated attacks on multiple fronts of this dreadful disease may lead to major breakthroughs that will significantly and meaningfully improve the therapeutic efficacy and clinical outcome for brain metastasis patients in dire needs.
Acknowledgments
This work was supported partially by R01CA112567-06 (DY), R01CA184836 (DY), METAvivor Research Grant (DY), China Medical University Research Fund (DY). D. Yu is the Hubert L. & Olive Stringer Distinguished Chair in Basic Science at MDACC.
Footnotes
We apologize for not being able to cite all the relevant original research and review articles due to space limitation.
References
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 4.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 5.Perin T, Canzonieri V, Memeo L, Massarut S. Breast metastasis of primary colon cancer with micrometastasis in the axillary sentinel node: a metastasis that metastasized? Diagn Pathol. 2011;6:45. doi: 10.1186/1746-1596-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen S, Dahlke M, Trang NT, Khoa MT, Rades D. Estimation of the Six-month Survival Probability After Radiosurgery for Brain Metastases from Kidney Cancer. Anticancer Res. 2015;35(7):4215–4217. [PubMed] [Google Scholar]
- 7.Chua C, Raaj J, Pan S, Farid M, Lee JF, Ho ZC, et al. Brain metastasis in sarcoma: Does metastasectomy or aggressive multi-disciplinary treatment improve survival outcomes. Asia Pac J Clin Oncol. 2016;12(1):e16–22. doi: 10.1111/ajco.12111. [DOI] [PubMed] [Google Scholar]
- 8.Lemke J, Barth TF, Juchems M, Kapapa T, Henne-Bruns D, Kornmann M. Long-term survival following resection of brain metastases from pancreatic cancer. Anticancer Res. 2011;31(12):4599–4603. [PubMed] [Google Scholar]
- 9.Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 10.Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong W, Jarvis C, Mackillop WJ. Estimating the need for palliative radiotherapy for brain metastasis: a benchmarking approach. Clin Oncol (R Coll Radiol) 2015;27(2):83–91. doi: 10.1016/j.clon.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Goncalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32(11):4655–4662. [PubMed] [Google Scholar]
- 13.Smedby KE, Brandt L, Backlund ML, Blomqvist P. Brain metastases admissions in Sweden between 1987 and 2006. Br J Cancer. 2009;101(11):1919–1924. doi: 10.1038/sj.bjc.6605373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8(6):344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Yu D. Microenvironment determinants of brain metastasis. Cell Biosci. 2011;1(1):8. doi: 10.1186/2045-3701-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 18.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 19.Daphu I, Sundstrom T, Horn S, Huszthy PC, Niclou SP, Sakariassen PO, et al. In vivo animal models for studying brain metastasis: value and limitations. Clin Exp Metastasis. 2013;30(5):695–710. doi: 10.1007/s10585-013-9566-9. [DOI] [PubMed] [Google Scholar]
- 20.Wu YJ, Muldoon LL, Gahramanov S, Kraemer DF, Marshall DJ, Neuwelt EA. Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. J Neurooncol. 2012;110(1):27–36. doi: 10.1007/s11060-012-0942-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 22.Debeb BG, Lacerda L, Anfossi S, Diagaradjane P, Chu K, Bambhroliya A, et al. miR-141-Mediated Regulation of Brain Metastasis From Breast Cancer. J Natl Cancer Inst. 2016;108(8) doi: 10.1093/jnci/djw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni J, Ramkissoon SH, Xie S, Goel S, Stover DG, Guo H, et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016;22(7):723–726. doi: 10.1038/nm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–983. doi: 10.1016/S1470-2045(16)30053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhang N, Hoffman RM, Zhao M. Surgically-Induced Multi-organ Metastasis in an Orthotopic Syngeneic Imageable Model of 4T1 Murine Breast Cancer. Anticancer Res. 2015;35(9):4641–4646. [PubMed] [Google Scholar]
- 26.Erin N, Kale S, Tanriover G, Koksoy S, Duymus O, Korcum AF. Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res Treat. 2013;139(3):677–689. doi: 10.1007/s10549-013-2584-0. [DOI] [PubMed] [Google Scholar]
- 27.Barajas RF, Jr, Cha S. Imaging diagnosis of brain metastasis. Prog Neurol Surg. 2012;25:55–73. doi: 10.1159/000331174. [DOI] [PubMed] [Google Scholar]
- 28.Waerzeggers Y, Rahbar K, Riemann B, Weckesser M, Schafers M, Hesselmann V, et al. PET in the diagnosis and management of patients with brain metastasis: current role and future perspectives. Cancer Biomark. 2010;7(4):219–233. doi: 10.3233/CBM-2010-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 30.Murrell DH, Zarghami N, Jensen MD, Chambers AF, Wong E, Foster PJ. Evaluating Changes to Blood-Brain Barrier Integrity in Brain Metastasis over Time and after Radiation Treatment. Transl Oncol. 2016;9(3):219–227. doi: 10.1016/j.tranon.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Brien ER, Kersemans V, Tredwell M, Checa B, Serres S, Soto MS, et al. Glial activation in the early stages of brain metastasis: TSPO as a diagnostic biomarker. J Nucl Med. 2014;55(2):275–280. doi: 10.2967/jnumed.113.127449. [DOI] [PubMed] [Google Scholar]
- 32.Poli GL, Bianchi C, Virotta G, Bettini A, Moretti R, Trachsel E, et al. Radretumab radioimmunotherapy in patients with brain metastasis: a 124I-L19SIP dosimetric PET study. Cancer Immunol Res. 2013;1(2):134–143. doi: 10.1158/2326-6066.CIR-13-0007. [DOI] [PubMed] [Google Scholar]
- 33.Sarmiento M. Use of confocal microscopy in the study of microglia in a brain metastasis model. Methods Mol Biol. 2013;1041:337–346. doi: 10.1007/978-1-62703-520-0_29. [DOI] [PubMed] [Google Scholar]
- 34.Saha D, Dunn H, Zhou H, Harada H, Hiraoka M, Mason RP, et al. In vivo bioluminescence imaging of tumor hypoxia dynamics of breast cancer brain metastasis in a mouse model. J Vis Exp. 2011;(56) doi: 10.3791/3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guldner IH, Yang L, Cowdrick KR, Wang Q, Alvarez Barrios WV, Zellmer VR, et al. An Integrative Platform for Three-dimensional Quantitative Analysis of Spatially Heterogeneous Metastasis Landscapes. Sci Rep. 2016;6:24201. doi: 10.1038/srep24201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung K, Wallace J, Kim SY, Kalyanasundaram S, Andalman AS, Davidson TJ, et al. Structural and molecular interrogation of intact biological systems. Nature. 2013;497(7449):332–337. doi: 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JY, Park K, Lim SH, Kim HS, Yoo KH, Jung KS, et al. Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget. 2015;6(41):43731–43742. doi: 10.18632/oncotarget.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee HW, Seol HJ, Choi YL, Ju HJ, Joo KM, Ko YH, et al. Genomic copy number alterations associated with the early brain metastasis of non-small cell lung cancer. Int J Oncol. 2012;41(6):2013–2020. doi: 10.3892/ijo.2012.1663. [DOI] [PubMed] [Google Scholar]
- 39.Li F, Sun L, Zhang S. Acquirement of DNA copy number variations in non-small cell lung cancer metastasis to the brain. Oncol Rep. 2015;34(4):1701–1707. doi: 10.3892/or.2015.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marzese DM, Witz IP, Kelly DF, Hoon DS. Epigenomic landscape of melanoma progression to brain metastasis: unexplored therapeutic alternatives. Epigenomics. 2015;7(8):1303–1311. doi: 10.2217/epi.15.77. [DOI] [PubMed] [Google Scholar]
- 41.Marzese DM, Scolyer RA, Huynh JL, Huang SK, Hirose H, Chong KK, et al. Epigenome-wide DNA methylation landscape of melanoma progression to brain metastasis reveals aberrations on homeobox D cluster associated with prognosis. Hum Mol Genet. 2014;23(1):226–238. doi: 10.1093/hmg/ddt420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salhia B, Kiefer J, Ross JT, Metapally R, Martinez RA, Johnson KN, et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One. 2014;9(1):e85448. doi: 10.1371/journal.pone.0085448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci U S A. 2011;108(42):17456–17461. doi: 10.1073/pnas.1114210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camacho L, Guerrero P, Marchetti D. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PLoS One. 2013;8(9):e73790. doi: 10.1371/journal.pone.0073790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida A, Okamoto N, Tozawa-Ono A, Koizumi H, Kiguchi K, Ishizuka B, et al. Proteomic analysis of differential protein expression by brain metastases of gynecological malignancies. Hum Cell. 2013;26(2):56–66. doi: 10.1007/s13577-012-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dun MD, Chalkley RJ, Faulkner S, Keene S, Avery-Kiejda KA, Scott RJ, et al. Proteotranscriptomic Profiling of 231-BR Breast Cancer Cells: Identification of Potential Biomarkers and Therapeutic Targets for Brain Metastasis. Mol Cell Proteomics. 2015;14(9):2316–2330. doi: 10.1074/mcp.M114.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sjobakk TE, Vettukattil R, Gulati M, Gulati S, Lundgren S, Gribbestad IS, et al. Metabolic profiles of brain metastases. Int J Mol Sci. 2013;14(1):2104–2118. doi: 10.3390/ijms14012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neagu MR, Gill CM, Batchelor TT, Brastianos PK. Genomic profiling of brain metastases: current knowledge and new frontiers. Chin Clin Oncol. 2015;4(2):22. doi: 10.3978/j.issn.2304-3865.2015.06.04. [DOI] [PubMed] [Google Scholar]
- 49.Saunus JM, Quinn MC, Patch AM, Pearson JV, Bailey PJ, Nones K, et al. Integrated genomic and transcriptomic analysis of human brain metastases identifies alterations of potential clinical significance. J Pathol. 2015;237(3):363–378. doi: 10.1002/path.4583. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paik PK, Shen R, Won H, Rekhtman N, Wang L, Sima CS, et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov. 2015;5(6):610–621. doi: 10.1158/2159-8290.CD-14-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015;5(11):1164–1177. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li F, Glinskii OV, Zhou J, Wilson LS, Barnes S, Anthony DC, et al. Identification and analysis of signaling networks potentially involved in breast carcinoma metastasis to the brain. PLoS One. 2011;6(7):e21977. doi: 10.1371/journal.pone.0021977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xing F, Sharma S, Liu Y, Mo YY, Wu K, Zhang YY, et al. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-alpha. Oncogene. 2015;34(37):4890–4900. doi: 10.1038/onc.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmad A, Sethi S, Chen W, Ali-Fehmi R, Mittal S, Sarkar FH. Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am J Transl Res. 2014;6(4):384–390. [PMC free article] [PubMed] [Google Scholar]
- 57.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73(4):1434–1444. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang SJ, Seol HJ, Park YM, Kim KH, Gorospe M, Nam DH, et al. MicroRNA-146a suppresses metastatic activity in brain metastasis. Mol Cells. 2012;34(3):329–334. doi: 10.1007/s10059-012-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Sullivan PS, Goodman JC, Gunaratne PH, Marchetti D. MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Res. 2011;71(3):645–654. doi: 10.1158/0008-5472.CAN-10-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Donzelli S, Mori F, Bellissimo T, Sacconi A, Casini B, Frixa T, et al. Epigenetic silencing of miR-145–5p contributes to brain metastasis. Oncotarget. 2015;6(34):35183–35201. doi: 10.18632/oncotarget.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang SJ, Lee HW, Kim HR, Song HJ, Lee DH, Lee H, et al. Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget. 2015;6(24):20434–20448. doi: 10.18632/oncotarget.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen LT, Xu SD, Xu H, Zhang JF, Ning JF, Wang SF. MicroRNA-378 is associated with non-small cell lung cancer brain metastasis by promoting cell migration, invasion and tumor angiogenesis. Med Oncol. 2012;29(3):1673–1680. doi: 10.1007/s12032-011-0083-x. [DOI] [PubMed] [Google Scholar]
- 63.Singh M, Garg N, Venugopal C, Hallett R, Tokar T, McFarlane N, et al. STAT3 pathway regulates lung-derived brain metastasis initiating cell capacity through miR-21 activation. Oncotarget. 2015;6(29):27461–27477. doi: 10.18632/oncotarget.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanniford D, Zhong J, Koetz L, Gaziel-Sovran A, Lackaye DJ, Shang S, et al. A miRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin Cancer Res. 2015;21(21):4903–4912. doi: 10.1158/1078-0432.CCR-14-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nasser S, Ranade AR, Sridhar S, Haney L, Korn RL, Gotway MB, et al. Biomarkers associated with metastasis of lung cancer to brain predict patient survival. Int J Data Min Bioinform. 2011;5(3):287–307. doi: 10.1504/ijdmb.2011.040385. [DOI] [PubMed] [Google Scholar]
- 66.Wu K, Sharma S, Venkat S, Liu K, Zhou X, Watabe K. Non-coding RNAs in cancer brain metastasis. Front Biosci (Schol Ed) 2016;8:187–202. doi: 10.2741/s457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L, Chen L, Wang Y, Jiang X, Xia H, Zhuang Z. Long noncoding RNA MALAT1 promotes brain metastasis by inducing epithelial-mesenchymal transition in lung cancer. J Neurooncol. 2015;121(1):101–108. doi: 10.1007/s11060-014-1613-0. [DOI] [PubMed] [Google Scholar]
- 68.de Oliveira Barros EG, Palumbo A, Jr, Mello PL, de Mattos RM, da Silva JH, Pontes B, et al. The reciprocal interactions between astrocytes and prostate cancer cells represent an early event associated with brain metastasis. Clin Exp Metastasis. 2014;31(4):461–474. doi: 10.1007/s10585-014-9640-y. [DOI] [PubMed] [Google Scholar]
- 69.Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Ben Menachem S, et al. Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol. 2015;236(1):116–127. doi: 10.1002/path.4509. [DOI] [PubMed] [Google Scholar]
- 70.Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jia W, Martin TA, Zhang G, Jiang WG. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013;33(6):2353–2359. [PubMed] [Google Scholar]
- 72.Blecharz KG, Colla R, Rohde V, Vajkoczy P. Control of the blood-brain barrier function in cancer cell metastasis. Biol Cell. 2015;107(10):342–371. doi: 10.1111/boc.201500011. [DOI] [PubMed] [Google Scholar]
- 73.Wilhelm I, Molnar J, Fazakas C, Hasko J, Krizbai IA. Role of the blood-brain barrier in the formation of brain metastases. Int J Mol Sci. 2013;14(1):1383–1411. doi: 10.3390/ijms14011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weidle UH, Niewohner J, Tiefenthaler G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genomics Proteomics. 2015;12(4):167–177. [PubMed] [Google Scholar]
- 75.Winkler F, Osswald M, Blaes J, Liao Y, Solecki G, Gommel M, et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1327. [DOI] [PubMed] [Google Scholar]
- 76.Fortin D. The blood-brain barrier: its influence in the treatment of brain tumors metastases. Curr Cancer Drug Targets. 2012;12(3):247–259. doi: 10.2174/156800912799277511. [DOI] [PubMed] [Google Scholar]
- 77.Wrobel JK, Toborek M. Blood-brain barrier remodeling during brain metastasis formation. Mol Med. 2016 doi: 10.2119/molmed.2015.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yonemori K, Tsuta K, Ono M, Shimizu C, Hirakawa A, Hasegawa T, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116(2):302–308. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- 79.Connell JJ, Chatain G, Cornelissen B, Vallis KA, Hamilton A, Seymour L, et al. Selective permeabilization of the blood-brain barrier at sites of metastasis. J Natl Cancer Inst. 2013;105(21):1634–1643. doi: 10.1093/jnci/djt276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adkins CE, Mohammad AS, Terrell-Hall TB, Dolan EL, Shah N, Sechrest E, et al. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin Exp Metastasis. 2016;33(4):373–383. doi: 10.1007/s10585-016-9784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lyle LT, Lockman PR, Adkins CE, Mohammad AS, Sechrest E, Hua E, et al. Alterations in Pericyte Subpopulations are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Huang WC, Zhang L, Zhang C, Lowery FJ, Ding Z, et al. SRC family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res. 2013;73(18):5764–5774. doi: 10.1158/0008-5472.CAN-12-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Do J, Foster D, Renier C, Vogel H, Rosenblum S, Doyle TC, et al. Ex vivo Evans blue assessment of the blood brain barrier in three breast cancer brain metastasis models. Breast Cancer Res Treat. 2014;144(1):93–101. doi: 10.1007/s10549-014-2854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J, Cai P, Shalviri A, Henderson JT, He C, Foltz WD, et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano. 2014;8(10):9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 85.Wan X, Zheng X, Pang X, Pang Z, Zhao J, Zhang Z, et al. Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget. 2016 doi: 10.18632/oncotarget.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release. 2016;238:281–288. doi: 10.1016/j.jconrel.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]