Despite the innumerable successes in curing patients with Hodgkin Lymphoma (HL) in the front-line setting still up to 20% of patients will experience relapsed or refractory disease. Salvage chemotherapy with regimens such as ICE (ifosfamide, carboplatin, and etoposide) followed by high-dose chemotherapy plus an autologous stem cell transplant (HDT/ASCT) historically leads to a 40–60% long-term event-free survival (EFS) rate [1,2]. ICE as an established regimen induces a computed tomography (CT)-assessed complete remission (CR) rate of 26% when given at baseline doses, while augmented dosing results in a positron emission tomography (PET)-assessed CR rate of 61% [3,4]. The importance of pre-ASCT CR in improving EFS after ASCT is well-described. Moskowitz et al. also showed a 51% improvement in post-ASCT EFS for those who had a negative PET prior to transplant, thus highlighting the imperative need to improve CR rates to salvage chemotherapy [4].
We sought therefore to meet this need through addition of a targeted agent to ICE. The proteasome inhibitor bortezomib had been shown to induce cell cycle arrest at G2-M phase and apoptosis in HL cells. Furthermore, data from trials combining chemotherapy with bortezomib in various disease entities has shown a synergistic response, with bortezomib potentially acting to overcome chemotherapy resistance. We initially conducted a pilot trial with bortezomib alone in relapsed/refractory HL, and then a phase 1 bortezomib plus baseline dosed ICE (BICE) trial which demonstrated an overall response rate (ORR) of 75% with all responding patients having PET negativity [5,6]. Given this favorable data we proceeded to further conduct a phase II randomized trial.
In this trial we evaluated the efficacy and safety of bortezomib plus ICE (BICE) versus ICE in the treatment of patients with relapsed/refractory HL prior to autologous transplant. The study was IRB-approved, and informed consent was provided. Patients in this single-center study were enrolled between November 2009 and December 2010. Eligibility criteria included patients with first relapse or refractory classical HL who had received a front-line anthracycline-containing regimen. Treatment was administered according to regimen described with the phase 1 trial with bortezomib 1.5 mg/m2 given on days 1 and 4. ICE was given according to baseline regimen dosing [6]. Pegfilgrastim was given on day 5 and patients received prophylaxis with ciprofloxacin, fluconazole, and valacyclovir. Treatment was administered on an inpatient basis and could be repeated every 14 days if absolute neutrophil count and platelet count recovered to ≥ 1.0 × 109/L and ≥ 90 × 109/L respectively. Staging via CTs and PET/CT scans were performed at baseline and after three cycles of treatment.
A Bayesian adaptive algorithm was anticipated to be used in this trial, with the randomization of the first 20 patients between ICE and BICE arms following a “1.1” allocation ratio and thereafter proceeding to randomize patients in favor of the treatment arm that was yielding the better response rates [7]. The primary endpoints included CT assessed complete remission (CR) rate, overall response rate (ORR), partial remission (PR) rate, progression-free survival (PFS), and overall survival (OS). Secondary endpoints included PET scan response. For PFS, time to relapse or death was calculated in months from date of administration of cycle 1 to relapse date or death date as appropriate. For overall survival analysis, time to death as calculated in months from cycle 1 to death date or last follow-up date if death did not occur. Patients were censored at the last follow-up date if neither relapse nor death occurred.
A total of 20 patients were enrolled, with 10 assigned to each arm (Table 1). The median age of patients was 30 (20 – 59), and 45% had primary refractory disease (six in the BICE arm and three in the ICE arm). Patients were well matched in both arms with regard to characteristics including gender, age, and disease status (refractory vs. relapsed). Repeat staging after three cycles demonstrated that by the 1999 IWG response criteria the ORR was similar in the two arms (70% for BICE and 60% of ICE), and the CT scan assessed CRs were 3/10 (30%) in the BICE arm vs. 1/10 (10%) in the ICE arm (p-value = 0.5820) [8]. PET negativity was achieved in 30% of the BICE patients as compared to 60% of the ICE patients. Of the patients who received additional salvage therapy with GND (gemcitabine, navelbine and doxorubicin), one (10%) had PD, one (10%) PR, and one (10%) SD. One patient with PD following BICE went on to receive six lines of salvage chemotherapy prior to obtaining CR and receiving ASCT. Three of 10 ICE patients with stable disease went on to receive additional salvage therapy with GND prior to ASCT [9]. One patient on the ICE arm was noted to have SD by CT imaging, but given PET negativity, received no further salvage chemotherapy. Enrollment was halted after the first 20 patients were treated given that ORR between the two arms were similar and PET scan-assessed CR rates favored the standard of care ICE arm. This paired with the introduction of other novel agents for relapsed classical Hodgkin lymphoma resulted in the decision to close enrollment to this trial early.
Table 1.
Patient characteristics.
| Patient characteristic | BICE (n =10) |
ICE (n = 10) |
|---|---|---|
| Age | ||
| <30 | 6 | 4 |
| >30 | 4 | 6 |
| Gender | ||
| Female | 3 | 7 |
| Male | 7 | 3 |
| Response to primary therapy | ||
| Relapsed | 4 | 7 |
| Refractory | 6 | 3 |
| Prior front-line chemotherapy | ||
| ABVD | 10 | 8 |
| BEACOPP | 0 | 1 |
| MOPP/ABVD | 0 | 1 |
| Prior radiotherapy | 3 | 2 |
| Mobilization chemotherapy regimen | ||
| Ifosfamide/etoposide | 7 | 8 |
| GND | 2 | 1 |
| Cyclophosphamide | 1 | 0 |
| Conditioning chemotherapy regimen | ||
| Gemcitabine/ busulfan/ melphalan | 9 | 9 |
| BEAM | 1 | 0 |
ABVD, adriamycin, bleomycin, vinblastine, dacarbazine; BEACOPP, bleomycin, etoposide, Adriamycin, cyclophosphamide, oncovin, procarbazine, prednisone; MOPP/ABVD, alternating cycles of mustagen, oncovin, procarbazine, and prednisone w/ABVD; GND, gemcitabine, navelbine doxil.
Nineteen of a total of 20 patients in the study went on to ASCT, with sufficient stem cell collection (average CD34 count of 5.38e6/kg). In regard to mobilization regimens, 7 of 10 patients in the BICE arm and 8 of 10 patients on the ICE arm received IE (ifosfamide and etoposide). Three patients (two in the BICE arm and one in the ICE arm) were mobilized directly with growth-factor after receiving salvage GND alone. Finally, one patient in the BICE arm was mobilized with high-dose cyclophosphamide. A majority of patients (16 of 20 patients, 80%) received the conditioning regimen of gemcitabine, busulfan and melphalan on protocol [10]. One of the remaining patients received a conditioning regimen of gemcitabine, busulfan, melphalen and SAHA on protocol, one standard-of care BEAM (carmustin, etoposide cytarabine and melphalen), and one gemcitabine, busulfan and melpahlen off protocol [11].
Common Terminology Criteria for Adverse Events (CTCAE) version 4 was utilized to score toxicities. Myelosuppression was the most common toxicity in both arms. Reversible grade 4 neutropenia and thrombocytopenia occurred respectively in 70% and 50% of the BICE patients compared to 40% and 10% of the ICE patients. One patient in each arm had febrile neutropenia. However, no patients were removed from the study on account of persistent toxicities, defined as grade 4 thrombocytopenia or neutropenia lasting longer than 2 weeks. Transient grade 1 and 2 transaminitis were seen in 80% vs. 50% of patients in the BICE vs. ICE arm, and grade 1 hyperbilirubinemia was seen in one patient treated with BICE. There was also only one case of reversible grade 1 peripheral sensory neuropathy in one patient who received BICE. Median days for re-treatment for cycle 2/3 was 20/21 days in the BICE arm and 21/20 days in the ICE arm.
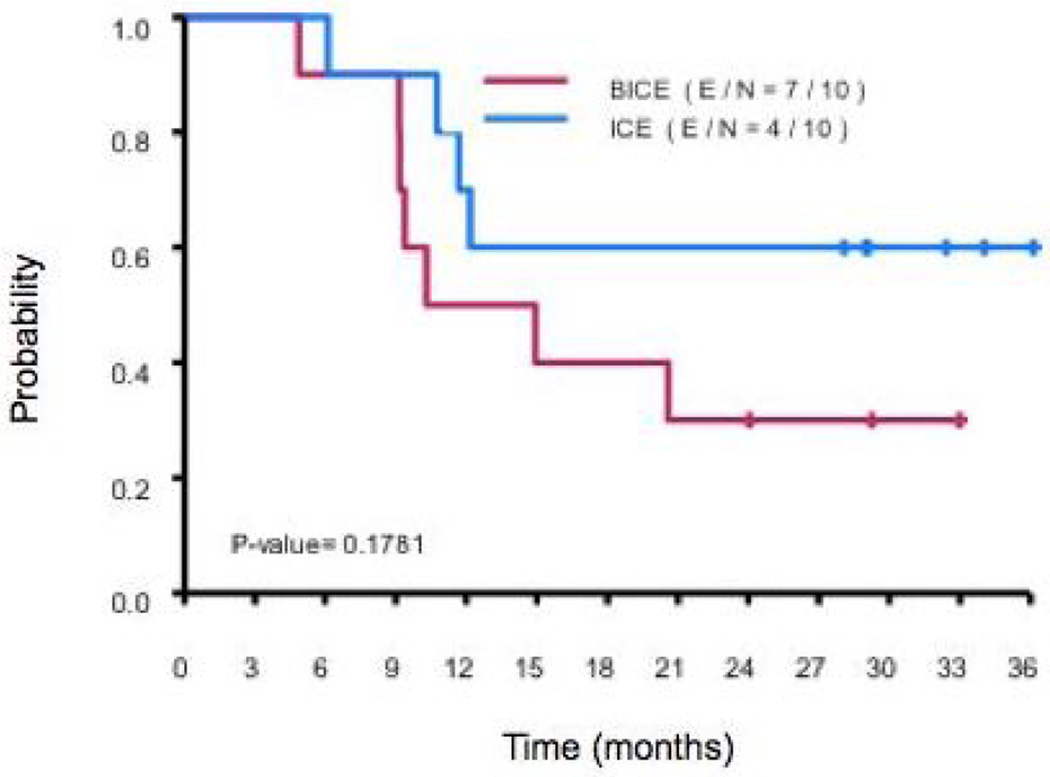
In the analysis, no significant associations were seen between patient characteristics and disease response. The median follow-up time for censored observation was 29.9 months (6.1–38.9 months). At 12 and 24 mos, PFS rate among patients treated with BICE was 0.5 (95% CI 00.27 and 0.93) and 0.3 (95% CI 0.12 and 0.77). Amongst those who received ICE, PFS was 0.7 (95% CI 0.47 and 1.00) and 0.6 (95% CI 0.36 and 1.00). In regard to OS at follow-up of 24 mos, the rate was 0.7 (95% CI 0.47 and 1.00) in patients treated with BICE vs. 0.89 (95% CI 0.71 and 1.00) in those treated with ICE. Utilizing univariate analysis, there was no significant difference in either PFS or OS (p-values of 0.1781 and 0.3154, respectively; Figure 1).
Figure 1.
PFS by treatment group.
Based on findings of this phase 2 trial, there is insufficient data to suggest superiority of BICE as compared to ICE as a salvage therapy for patients with relapsed/refractory HL. Although the study was randomized, the small sample size prevented any definitive comparison between the cohorts. A potential confounder in our study may be the fact that a greater number of patients with refractory disease were in the group treated with BICE (6 vs. 3), although the total number was likely not large enough to reach significance (p-value 0.3698). In regard to toxicity, BICE appears to be well tolerated. Although more grade 3 and 4 hematologic toxicities (37% vs. 28%) occurred in the BICE arm, they were reversible, and comparable occurrences of neutropenic fever were seen in both groups. Given the lack of significant improvement in outcomes for BICE- as compared to ICE-treated patients in the first 20 patients enrolled and other available novel agents such as panobinostat for combination with ICE, a combination for which we have recently reported positive results for, we made the decision to halt enrollment to this trial [12]. In conclusion, given the recent approval of brentuximab vedotin and other novel therapeutic agents, the role of bortezomib in the future of Hodgkin lymphoma treatment will need further evaluation.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 2.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz CH, Matasar MJ, Zelenetz AD, et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–1670. doi: 10.1182/blood-2011-10-388058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younes A, Pro B, Fayad L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood. 2006;107:1731–1732. doi: 10.1182/blood-2005-09-3731. [DOI] [PubMed] [Google Scholar]
- 6.Fanale M, Fayad L, Pro B, et al. Phase I study of bortezomib plus ICE (BICE) for the treatment of relapsed/refractory Hodgkin lymphoma. Br J Haematol. 2011;154:284–286. doi: 10.1111/j.1365-2141.2011.08618.x. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, Eick SG. Adaptive assignment versus balanced randomization in clinical trials: a decision analysis. Stat Med. 1995;14:231–246. doi: 10.1002/sim.4780140302. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 10.Nieto Y, Anderlini P, Popat U, et al. Gemcitabine, busulfan and melphalan (GemBuMel) is a new high-dose chemotherapy (HDC) regimen with high activity in refractory Hodgkin’s lymphoma (HL) patients receiving an autologous stem-cell transplant (ASCT): a contemporaneous comparison with BEAM and busulfan/melphalan (BuMel) ASH Annual Meeting Abstracts. 2010;116:690. [Google Scholar]
- 11.Nieto Y, Thall P, Valdez B, et al. Vorinostat (SAHA) combined with high-dose gemcitabine/busulfan/melphalan (SAHA/Gem/Bu/Mel) with autologous stem-cell transplant (ASCT) in patients with refractory lymphomas. Blood. 2013;122 doi: 10.1016/j.bbmt.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oki Y, Fanale MA, Westin JR, et al. A phase I study of panobinostat in combination with ICE (ifosfamide, carboplatin and etoposide) in patients with relapsed or refractory classical Hodgkin lymphoma (cHL) Blood. 2013;122:252. [Google Scholar]