Abstract
Advanced, noninvasive imaging has revolutionized our understanding of language networks in the brain and is reshaping our approach to the presurgical evaluation of patients with epilepsy. Functional magnetic resonance imaging (fMRI) has had the greatest impact, unveiling the complexity of language organization and reorganization in patients with epilepsy both pre- and postoperatively, while volumetric MRI and diffusion tensor imaging have led to a greater appreciation of structural and microstructural correlates of language dysfunction in different epilepsy syndromes. In this article, we review recent literature describing how unimodal and multimodal imaging has advanced our knowledge of language networks and their plasticity in epilepsy, with a focus on the most frequently studied epilepsy syndrome in adults, temporal lobe epilepsy (TLE). We also describe how new analytic techniques (i.e., graph theory) are leading to a refined characterization of abnormal brain connectivity, and how subject-specific imaging profiles combined with clinical data may enhance the prediction of both seizure and language outcomes following surgical interventions.
1. Introduction
Temporal lobe epilepsy (TLE) is the most common type of localization-related epilepsy in adults, and a frequent cause of chronic, intractable seizures (Wiebe, 2000). Up to 70% of patients suffering from TLE have mesial temporal sclerosis (MTS), which is characterized by hippocampal atrophy and increased signal on MR, with significant neuronal loss seen on histological examination (Malmgren & Thom, 2012). In addition, patients with TLE suffer frequent cognitive comorbidities that range from mild impairments in memory to more severe impairments across multiple cognitive domains (B. Hermann, Seidenberg, Lee, Chan, & Rutecki, 2007). Along with memory, language impairment is one of the most common and pervasive cognitive complaints in adults with TLE, and it is characterized by impairments in visual and auditory naming, fluency, and occasionally comprehension (Bartha, Benke, Bauer, & Trinka, 2005; Devinsky, Perrine, Llinas, Luciano, & Dogali, 1993; Hamberger, Goodman, Perrine, & Tamny, 2001; Jaimes-Bautista, Rodriguez-Camacho, Martinez-Juarez, & Rodriguez-Agudelo, 2015; Metternich, Buschmann, Wagner, Schulze-Bonhage, & Kriston, 2014; Oyegbile et al., 2004; Schwartz, Resor, De La Paz, & Goodman, 1998). When seizures arise from the language-dominant hemisphere, patients with TLE are also more likely to show atypical patterns of language representation in the brain that likely reflect reorganization of function in response to injury. Organization and re-organization of language has been studied extensively in patients with TLE using invasive methods, including the intracarotid amobarbital procedure (IAP) and electrical stimulation mapping (ESM). However, with the emergence of newer imaging techniques, we are now able to more fully appreciate the complexity of re-organization within language networks that result from early injury to the brain, years of chronic seizure activity, and/or surgical interventions.
In this review we highlight how functional magnetic resonance imaging (fMRI; both task-based and resting-state), diffusion tensor imaging (DTI), and volumetric MRI have advanced our understanding of preoperative language networks in TLE, aided in the prediction of postoperative language decline, and provided insight into network re-organization following surgical intervention. We will also describe how newer analytic models (i.e., graph theory) applied to data from these techniques are reshaping our understanding of language networks in patients with both TLE and other epilepsy syndromes. Finally, we discuss how future research that integrates advanced imaging with other diagnostic methods and clinical information may enhance the prediction of individual patient outcomes and answer other fundamental questions related to network dysfunction and plasticity in epilepsy. It should be acknowledged there are many other important imaging methodologies that have contributed vastly to this literature. In particular, magnetoencephalography (MEG) and magnetic source imaging (MSI) have provided exquisite information on the spatiotemporal characteristics of language processing and reorganization in epilepsy, and these methods have generally shown strong concordance with the IAP (Billingsley-Marshall et al., 2007; D'Arcy et al., 2013; Doss, Zhang, Risse, & Dickens, 2009; Findlay et al., 2012; Hirata et al., 2010; Hirata et al., 2004; Maestu et al., 2002; McDonald et al., 2009; Papanicolaou et al., 2004; Pataraia, Billingsley-Marshall, et al., 2005; Pataraia, Lindinger, Deecke, Mayer, & Baumgartner, 2005; Pataraia et al., 2004; Rezaie et al., 2014; Simos et al., 1999; Tanaka et al., 2013; Van Poppel et al., 2012). However, to provide sufficient depth, we choose to focus the current review on the most commonly used and widely available methods in use at most epilepsy surgical centers. A comprehensive review of all noninvasive modalities is beyond the scope of this article, but for excellent reviews on the use of MEG/MSI for language mapping, see (Breier, Billingsley-Marshall, Pataraia, Castillo, & Papanicolaou, 2006; Frye, Rezaie, & Papanicolaou, 2009; Pirmoradi, Beland, Nguyen, Bacon, & Lassonde, 2010; Wheless et al., 2004). In addition, because the nature of the language (and cognitive) deficits in pediatric TLE have not been well-specified and there is a paucity of imaging studies examining TLE during childhood, the current review is focused predominantly on adult TLE.
2. Traditional assessment of language lateralization and localization in TLE—The “gold” standards
Much of what was first discovered about language in TLE resulted from surgical planning procedures in medically refractory patients preparing to undergo anterior temporal lobectomy (ATL), a surgical intervention with an approximately 70% success rate (Engel, 2003). This procedure typically involves removal of the anterior portion of the hippocampus, parahippocampal gyrus and amygdala, along with variable resection of the adjacent temporal lobe white matter and lateral neocortex (Wiebe et al., 2001). Since multiple temporal lobe structures targeted for resection play a significant role in language, 25 to 60% of adults who undergo ATL will develop severe dysnomia (Sabsevitz et al., 2003). This decline can be severe and is most common following dominant-hemisphere ATL, but it has also been documented in patients after nondominant ATL (Jabbour, Hempel, Gates, Zhang, & Risse, 2005; Loring et al., 1990).
Traditionally, two invasive procedures have been performed in most epilepsy surgical centers to minimize the risk of postoperative language decline. The first is the IAP, which is performed primarily to lateralize language functions by inducing a temporary “lesion” thought to mimic surgery. The second procedure, ESM, is used to more precisely localize a patient's language areas by applying electrical stimulation to the cortical surface while the patient performs a simple language task, in order to identify language-crucial areas, with the goal of sparing these regions during a surgical resection. These procedures are described in detail in Hamberger and Cole (Hamberger & Cole, 2011).
Despite the invasiveness nature of the IAP and ESM and their widespread use in clinical practice, there is still limited evidence that the use of either method has substantially improved language or memory outcomes following ATL (Binder, 2011b; Hamberger, 2007). Furthermore, performing these procedures with pediatric or low-functioning populations is considerably more difficult, due in part to diminished compliance and the resultant decrease in reliability of the procedures, which is concerning because the age of epilepsy surgery is steadily decreasing (Williams, Abbott, & Manson, 1992). These concerns are among those that have motivated the search for alternative, noninvasive methods that can supplant or complement the IAP and ESM. We introduce several of these technologies below.
3. FMRI and language networks in TLE
Functional magnetic resonance imaging (fMRI) has evolved as the most popular noninvasive technique for investigating the neural underpinnings of language function in patients with neurological disorders and healthy controls. FMRI makes use of an applied magnetic field to measure hemodynamic responses related to neural activity (Ogawa, Lee, Kay, & Tank, 1990). Neuronal activation increases cerebral blood flow to the local brain region, and the active region is supplied with oxygen-rich blood, resulting in an increased ratio of oxygenated to deoxygenated hemoglobin. Differences in the magnetic properties of oxygenated and deoxygenated hemoglobin lead to a change in magnetic resonance signal in the activated region. This blood oxygen level dependence (BOLD) functions as an endogenous contrast agent for MR imaging.
Although traditionally viewed as a research tool, fMRI has now assumed an important role in the clinical evaluation of patients with epilepsy. It is not only seen as less invasive and more cost-effective than the IAP (Medina, Aguirre, Bernal, & Altman, 2004), but it is considered better able to examine intrahemispheric localization of language regions and it generates a continuous measure of language lateralization (Duncan, 2010). FMRI can also lateralize specific regions of interest for surgical consideration (Janecek et al., 2013; Sabsevitz et al., 2003). Further, the ability to administer and repeat multiple tasks, if necessary, is valuable for some patients, and the use of a panel of tasks that tap expressive and receptive language abilities is possible, which allows for more precise localization of a range of language functions (Gaillard et al., 2004; Lehericy et al., 2000).
3.1 Concordance between fMRI and the IAP
With IAP viewed as the gold standard for determining language lateralization in patients with TLE, the majority of fMRI studies have been designed to evaluate overall concordance with the IAP. For these studies, the metric most often used is an fMRI language laterality index (LI) (Ruff et al., 2008). The equation LI = ([Vl-Vr]/[Vl+Vr]) × 100, where V represents the number of activated voxels for the left or right hemispheres, is typically used to determine laterality (Binder et al., 1996). The resulting LI ranges from +100 (strong left hemisphere dominance) to -100 (strong right hemisphere dominance). While IAP results are typically categorical in nature (left, bilateral, or right language dominance), the fMRI LI is measured along a graded continuum, which can more precisely estimate the degree of left and right hemisphere contributions to language functioning (Seghier, Lazeyras, Pegna, Annoni, & Khateb, 2008).
Most fMRI studies have used either language production tasks (i.e., verb generation or word-stem completion) or language comprehension tasks (i.e., semantic decision or rhyme detection) to probe language lateralization in epilepsy and have reported relatively high concordance rates between fMRI and IAP, typically ranging between 80 and 90% (Bauer, Reitsma, Houweling, Ferrier, & Ramsey, 2014). In one of the largest studies to date (n=229), Janecek et al. (Janecek et al., 2013) found a concordance rate of 86% using a semantic decision task, which is comparable to an overall 85% concordance rate based on other studies (average of 22 studies weighted by sample size: (Adcock, Wise, Oxbury, Oxbury, & Matthews, 2003; Arora et al., 2009; M. Baciu et al., 2001; Bahn et al., 1997; Benke et al., 2006; Binder et al., 1996; Carpentier et al., 2001; Deblaere et al., 2004; Desmond et al., 1995; Gaillard et al., 2002; Gaillard et al., 2004; Hertz-Pannier et al., 1997; Lehericy et al., 2000; Liegeois et al., 2002; Rutten, Ramsey, van Rijen, Alpherts, & van Veelen, 2002; Sabbah et al., 2003; Spreer et al., 2002; Szaflarski et al., 2008; Woermann et al., 2003; Worthington et al., 1997; Yetkin et al., 1998). In a recent meta-analytic review of 22 studies using either production or comprehension tasks, Bauer et al. (2014) found an overall 81% concordance rate between fMRI and the IAP. Concordance rates in Bauer et al. were higher in patients with strong left hemisphere language dominance (94%), but much lower for patients with atypical language dominance (51%). Interestingly, neither the type of task nor the specific regions of interest (ROIs) used (i.e., frontal versus temporo-parietal versus hemispheric ROIs) affected concordance rates (Bauer et al., 2014). A second meta-analysis by Dym et al. reported a sensitivity for fMRI of 83.5% and a specificity of 88.1% when compared to the IAP (Dym, Burns, Freeman, & Lipton, 2011). Unlike Bauer et al., Dym et al. found higher specificity with the use of word generation tasks compared to semantic decision tasks (95.6% vs 69.5%) and a higher sensitivity for global evaluation with fMRI relative to the use of a frontal or temporo-parietal ROI approach.
Several issues may limit concordance rates between fMRI and the IAP in assessing language dominance. The variety of tasks used in fMRI and lack of standardized protocols has restricted uniformity in assessing language functioning. As noted above, some studies have also found differences in concordance rates based on whether global versus regional ROIs are used (Benke et al., 2006; Deblaere et al., 2004). At a more intrinsic level, IAP is a ‘negative’ or lesion-type paradigm, while fMRI is a ‘positive’ or activation-based technique that may implicate regions that are participatory but not essential for language. As noted, the presence of atypical or bilateral language dominance can also affect concordance rates (Arora et al., 2009; Benke et al., 2006) in that fMRI is more likely than IAP to conclude bilateral language dominance in cases where disparity arises (Gaillard et al., 2004). This has led some to the conclusion that fMRI is a reliable method for determining language lateralization in patients with typical (left) language function, but that further workup is warranted in patients who show atypical language dominance on fMRI. However, as noted by Bauer et al., (2014) the IAP may be best considered a “silver standard” and determining which technique provides the most accurate prediction of postoperative language outcome should be the definitive test (Bauer et al., 2014).
3.2 Patterns of language organization revealed by fMRI
IAP studies have demonstrated that patients with left-handedness, left hemisphere seizure onset and an early left hemisphere lesion or MTS are more likely to demonstrate atypical language dominance. It has been proposed that chronic epilepsy activity affects brain structure and function gradually, and these disturbances can shift or re-route language pathways during development to the nondominant hemisphere (Duchowny et al., 1996; Janszky, Mertens, Janszky, Ebner, & Woermann, 2006). Because language is comprised of a number of discrete functions that have the potential to reorganize differently, there are a variety of ways in which this insidious process may shift language, and recent fMRI studies have evinced a myriad of different language patterns that may emerge as the result of an early lesion and/or years of chronic seizures.
Atypical language organization in epilepsy patients has traditionally been conceived of as following three general and overarching patterns. The first is a right hemisphere dominance, with core language expression and comprehension networks in the typically-dominant left hemisphere shifting to the right hemisphere, most often to homologous regions (Gaillard et al., 2002; Gaillard et al., 2004; Gaillard et al., 2007; Thivard et al., 2005). A second pattern of atypical organization is crossed or bilateral dominance, meaning that only some language functions have moved to the right hemisphere, with some language representation preserved in the left hemisphere (M. V. Baciu et al., 2003; Lee et al., 2008; Thivard et al., 2005). For example, a frontal language region subserving production may be right-lateralized, but a temporal language region subserving comprehension may be left-lateralized. Indeed, Thivard et al., (2005) used both a language production and a language comprehension task in fMRI to show that epilepsy-related language reorganization can affect frontal and temporal language areas differently, with 4/7 TLE patients (3 left and 1 right) exhibiting right lateralization in temporal lobe regions subserving comprehension but symmetric or left-sided activation in prefrontal regions subserving language production (Thivard et al., 2005). When language functions are represented in both hemispheres there may also be symmetric representation for some or all language regions. The third pattern of atypical organization is intrahemispheric reorganization, with language regions shifting within the same hemisphere that contains the seizure onset zone, most often to regions adjacent to typical perisylvian cortex within the frontal or temporal lobe (Bell et al., 2002; Brazdil, Zakopcan, Kuba, Fanfrdlova, & Rektor, 2003; Mbwana et al., 2009).
Recent work by Berl et al. (2014), however, indicates that this classic three-pattern classification is oversimplified (Berl et al., 2014). These investigators expanded the classification of language organization profiles, hypothesizing 15 possible patterns of language representation (See Figure 1). Using a data-driven clustering method in 220 epilepsy patients performing an auditory semantic decision task, they identified nine unique fMRI language patterns. Consistent with previous studies, they showed that right lateralization is more common in patients with a left-sided seizure focus; however, they also reported on three patients with complete right-sided language dominance who had a right-sided seizure focus. This finding supports the idea that, while infrequent, patients with a right hemisphere seizure focus may also have atypical language dominance. Among the nine patterns identified, Berl and colleagues described two patterns of crossed dominance: activation of left inferior frontal gyrus (IFG; Broca's area) with right Wernicke's Area (WA), and vice versa. It was more common in this study for patients to have bilateral IFG activation than bilateral WA activation: there was only one cluster of patients with bilateral WA lateralization, and the patients in this cluster were also more likely to show bilateral IFG activation. Notably, in terms of clinical correlates, patients who were left-handed were more likely than right-handers to show right lateralized IFG, while patients with an early age of seizure onset were more likely to show bilateral WA, and patients with a left-sided seizure focus were more likely to show a unilateral right (IFG and WA) pattern of language dominance. Although the reason for these associations has not been well-studied, Berl et al. hypothesized that the association between left-handedness and IFG laterality could reflect the proximity of the IFG to motor cortex. These results support and expand upon previous fMRI studies that have shown a higher rate of atypical language dominance, specifically interhemispheric shift, among TLE patients with an earlier age of seizure onset, left or mixed handedness, and left-sided seizure focus (Berl et al., 2005; Gaillard et al., 2007; Szaflarski et al., 2002). Conversely, patients with space-occupying lesions may be more likely to show intrahemispheric reorganization of language within the temporal lobe (Hamberger, McClelland, McKhann, Williams, & Goodman, 2007).
Figure 1.
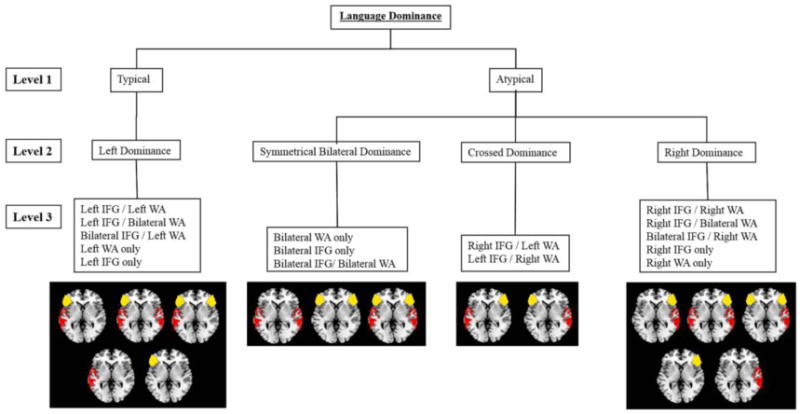
Hierarchy of 15 typical and atypical language patterns based on categorical criteria in both patients and controls. Language is classified into 3 levels: typical=atypical, language dominance (left, bilateral, crossed, or right), and individual activation patterns. Regions of interest are left lateralized if lateralization index (LI) ≥ 0.20, bilateral if LI < |0.20|, and right lateralized if LI ≤ −20.20. IFG = inferior frontal gyrus; WA = Wernicke area. Figure modified with author's permission.
Berl et al.'s description of the many different patterns of language reorganization and crossed dominance demonstrates the need for assessment of language function on a regional and function-specific level, rather than on a more global, hemispheric one, as some aspects of language may reorganize while others do not (Berl et al., 2014; Hamberger et al., 2007). It is of note that much of the literature in fMRI and epilepsy continues to treat language as a monolithic construct in which all aspects of language either shift to the right hemisphere or remain left-lateralized. Conversely, Berl et al.'s work overcomes this limitation by presenting language as a multivariate construct made up of different components and discrete functionalities that may reorganize together or independently.
3.3 Utility of FMRI versus IAP for predicting language outcome after ATL
As noted above, the most common language deficits reported in patients with TLE include impairments in visual and auditory naming, auditory comprehension, and fluency. Of these, naming is at highest risk for additional postsurgical decline, with 25 to 60% of patients who undergo dominant hemisphere ATL developing some degree of dysnomia (Baxendale, 2002; B. P. Hermann et al., 1999; Langfitt & Rausch, 1996; Sabsevitz et al., 2003; Seidenberg et al., 1998; Stafiniak et al., 1990). Despite the well-established literature describing language decline following left ATL and a wealth of research on concordance rate between fMRI and IAP, surprisingly few studies have examined the ability of fMRI versus the IAP to predict postoperative naming decline (Bonelli et al., 2012; Doucet, Rider, et al., 2015; Sabsevitz et al., 2003). This is particularly important given that the many different patterns of atypical language organization described above would differentially alter risk for language decline after epilepsy surgery. In the only study comparing fMRI and the IAP, Sabsevitz et al. (2003) found both fMRI and IAP laterality to predict naming decline following ATL (Sabsevitz et al., 2003). Furthermore, both modalities explained more variance in naming decline than age of seizure onset or preoperative naming performance. FMRI results accounted for 41% of the variance in naming decline, where LI toward the left hemisphere was associated with greater decline and LI toward the right hemisphere was associated with little to no decline. Both fMRI temporal lobe LI and IAP LI demonstrated 100% sensitivity in predicting naming deficit, and fMRI showed a higher specificity than IAP (57% versus 43%). In addition, recent data from the same group has shown that that fMRI language lateralization is a better predictor of verbal memory decline than memory results from IAP (Binder et al., 2008). Although the reason for this is unclear, Binder et al. suggest that the verbal episodic memory encoding system is likely to be co-lateralized with language (i.e., the type of material encoded by the left or right MTL depends on the type of information it receives from the ipsilateral neocortex). Thus, language lateralization should be a reliable indicator of verbal memory lateralization and therefore, a reliable predictor of verbal memory decline. These data suggest that fMRI language lateralization may have clinical utility beyond the prediction of language outcome, and a prospective study is currently evaluating this potential application.
3.4 FMRI and postoperative language reorganization
Although preoperative reorganization of language has received considerable attention in the epilepsy literature, an emerging area of interest involves the potential for postoperative reorganization of language networks after ATL. There are even some data to suggest a reversal of atypical language dominance after successful seizure control has been achieved, indicating that atypical dominance due to epileptic activity may represent a temporary, adaptive response in some patients. Helmstaedter et al. (2006) reviewed three patients with left TLE who were right language dominant based on an fMRI semantic decision task and underwent left selective amygdalohippocampectomy. Of these patients, the one with the youngest age of onset demonstrated a postoperative shift of language dominance to the left hemisphere in both temporal (receptive) and frontal (expressive) perisylvian regions (Helmstaedter, Fritz, Gonzalez Perez, Elger, & Weber, 2006).
Other studies have used fMRI to examine reorganization of language following left versus right ATL. Backes et al. (2005) reported greater bilateral representation in patients after left ATL when compared to right ATL and controls (Backes et al., 2005). However, no preoperative neuroimaging was available for comparison. Wong et al. (2009) examined fMRI language activations in 19 patients with TLE before and after ATL during a covert verb generation task (Wong et al., 2009). Prior to ATL, patients with right and left TLE showed more bilateral language responses relative to controls. Following ATL, patients with left TLE only showed reduced activity in the left middle frontal gyrus and right IFG, whereas no difference was observed in the patients with right TLE. However, in the right TLE patients, the relationship between language performances and language activations shifted away from typical language regions to the anterior cingulate cortex (ACC). Although unexpected, the investigators propose that this shift to regions not specific for language, i.e., the ACC, may indicate an increased reliance on a more general cognitive control and response selection network needed for verb generation. These findings suggest that the language networks in left versus right TLE exhibit different reorganization after ATL, and this reorganization affects naming performance.
In a second longitudinal study, Bonelli et al. (2012) examined the association between preoperative functional lateralization and postoperative reorganization (n=44) and found that patients with left TLE who did not show evidence of significant postoperative naming decline recruited the residual left posterior hippocampus for word retrieval, while those with postoperative decline relied on contralateral frontal regions. They also observed greater postoperative bilateral middle and inferior frontal fMRI activation in left TLE patients, which was not observed in right TLE patients. Their findings suggest that interhemispheric reorganization of naming to contralateral frontal regions does occur in some patients, but that this strategy may be less effective than intrahemispheric utilization of the (ipsilateral) posterior hippocampus (Bonelli et al., 2012). The findings of Bonelli et al. also support Binder's evidence for a close correspondence between language and verbal memory outcomes following ATL, which are likely subserved by a single semantic network (Binder, 2011b). While Bonelli et al. (2012) point to greater benefit from intra- versus interhemispheric reorganization in the context of naming, Kim et al. (2010) demonstrated greater benefits to interhemispheric reorganization and the recruitment of right hemisphere regions post-surgery for preserving word generation. Thus, the circumstances under which inter- versus intrahemispheric reorganization occurs postoperatively are still unclear, as is the relative benefit of each of these patterns to different components of language outcome (Kim et al., 2010).
3.5 Resting-state fMRI (rs-fMRI) and language
Although task-based fMRI is intuitively appealing, and there is accumulating evidence for the predictive value of semantic decision and comprehension tasks for mapping temporal lobe language functions in epilepsy (Binder, 2011a, 2011b), the demand of a task may affect results, as patient cooperation and performance may fluctuate. Therefore, functional connectivity (FC) as measured by rs-fMRI is being explored as an alternative strategy for probing language networks. Unlike task-based fMRI, which shows the activation of brain areas in response to specific language tasks, rs-fMRI purports to measure intrinsic activity, or the neural and metabolic activity of the brain at rest (when no task is performed). FC measures are then derived by measuring BOLD coherence among cortical regions, from which network “cooperation” or connectivity is assumed (Friston, Frith, Liddle, & Frackowiak, 1993; Pereira et al., 2010). Although there is some concern that rs-fMRI maps may, in part, reflect neural artifact (Birn, Diamond, Smith, & Bandettini, 2006; Cordes et al., 2001; Shmueli et al., 2007) other data suggests that these synchronous fluctuations in BOLD signal do in fact represent the functional connectedness of cortical areas and are observed across the whole cortex and in different cognitive networks (i.e., for attention, language, or episodic memory) (see (Biswal, Van Kylen, & Hyde, 1997; Bressler, 1995) for a review).
Given the appeal of rs-fMRI in terms of both the ease and efficiency with which it can be collected, many studies are currently underway to determine the clinical utility of rs-fMRI measures and to explore its potential to better delineate language networks in TLE. However, the value of rs-fMRI as compared to task-based fMRI in TLE is still debated. Waites et al (2006) examined language networks in 17 patients with left TLE using both task-based (i.e., word fluency) and rs-fMRI. Four seed regions were derived from the task-based data in 30 healthy controls [left IFG, left middle frontal gyrus (MFG), dorsal ACC, and posterior cingulate cortex (PCC)] and used in the rs-fMRI analysis. On task-based fMRI, patients and controls showed similar patterns of activation; however, the rs-FC of patients differed from that of controls, with left TLE patients showing reduced FC among language regions compared to controls. While the controls showed significant connectivity between the seed regions and the majority of typical language regions, the patients only showed connectivity of each seed region with voxels near the seed, and in some cases with a seed in the contralateral homologous region (Figure 2). This study suggests that rs-FC can be effective in revealing altered patterns of connectivity that may not be obvious in many patients on task-based fMRI (Waites, Briellmann, Saling, Abbott, & Jackson, 2006). Doucet et al. (2015) studied 55 patients with TLE and reached a similar conclusion, i.e., that rs-FC data is perhaps more sensitive than task-based fMRI for detecting altered language network connectivity. Similar to Waites et al. (2006), the LI drawn from task-based fMRI did not show significant differences between the patients and controls. However, the strength of left hemisphere dominance was associated with the following rs-FC patterns, as depicted in Figure 3: (1) higher left intrahemispheric rs-FC (panel A: red line) and lower interhemispheric rs-FC (panel A: blue line) emerging from the left seed region, as well as (2) lower right intrahemispheric rs-FC (panel B: blue line) and higher interhemispheric FC (panel B: red line) emerging from the right seed region. These associations were observed in both patients with right and left TLE (Doucet, Pustina, et al., 2015). There is also some emerging evidence that rs-fMRI may help predict postsurgical language outcomes when data are analyzed using new analytic techniques (see discussion of graph theory below). In summary, rs-FC has emerged as a promising tool for probing network connectivity in patients with epilepsy, and this technique could offer some advantages over task-based fMRI for quantifying language network disruption in TLE. However, it is important to note that although rs-fMRI may be sensitive to epilepsy-related disruption within language networks on a group basis, unlike task-based fMRI, its sensitivity and specificity for determining language dominance on an individual basis is less well-established, and requires further validation.
Figure 2.
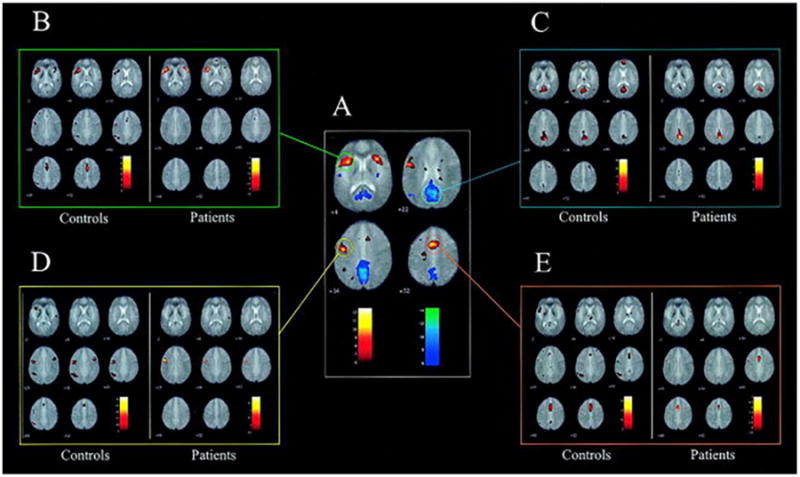
Connectivity maps of controls and patients with left temporal lobe epilepsy (TLE) for four seed regions. (A) Brain activity during the language production in a group of 30 healthy controls. Results are overlaid onto the mean echo planar imaging of the 30 subjects (activation in red-yellow, deactivation in blue-white). Group analysis is performed using a mixed effects model and thresholded at p value less than 0.05, corrected for multiple comparisons. The approximate locations of the four seed regions are shown as colored rings: anterior cingulate cortex (ACC) (red), posterior cingulate cortex (PCC) (blue), left IFG (green), and left middle frontal gyrus (MFG) (yellow). (B–E) The results of performing functional connectivity analysis on a separate cohort of 8 healthy controls (left panels), together with a group of 17 patients with left TLE (right panels). Regions functionally connected (correlated) with the ROI average signal time course in the left IFG (B), PCC (C), left MFG (D) and ACC (E).
Figure 3.
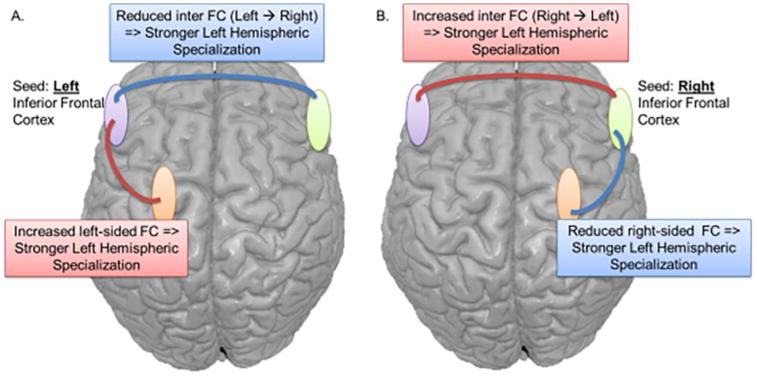
Schematic depiction of the rsFC relations associated with stronger left hemispheric specialization for language, shown in separate panels for the left- (A) and right- (B) sided seeds. Note, weaker hemispheric specialization (weaker laterality) is associated with the inverse rsFC patterns emerging from the seeds. Importantly, both our left and right TLE patients displayed these general patterns. Blue lines represent reduced rsFC with stronger specialization. Red lines represent higher rsFC with stronger specialization.
4. DTI and language networks in TLE
Although fMRI has had the greatest influence on our current models of language organization and reorganization in TLE of any imaging modality, DTI has also provided unique insight into language networks and how they are disrupted in TLE. In particular, DTI tractography has shown that several white matter tracts, including the arcuate fasciculus (ARC), uncinate fasciculus (UNC), inferior longitudinal fasciculus (ILF), and the inferior frontal occipital fasciculus (IFOF) are all important components of a complex language network (see Figure 4). In particular, the ARC is a long association tract well known to be important in language functioning that connects areas involved in language comprehension in the posterior superior temporal gyrus (i.e., Wernicke's area) to areas involved in language expression in the IFG (i.e., Broca's area) (Upadhyay, Hallock, Ducros, Kim, & Ronen, 2008). The UNC is a curved fiber that connects the anterior temporal lobe to the medial and lateral frontal cortex (Hasan et al., 2009) and has been implicated in the transmission of information related to semantic language content and confrontation naming (Lu et al., 2002). The ILF joins posterior occipitotemporal regions, including fusiform cortex, to the temporal pole (Ashtari, 2012) and may participate in the transfer of information about objects, faces, and written words. Finally, the IFOF is a long fiber tract that originates in occipitotemporal regions, courses through the temporal lobe medial to the ILF, and terminates in orbitofrontal and frontopolar regions (Caverzasi, Papinutto, Amirbekian, Berger, & Henry, 2014). This fiber tract has also been implicated in semantic processing and visual object naming (Mandonnet, Nouet, Gatignol, Capelle, & Duffau, 2007; McDonald et al., 2008). Thus, the ARC, UNC, ILF and IFOF may all be facets of a complex network of fibers involved in lexical-semantic processing, where damage to one or more of these fiber tracts can lead to impairment in specific language skills.
Figure 4.
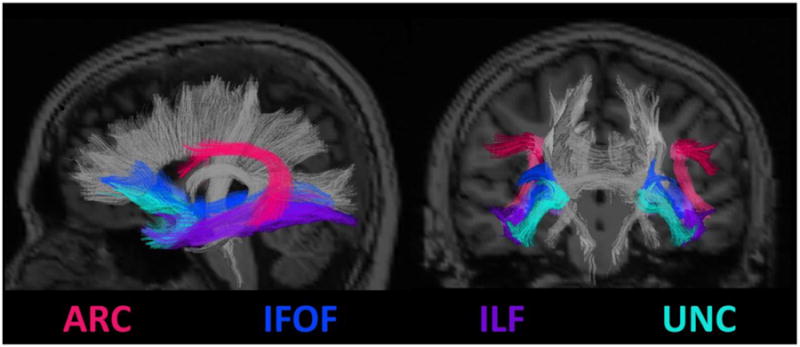
DTI fiber tracts implicated in language processing generated from a probabilistic fiber track atlas. ARC, arcuate fasciculus; ILF, inferior longitudinal fasciculus; UNC, uncinate fasciculus; IFOF, inferior fronto-occipital fasciculus.
4.1 DTI correlates of language performance in TLE
A majority of studies have examined how DTI-derived metrics, typically fractional anisotropy (FA) or mean diffusivity (MD), correlate with impairment on neuropsychological measures of language. McDonald et. al. (2008) applied probabilistic tractography to patients with TLE and demonstrated that increased MD of the left ARC and UNC, along with decreased FA of bilateral ARC, UNC, and left IFOF were related to poorer visual naming performances. Not surprisingly, the strongest correlation was observed between FA of the left ARC and naming, and this association held even after controlling for left hippocampal volume. This group also found a strong correlation between integrity of the right ARC and visual naming in TLE. These data are commensurate with studies demonstrating right hemisphere contributions to language processing (Powell et al., 2007), and with studies suggesting partial reorganization of language to the right hemisphere in some patients with left TLE (Pustina et al., 2014). Despite frontotemporal white matter correlations with naming, their study did not find an association between fiber tract compromise and phonemic fluency impairments in their patient cohort. Conversely, Wang et al. (2010) used a voxel-based approach and found that lower FA in regions of the left frontal lobe and right occipital lobe were associated with poorer semantic fluency in patients with TLE (Wang et al., 2010). Although the reason for these divergent findings is unclear, it is possible that the use of different fluency measures (i.e., phonemic versus semantic) or the use of different DTI approaches (tract-based versus voxel-based) may explain these disparate outcomes.
Beyond the examination of DTI alone, Kucukboyaci et al. (2014) took a multimodal imaging approach to understanding language impairment in TLE by examining the relative contributions of DTI versus volumetric MRI-based measures to predicting visual and auditory naming performances. Based on previous research implicating a network of frontotemporal fiber tracts in language performance (McDonald et al., 2008), principal components analysis was used to leverage the shared variance among multiple frontotemporal fiber tracts to evaluate language functioning in patients with TLE. This analysis showed that after controlling for the effects of left hippocampal volume, reduced frontotemporal FA was associated with poorer visual naming while increased MD was associated with poorer auditory naming. Neither cortical thickness nor gray-white contrast within frontotemporal regions explained language performances in TLE (Kucukboyaci et al., 2014). These data support previous research demonstrating the value of DTI for understanding language performances and provide some evidence that measures of microstructural white matter integrity could be more sensitive than measures obtained from volumetric MRI for understanding language impairment in TLE. However, additional studies directly comparing the two modalities are needed to reach any firm conclusions regarding their relative predictive value.
4.2 DTI and concordance with fMRI and the IAP
A second approach to probing language networks in patients with TLE has been to examine the degree to which asymmetries in frontotemporal fiber tract FA/MD values are associated with asymmetries in fMRI language activations and results from the IAP. This approach is based on research demonstrating that in healthy individuals, a leftward asymmetry exists in frontotemporal FA and tract volumes(including the ARC, UNC, and IFOF), which correlates with typical leftward asymmetries in fMRI language activations (Powell et al., 2006; Vernooij et al., 2007). Thus, deviations from this expected asymmetry may imply language reorganization in TLE. Powell et al. (Powell et al., 2007) combined fMRI and probabilistic tractography in a cohort of patients with TLE to study language reorganization. Using fMRI tasks of verb generation and reading comprehension, they demonstrated that patients with left TLE had less lateralized language activation patterns relative to patients with right TLE and healthy controls. Reductions in language asymmetry were coupled with reductions in FA asymmetry within frontotemporal fibertracts. The authors interpreted this finding as evidence of both structural and functional reorganization of language in patients with left TLE. Rodrigo et al. (Rodrigo et al., 2008) used a similar approach and found a leftward asymmetry in FA of the ARC that was associated with leftward fMRI language activations in patients with right TLE and controls. However, this relationship did not hold for patients with left TLE. Despite reduced microstructural and functional asymmetry of the ARC in patients with left TLE, there was a lack of coherence between functional and DTI measures, which underscores the complexity of language reorganization in patients with left TLE. In a third multimodal imaging study, Ellmore et al. (2010) examined whether white matter microstructure of several frontotemporal fiber tracts, including the ARC, UNC, and ILF, could be used to predict asymmetric language representation, as determined by the IAP (Ellmore et al., 2010). They found that laterality indices computed from asymmetry of the ARC, but not other pathways, correctly classified 82.6% (19 of 23) of patients, whereas prefrontal fMRI LI correctly classified 86.9% (20 of 23) of patients when using IAP language indices as the gold standard. However, when FA of the ARC was combined with fMRI language activation patterns and handedness, 95.6% (22 of 23) of patients were correctly classified, suggesting that these measures may provide complementary information for lateralization language in TLE. It is of interest that in cases where fMRI or ARC LI was discordant with the IAP, fMRI was more likely to strongly lateralize patients (albeit in the direction opposite the IAP), whereas DTI metrics were more likely to be inconclusive (low laterality magnitude, but correct sign). Furthermore, in 2 of the 3 cases incorrectly classified by fMRI, ARC LI correctly classified the patient. These data suggest that although fMRI may have slightly stronger concordance with the IAP overall, DTI measures may add significant value and provide greater confidence of language lateralization in TLE.
In summary, these studies indicate that increased FA and decreased MD of left frontotemporal fiber tracts are associated with better performances on measures of language functioning in patients with TLE. However, there appears to be a decreased asymmetry within language networks in many patients with left TLE that is reflected in both functional and microstructural measures and may indicate a partial or complete language reorganization associated with chronic TLE. Asymmetry of the ARC may show the strongest concordance with both fMRI and IAP laterality, whereas there is disagreement among studies as to the value of UF, ILF, and IFOF asymmetry in this regard. Although the relative value of fMRI versus ARC asymmetry for predicting IAP laterality is not well established, the extant data suggest that these measures may provide complementary information related to language lateralization in TLE.
4.3 DTI and postoperative language outcome and reorganization
As with fMRI, few studies have addressed the ability of DTI to examine postoperative language outcome and reorganization following ATL. Yogarajah et al. (2010) measured white matter networks and language performances before and after ATL in patients with TLE and found widespread decreases in FA surrounding the resection zone, in addition to increases in FA after left ATL in a cluster of regions that included the ipsilateral external capsule, posterior limb of the internal capsule, and corona radiata. Mean pre- and postoperative FA and parallel diffusivity in this cluster were significantly correlated with postoperative verbal fluency and naming scores in patients with left TLE. Specifically, greater increases in parallel diffusivity following left ATL were associated with smaller declines in language performances. The authors suggest that this cluster of altered diffusion following left ATL corresponds to a ventromedial language network located medial to the dorsolateral language network, which may be less susceptible to injury following ATL and have a greater propensity for structural reorganization (Yogarajah et al., 2010). Pustina et al. (2014) used DTI to measure white matter changes before and up to several years after ATL, examining the relationship between changes in FA and verbal fluency. In patients with left TLE, better preoperative phonemic fluency was related to higher FA in the left superior corona radiata, right SLF, and right UNC. However, better postoperative phonemic and semantic fluency were associated with higher FA of the right SLF only. Given the relationship of all three clusters with language performance before surgery but only the right SLF after surgery, the authors suggest that an adaptive interhemispheric shift occurs following left ATL (Pustina et al., 2014). Thus, although left ATLs increase risk for postoperative language decline, there is a high likelihood of functional and structural reorganization to the contralateral side that may help to preserve language in many patients.
A question of high clinical interest is the comparative value of DTI versus fMRI for predicting postoperative language outcomes and reorganization in TLE. To our knowledge, only one study has addressed this question. In a cohort of 15 patients, Osipowicz et al. (2015) concluded that rather than any one measure, a combination of DTI, fMRI, and rsfMRI metrics provided the best predictive model, explaining 52% of the variance in post-operative semantic fluency decline and correctly classifying 87% of patients as having good versus poor fluency outcome. Among the imaging measures, DTI had the highest beta value, explaining 32% of the variance in fluency decline. Importantly, this study found that the pre to postoperative change in these imaging variables was more predictive than baseline imaging values or baseline clinical variables (i.e., duration of epilepsy, age, baseline fluency score, and baseline Verbal IQ) (Osipowicz, Sperling, Sharan, & Tracy, 2015). These data suggest that although preoperative imaging and clinical factors are important predictors of language outcome, the degree of postoperative reorganization that ensues within language networks may be the most important determinant of outcome.
Taken together, studies using DTI to examine language networks in patients with TLE have supported the idea that multiple frontotemporal fiber tracts play a role in naming, fluency and reading comprehension. However, the left ARC remains the fiber tract most frequently associated with language performances in TLE and shows the strongest concordance with the IAP and fMRI. Although there does not appear to be a one-to-one correspondence between functional and microstructural asymmetries, patients with left TLE are more likely to have less left-lateralized DTI and fMRI patterns, consistent with the concept of interhemispheric language reorganization in TLE. As with language studies using fMRI, DTI studies have also provided evidence that postoperative reorganization and adaptive changes may occur following left ATL, and this reorganization may occur within intrahemispheric (Yogarajah et al., 2010) or interhemispheric (Pustina et al., 2014) networks. Although a few studies have addressed the relative and/or combined value of fMRI and DTI for predicting language lateralization and outcomes in TLE, this represents a sizable gap in the literature in need of great attention. In addition, the degree to which postoperative language reorganization drives language outcomes is of high clinical importance, but nearly unchartered to date.
5. Volumetric MRI correlates of language impairment in TLE
Volumetric MRI has long been used to quantify structural pathology in patients with TLE, but it is less frequently employed relative to fMRI and DTI for examining specific language deficits. Seidenberg et al. (2005) investigated the relative contribution of hippocampal and extrahippocampal temporal lobe volumes to visual naming impairments in 53 patients with TLE and reported the following key findings: (1) a stronger relationship between left compared to right temporal lobe volumes and visual naming performance; (2) left temporal lobe white matter volume and left hippocampal volume contributed uniquely to visual naming performance; and (3) left temporal lobe white matter volume but not left hippocampal volume predicted recognition naming performance (Seidenberg, Geary, & Hermann, 2005). Similarly, Alessio et al. (2006) found that left hippocampal volume loss and perirhinal volume asymmetry independently predicted semantic fluency and visual naming impairments in patients with TLE (Alessio et al., 2006). These volumetric data are supported by studies showing that poorer visual naming is observed in patients with left hippocampal sclerosis compared to those with structurally normal hippocampi (Seidenberg et al., 1998), and 1H-MRS spectroscopy data showing significant correlations between hippocampal metabolism and visual naming performance (Martin et al., 1999; Sawrie et al., 2000). Interestingly, no studies have reported an association between hippocampal volume and auditory naming performance in TLE, leading researchers to conclude that although the hippocampus may be part of a temporal lobe network involved in visual naming, its contribution is more likely related to visual object processing rather than to linguistic mechanisms (for a review, see (Hamberger, 2015)).
In addition to studies focused exclusively on temporal lobe pathology, Hermann et al. (2003) demonstrated an association between total cerebral white matter volumes and phonemic fluency, but not naming, in 58 patients with TLE. It is of note that gray matter volumes were not related to neuropsychological measures in their study (B. Hermann et al., 2003). Using both stereological analysis of prefrontal subfields and voxel-based morphometry (VBM) of prefrontal cortex, Keller et al. (2009) found associations between left hippocampal and left prefrontal volumes and phonemic fluency using stereology–based, but not VBM analysis. Although the reason for these discrepant findings is not clear, the authors note fundamental differences in the regions of brain morphology assessed between stereology and VBM (Keller, Baker, Downes, & Roberts, 2009). Analysis of prefrontal structure using stereology quantifies regional volumes of gray and white matter together, whereas VBM analyzes regional distribution of only gray matter. Thus, it may be that reductions in white matter volume are the primary factor leading to language impairments in these studies. These data and those of Hermann et al. (2003) complement DTI work and suggest that white matter networks may be more important to language proficiency than specific cortical regions.
Very few studies have examined volumetric MRI in multimodal models of language impairment in TLE. As described above, Kucukboyaci et al. (2014) combined volumetric MRI with DTI and revealed that frontotemporal white matter integrity, but not cortical surface features (i.e., regional thickness, volume, or gray-white contrast) contributed to visual and auditory naming deficits in TLE. However, in a recent VBM study combining volumetric MRI and fMRI, Labudda et al. (2012) investigated language impairment in 20 left TLE patients with typical and 20 with atypical (right-sided) language on fMRI and found that patients with atypical language lateralization had increases of grey matter volumes, mainly within right-sided temporo-lateral cortex and less significantly within frontal brain regions compared to patients with typical language lateralization. In addition, the degree of atypical fronto-temporal language activation correlated with right-sided temporal and frontal gray matter volumes. However, patients with atypical language lateralization did not differ in terms of language performance from patients with typical language dominance (Labudda, Mertens, Janszky, Bien, & Woermann, 2012). These findings complement those from multimodal studies of fMRI-DTI, suggesting that structural changes underlie functional reorganization within language networks in TLE.
In summary, volumetric MRI studies of language impairment in TLE have supported the role of left lateral frontotemporal and medial temporal lobe structures, including the hippocampus, in naming and fluency performances. Furthermore, there is some evidence that atypical language dominance may be accompanied by structural shifts to right homologous language regions in some patients with left TLE and that these shifts may provide the basis for more successful language reorganization.
6. New analytic models for evaluating network disruption in TLE: Emerging contributions of graph theory
The goal of graph theoretical approaches to brain networks is to quantify structural and functional systems in the brain in order to analyze neural network organization [see (Albert & Barabási, 2002) for more comprehensive overview of modeling complex networks]. It utilizes a mathematical framework derived from functional, structural, or diffusion data to analyze interconnectivity (also referred to as vertices or edges) between brain regions (or nodes). In a network, nodes are linked with other nodes by edges, and a brain network formed by the interconnecting edges and nodes is referred to as a connectome. The complete mapping of the connectome allows for the generation of a connectivity matrix that encompasses all possible connections among the interconnected nodes. Graph theoretical parameters such as clustering coefficient, or the density of the connections around specific nodes, are commonly used to quantify the topological properties of the human brain connectome. Another commonly employed parameter is path length, which is the average number of shortest paths between any two nodes within the network. A network with short path length denotes efficiency as information can travel across the network more quickly. Different interconnectivity between nodes can characterize the network's topology. As an example, a small-world network has the characteristics of high clustering coefficient and low path length and is both locally and globally efficient. In essence, graph theory creates a framework of network organization that can be analyzed to characterize and predict change in network topology and measure overall network resilience (Bernhardt, Bonilha, & Gross, 2015).
Recent studies applying graph theory to structural and functional imaging data have supported the evolving concept of epilepsy as a network disorder and provided evidence for alterations in whole brain network topology in patients with TLE and extratemporal epilepsy (Bernhardt, Bernasconi, Hong, Dery, & Bernasconi, 2015; Bernhardt, Chen, He, Evans, & Bernasconi, 2011; Liao et al., 2010; Vaessen et al., 2012). Bernhardt et al. (2011) examined structural network organization and whole brain pathological interactions in TLE using MR-based cortical thickness correlations. While both healthy controls and patients with TLE exhibited short path length and a high degree of clustering consistent with small-world topology, patients with TLE showed increased clustering and path length and an altered distribution of network hubs, indicative of altered brain structural network topology. Their findings provide further evidence that epileptogenic networks disrupt whole-brain structural network organization (Bernhardt et al., 2011). Bernhardt et al. (2015) examined the limbic subnetwork in the mesiotemporal lobe in TLE and found increased clustering and path length, characterized by increased within-structure connectivity and decreased connectivity between the hippocampal and amygdala structures. They found that increased path length and decreased connectivity in preoperative network topology between the hippocampus and other structures was predictive of postoperative seizure freedom (Bernhardt, Bernasconi, et al., 2015). Using rs-fMRI data, Pedersen et al. (2015) similarly found increased clustering and local efficiency as well as isolation of network nodes in patients with focal extratemporal epilepsy compared to healthy controls (Pedersen, Omidvarnia, Walz, & Jackson, 2015). In contrast, graph theoretical analysis based on DTI data has yielded increased path length but decreased clustering coefficient in TLE (Vaessen et al., 2012). In a meta-analysis examining 13 studies on focal epilepsy and brain network organization, van Diessen et al. (2014) reviewed available literature which appear to corroborate several points: 1) there is an increase in path length and clustering coefficient in patients with focal epilepsy when compared to healthy control, 2) there is also an increase in segregation and regularized arrangement in network organization along with a decrease in global integration and connectivity, and 3) epileptic seizures affect structural and functional integrity of brain networks (Diessen, Diederen, Braun, Jansen, & Stam, 2013).
Despite an emerging literature on the utilization of graph theory to better understand disruptions in network connectivity in epilepsy, very few papers have examined the use of graph theory for probing language networks and predicting postoperative language decline following ATL. Doucet et al. (2015) examined the utility of rs-FC graph-theory measures for predicting cognitive outcomes following ATL and found that higher integration of the contralateral hippocampus with the rest of the brain was a strong predictor of better overall cognitive outcomes. With respect to language, random network organization and low integration predicted poorer expressive language outcome, explaining 73% of the variance in language decline following both left and right ATL (Doucet, Pustina, et al., 2015). In a study that examined localization-related epilepsy in children, Eddin et al. (2014) derived graph theory measures from fMRI data and examined global and local language networks. They found globally decreased efficiency in language network connectivity in pediatric patients when compared to controls. While small-world network topology was observed in both the pediatric patients and the control group, network activation in the pediatric epilepsy group was more random. Overall, the control group was observed to efficiently activate a smaller, partitioned network to complete a language task while the pediatric epilepsy group utilized the whole brain for the same task (Eddin et al., 2014). Kellerman et al. (2015) similarly found decreased efficiency and organization as well as poor segregation of cognitive network modules in the pediatric epilepsy population (Kellermann, Bonilha, Lin, & Hermann, 2015). Though still emerging, current literature into global network architecture in pediatric epilepsy appears to suggest an overall decrease in small-world topology, for which there is an associated reduction in global integration of local network and overall inefficiency in network activation. Despite the appeal of graph theory and early evidence for its clinical utility, this new analytic tool is still in development, and many questions remain regarding what different network measures imply for brain function. Thus, determining which graph theory measures best correlate with language performances and predict language outcomes in TLE and other epilepsy syndromes is a particularly important clinical goal.
7. Conclusions and Future Directions
Advanced neuroimaging has transformed our approach to the preoperative evaluation of patients with epilepsy and shifted our understanding away from one of strict localization and lateralization of language functions to one that focuses on complex language networks with variable patterns of language disruption in TLE. Furthermore, data from structural, functional, and diffusion imaging have provided evidence that these language networks can reorganize in response to an early cerebral insult or even following surgical intervention. Evidence for these adaptive changes and network plasticity is exciting given the potential implications for ameliorating language deficits over time as new behavioral, pharmacological, or medical (e.g., stimulation) interventions become available. Careful characterization of these language network changes is critical, as it could facilitate the matching of patients to surgical (i.e., ATL versus laser ablation) and rehabilitative interventions. However, proper characterization of network changes revealed by neuroimaging is complicated by the heterogeneity of language (re)organization in individual patients with TLE and by the dearth of true longitudinal studies, which can minimize inter-subject variability and provide information critical for characterizing and explaining changes that occur in the brain over time.
In addition, if neuroimaging is to make a substantive contribution to the clinical care of epilepsy patients, a better understanding of the significance of the imaging “signals” is imperative. In particular, what do different network abnormalities actually signify for language function? Do they reflect dysfunction/pathology, or compensatory changes that have the effect of improving language function? And what can the “signals” tell us about the specific functional mechanisms that underlie the change? For example, do they represent the re-activation of previously latent network connections for language (e.g., that may have been relevant for language at some point earlier in development), or do they represent the recruitment of novel regions and connections that indirectly support language function (e.g., recruitment of regions underlying more globally-relevant processes such as attention, that can provide ‘top-down’ modulation to support language function)? How do the answers to these questions differ for different components of language, given that language is not a monolithic construct (Bartha-Doering & Trinka, 2014), and different components of language (e.g., naming, reading, comprehension, repetition, etc.) may dissociate at the behavioral level and at the neuroanatomical level? Although some have proposed a conceptual framework for beginning to address these kinds of questions (Tracy & Osipowicz, 2011), the data needed to derive these answers is not readily available. In particular, future studies that thoughtfully relate network findings to clinically relevant measures of the different components of language function are greatly needed (M. Baciu & Perrone-Bertolotti, 2015; Protzner & McAndrews, 2011).
Further, despite the widespread availability of advanced imaging techniques and their increased use in clinical care, the relative value of DTI, fMRI, and volumetric MRI for predicting postoperative language outcomes (alone or in combination) has not been established, and the reason for occasional discordances among structural and functional imaging and the IAP remains unknown. The lack of progress in answering these questions, in addition to others, may explain why our ability to prevent or even mitigate language decline for individual patients following surgical intervention has not dramatically improved.
Along these lines, there is an increased need to shift our approach away from a focus on group neuroimaging analyses to one that focuses on patient-specific profiles. One example is a greater emphasis on systematically integrating language data derived from multimodal imaging (i.e., DTI and fMRI) with patient-specific clinical (i.e., preoperative language scores; age of seizure onset) and demographic (i.e., handedness; gender) characteristics. This approach is only beginning to emerge in epilepsy research and algorithms are now being developed that can be easily implemented in routine clinical care (see (Binder et al., 2008)). However, before this approach can be fully relied upon, we must better understand how variability at the individual patient level relates to differences at the network level; given that within the syndrome of TLE there is heterogeneity at all levels of analysis (e.g., clinical/demographic, behavioral/cognitive, brain structure/function), this is no small task.
As they mature, new analytic methods that provide an intricate characterization of topological aspects of abnormal brain connectivity and subject-specific profiles of network properties (i.e., graph theory) may challenge existing models of language disruption in TLE. Together with improvements in imaging hardware and data acquisition, these advancements show great promise for enhancing the prediction of both seizure and language outcomes following surgical interventions (see (Bernhardt, Bonilha, et al., 2015) for a review).
Highlights.
Non-invasive imaging has greatly advanced our understanding of language networks in epilepsy
FMRI is the most popular method for characterizing language organization and reorganization
Volumetric MRI and DTI provide structural and microstructural quantification of language network damage
Multimodal imaging and new analytic techniques may further refine models of language network disruption
Acknowledgments
The work was supported by the National Institute of Neurological Disorders and Stroke (R01NS065838 to C.R.M.). We thank Brandon Kopald for helpful comments on the manuscript.
Footnotes
Disclosure: Authors report no conflicts of interest and confirm that no competing financial interests exist.
This review article has not been published previously and the manuscript is not under consideration for publication in another journal. All authors listed on this manuscript have contributed significantly to the work reported. All authors have seen and agree with the contents of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Albert R, Barabási AL. Statistical mechanics of complex networks. Reviews of modern physics. 2002;74(1):47. [Google Scholar]
- Alessio A, Bonilha L, Rorden C, Kobayashi E, Min LL, Damasceno BP, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8(3):593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Ashtari M. Anatomy and functional role of the inferior longitudinal fasciculus: a search that has just begun. Dev Med Child Neurol. 2012;54(1):6–7. doi: 10.1111/j.1469-8749.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- Baciu M, Kahane P, Minotti L, Charnallet A, David D, Le Bas JF, et al. Functional MRI assessment of the hemispheric predominance for language in epileptic patients using a simple rhyme detection task. Epileptic Disord. 2001;3(3):117–124. [PubMed] [Google Scholar]
- Baciu M, Perrone-Bertolotti M. What do patients with epilepsy tell us about language dynamics? A review of fMRI studies. Rev Neurosci. 2015;26(3):323–341. doi: 10.1515/revneuro-2014-0074. [DOI] [PubMed] [Google Scholar]
- Baciu MV, Watson JM, McDermott KB, Wetzel RD, Attarian H, Moran CJ, et al. Functional MRI reveals an interhemispheric dissociation of frontal and temporal language regions in a patient with focal epilepsy. Epilepsy Behav. 2003;4(6):776–780. doi: 10.1016/j.yebeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Backes WH, Deblaere K, Vonck K, Kessels AG, Boon P, Hofman P, et al. Language activation distributions revealed by fMRI in post-operative epilepsy patients: differences between left- and right-sided resections. Epilepsy Res. 2005;66(1-3):1–12. doi: 10.1016/j.eplepsyres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bahn MM, Lin W, Silbergeld DL, Miller JW, Kuppusamy K, Cook RJ, et al. Localization of language cortices by functional MR imaging compared with intracarotid amobarbital hemispheric sedation. AJR Am J Roentgenol. 1997;169(2):575–579. doi: 10.2214/ajr.169.2.9242780. [DOI] [PubMed] [Google Scholar]
- Bartha-Doering L, Trinka E. The interictal language profile in adult epilepsy. Epilepsia. 2014;55(10):1512–1525. doi: 10.1111/epi.12743. [DOI] [PubMed] [Google Scholar]
- Bartha L, Benke T, Bauer G, Trinka E. Interictal language functions in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(6):808–814. doi: 10.1136/jnnp.2004.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PR, Reitsma JB, Houweling BM, Ferrier CH, Ramsey NF. Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. J Neurol Neurosurg Psychiatry. 2014;85(5):581–588. doi: 10.1136/jnnp-2013-305659. [DOI] [PubMed] [Google Scholar]
- Baxendale S. The role of functional MRI in the presurgical investigation of temporal lobe epilepsy patients: a clinical perspective and review. J Clin Exp Neuropsychol. 2002;24(5):664–676. doi: 10.1076/jcen.24.5.664.1005. [DOI] [PubMed] [Google Scholar]
- Bell B, Hermann B, Seidenberg M, Davies K, Cariski D, Rosenbek J, et al. Ipsilateral Reorganization of Language in Early-Onset Left Temporal Lobe Epilepsy. Epilepsy Behav. 2002;3(2):158–164. doi: 10.1006/ebeh.2002.0322. [DOI] [PubMed] [Google Scholar]
- Benke T, Koylu B, Visani P, Karner E, Brenneis C, Bartha L, et al. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada Test. Epilepsia. 2006;47(8):1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65(10):1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- Berl MM, Zimmaro LA, Khan OI, Dustin I, Ritzl E, Duke ES, et al. Characterization of atypical language activation patterns in focal epilepsy. Ann Neurol. 2014;75(1):33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Hong SJ, Dery S, Bernasconi A. Subregional Mesiotemporal Network Topology Is Altered in Temporal Lobe Epilepsy. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Bonilha L, Gross DW. Network analysis for a network disorder: The emerging role of graph theory in the study of epilepsy. Epilepsy Behav. 2015;50:162–170. doi: 10.1016/j.yebeh.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21(9):2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Billingsley-Marshall RL, Clear T, Mencl WE, Simos PG, Swank PR, Men D, et al. A comparison of functional MRI and magnetoencephalography for receptive language mapping. J Neurosci Methods. 2007;161(2):306–313. doi: 10.1016/j.jneumeth.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Binder JR. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2011a;20(2):214–222. doi: 10.1016/j.yebeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR. Preoperative prediction of verbal episodic memory outcome using FMRI. Neurosurg Clin N Am. 2011b;22(2):219–232, ix. doi: 10.1016/j.nec.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49(8):1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46(4):978–984. doi: 10.1212/wnl.46.4.978. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10(4-5):165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Bonelli SB, Thompson PJ, Yogarajah M, Vollmar C, Powell RH, Symms MR, et al. Imaging language networks before and after anterior temporal lobe resection: results of a longitudinal fMRI study. Epilepsia. 2012;53(4):639–650. doi: 10.1111/j.1528-1167.2012.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazdil M, Zakopcan J, Kuba R, Fanfrdlova Z, Rektor I. Atypical hemispheric language dominance in left temporal lobe epilepsy as a result of the reorganization of language functions. Epilepsy Behav. 2003;4(4):414–419. doi: 10.1016/s1525-5050(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Breier JI, Billingsley-Marshall R, Pataraia E, Castillo EM, Papanicolaou AC. Magnetoencephalographic studies of language reorganization after cerebral insult. Arch Phys Med Rehabil. 2006;87(12 Suppl 2):S77–83. doi: 10.1016/j.apmr.2006.07.271. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Large-scale cortical networks and cognition. Brain Res Brain Res Rev. 1995;20(3):288–304. doi: 10.1016/0165-0173(94)00016-i. [DOI] [PubMed] [Google Scholar]
- Carpentier A, Pugh KR, Westerveld M, Studholme C, Skrinjar O, Thompson JL, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001;42(10):1241–1254. doi: 10.1046/j.1528-1157.2001.35500.x. [DOI] [PubMed] [Google Scholar]
- Caverzasi E, Papinutto N, Amirbekian B, Berger MS, Henry RG. Q-ball of inferior fronto-occipital fasciculus and beyond. PLoS One. 2014;9(6):e100274. doi: 10.1371/journal.pone.0100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- D'Arcy RC, Bardouille T, Newman AJ, McWhinney SR, Debay D, Sadler RM, et al. Spatial MEG laterality maps for language: clinical applications in epilepsy. Hum Brain Mapp. 2013;34(8):1749–1760. doi: 10.1002/hbm.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deblaere K, Boon PA, Vandemaele P, Tieleman A, Vonck K, Vingerhoets G, et al. MRI language dominance assessment in epilepsy patients at 1.0 T: region of interest analysis and comparison with intracarotid amytal testing. Neuroradiology. 2004;46(6):413–420. doi: 10.1007/s00234-004-1196-0. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Sum JM, Wagner AD, Demb JB, Shear PK, Glover GH, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995;118(Pt 6):1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann Neurol. 1993;34(5):727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Diessen E, Diederen SJ, Braun KP, Jansen FE, Stam CJ. Functional and structural brain networks in epilepsy: what have we learned? Epilepsia. 2013;54(11):1855–1865. doi: 10.1111/epi.12350. [DOI] [PubMed] [Google Scholar]
- Doss RC, Zhang W, Risse GL, Dickens DL. Lateralizing language with magnetic source imaging: validation based on the Wada test. Epilepsia. 2009;50(10):2242–2248. doi: 10.1111/j.1528-1167.2009.02242.x. [DOI] [PubMed] [Google Scholar]
- Doucet GE, Pustina D, Skidmore C, Sharan A, Sperling MR, Tracy JI. Resting-state functional connectivity predicts the strength of hemispheric lateralization for language processing in temporal lobe epilepsy and normals. Hum Brain Mapp. 2015;36(1):288–303. doi: 10.1002/hbm.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet GE, Rider R, Taylor N, Skidmore C, Sharan A, Sperling M, et al. Presurgery resting-state local graph-theory measures predict neurocognitive outcomes after brain surgery in temporal lobe epilepsy. Epilepsia. 2015;56(4):517–526. doi: 10.1111/epi.12936. [DOI] [PubMed] [Google Scholar]
- Duchowny M, Jayakar P, Harvey AS, Resnick T, Alvarez L, Dean P, et al. Language cortex representation: effects of developmental versus acquired pathology. Ann Neurol. 1996;40(1):31–38. doi: 10.1002/ana.410400108. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Imaging in the surgical treatment of epilepsy. Nat Rev Neurol. 2010;6(10):537–550. doi: 10.1038/nrneurol.2010.131. [DOI] [PubMed] [Google Scholar]
- Dym RJ, Burns J, Freeman K, Lipton ML. Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test?: a meta-analysis. Radiology. 2011;261(2):446–455. doi: 10.1148/radiol.11101344. [DOI] [PubMed] [Google Scholar]
- Eddin AS, Wang J, Wu W, Sargolzaei S, Bjornson B, Jones RA, et al. The effects of pediatric epilepsy on a language connectome. Hum Brain Mapp. 2014;35(12):5996–6010. doi: 10.1002/hbm.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O'Neill TJ, et al. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. Neuroimage. 2010;49(3):2033–2044. doi: 10.1016/j.neuroimage.2009.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr A Greater Role for Surgical Treatment of Epilepsy: Why and When? Epilepsy Curr. 2003;3(2):37–40. doi: 10.1046/j.1535-7597.2003.03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay AM, Ambrose JB, Cahn-Weiner DA, Houde JF, Honma S, Hinkley LB, et al. Dynamics of hemispheric dominance for language assessed by magnetoencephalographic imaging. Ann Neurol. 2012;71(5):668–686. doi: 10.1002/ana.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Frye RE, Rezaie R, Papanicolaou AC. Functional Neuroimaging of Language Using Magnetoencephalography. Phys Life Rev. 2009;6(1):1–10. doi: 10.1016/j.plrev.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59(2):256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69(18):1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17(4):477–489. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ. Object naming in epilepsy and epilepsy surgery. Epilepsy Behav. 2015;46:27–33. doi: 10.1016/j.yebeh.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Cole J. Language organization and reorganization in epilepsy. Neuropsychol Rev. 2011;21(3):240–251. doi: 10.1007/s11065-011-9180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, Tamny T. Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56(1):56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, McClelland S, 3rd, McKhann GM, 2nd, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48(3):531–538. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Fritz NE, Gonzalez Perez PA, Elger CE, Weber B. Shift-back of right into left hemisphere language dominance after control of epileptic seizures: evidence for epilepsy driven functional cerebral organization. Epilepsy Res. 2006;70(2-3):257–262. doi: 10.1016/j.eplepsyres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Bell B, Rutecki P, Sheth RD, Wendt G, et al. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J Int Neuropsychol Soc. 2003;9(3):353–362. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13(1):12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13(1):3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48(4):1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Hirata M, Goto T, Barnes G, Umekawa Y, Yanagisawa T, Kato A, et al. Language dominance and mapping based on neuromagnetic oscillatory changes: comparison with invasive procedures. J Neurosurg. 2010;112(3):528–538. doi: 10.3171/2009.7.JNS09239. [DOI] [PubMed] [Google Scholar]
- Hirata M, Kato A, Taniguchi M, Saitoh Y, Ninomiya H, Ihara A, et al. Determination of language dominance with synthetic aperture magnetometry: comparison with the Wada test. Neuroimage. 2004;23(1):46–53. doi: 10.1016/j.neuroimage.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Jabbour RA, Hempel A, Gates JR, Zhang W, Risse GL. Right hemisphere language mapping in patients with bilateral language. Epilepsy Behav. 2005;6(4):587–592. doi: 10.1016/j.yebeh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jaimes-Bautista AG, Rodriguez-Camacho M, Martinez-Juarez IE, Rodriguez-Agudelo Y. Semantic Processing Impairment in Patients with Temporal Lobe Epilepsy. Epilepsy Res Treat. 2015;2015:746745. doi: 10.1155/2015/746745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecek JK, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, M ER, et al. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47(5):921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Keller SS, Baker G, Downes JJ, Roberts N. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav. 2009;15(2):186–195. doi: 10.1016/j.yebeh.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Kellermann TS, Bonilha L, Lin JJ, Hermann BP. Mapping the landscape of cognitive development in children with epilepsy. Cortex. 2015;66:1–8. doi: 10.1016/j.cortex.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Kang E, Kim JS, Song IC, Chung CK. Functional reorganization associated with semantic language processing in temporal lobe epilepsy patients after anterior temporal lobectomy : a longitudinal functional magnetic resonance image study. J Korean Neurosurg Soc. 2010;47(1):17–25. doi: 10.3340/jkns.2010.47.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukboyaci NE, Kemmotsu N, Leyden KM, Girard HM, Tecoma ES, Iragui VJ, et al. Integration of multimodal MRI data via PCA to explain language performance. Neuroimage Clin. 2014;5:197–207. doi: 10.1016/j.nicl.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labudda K, Mertens M, Janszky J, Bien CG, Woermann FG. Atypical language lateralisation associated with right fronto-temporal grey matter increases--a combined fMRI and VBM study in left-sided mesial temporal lobe epilepsy patients. Neuroimage. 2012;59(1):728–737. doi: 10.1016/j.neuroimage.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Langfitt JT, Rausch R. Word-finding deficits persist after left anterotemporal lobectomy. Arch Neurol. 1996;53(1):72–76. doi: 10.1001/archneur.1996.00550010090021. [DOI] [PubMed] [Google Scholar]
- Lee D, Swanson SJ, Sabsevitz DS, Hammeke TA, Scott Winstanley F, Possing ET, et al. Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav. 2008;13(2):350–356. doi: 10.1016/j.yebeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54(8):1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, Baldeweg T. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17(4):1861–1867. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, et al. Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia. 1990;28(8):831–838. doi: 10.1016/0028-3932(90)90007-b. [DOI] [PubMed] [Google Scholar]
- Lu LH, Crosson B, Nadeau SE, Heilman KM, Gonzalez-Rothi LJ, Raymer A, et al. Category-specific naming deficits for objects and actions: semantic attribute and grammatical role hypotheses. Neuropsychologia. 2002;40(9):1608–1621. doi: 10.1016/s0028-3932(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Maestu F, Ortiz T, Fernandez A, Amo C, Martin P, Fernandez S, et al. Spanish language mapping using MEG: a validation study. Neuroimage. 2002;17(3):1579–1586. doi: 10.1006/nimg.2002.1235. [DOI] [PubMed] [Google Scholar]
- Malmgren K, Thom M. Hippocampal sclerosis--origins and imaging. Epilepsia. 2012;53(4):19–33. doi: 10.1111/j.1528-1167.2012.03610.x. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130(Pt 3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Martin RC, Hugg JW, Roth DL, Bilir E, Gilliam FG, Faught E, et al. MRI extrahippocampal volumes and visual memory: correlations independent of MRI hippocampal volumes in temporal lobe epilepsy patients. J Int Neuropsychol Soc. 1999;5(6):540–548. doi: 10.1017/s1355617799566083. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132(Pt 2):347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Thesen T, Hagler DJ, Jr, Carlson C, Devinksy O, Kuzniecky R, et al. Distributed source modeling of language with magnetoencephalography: application to patients with intractable epilepsy. Epilepsia. 2009;50(10):2256–2266. doi: 10.1111/j.1528-1167.2009.02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina LS, Aguirre E, Bernal B, Altman NR. Functional MR imaging versus Wada test for evaluation of language lateralization: cost analysis. Radiology. 2004;230(1):49–54. doi: 10.1148/radiol.2301021122. [DOI] [PubMed] [Google Scholar]
- Metternich B, Buschmann F, Wagner K, Schulze-Bonhage A, Kriston L. Verbal fluency in focal epilepsy: a systematic review and meta-analysis. Neuropsychol Rev. 2014;24(2):200–218. doi: 10.1007/s11065-014-9255-8. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipowicz K, Sperling MR, Sharan AD, Tracy JI. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. J Neurosurg. 2015:1–9. doi: 10.3171/2014.9.JNS131422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Castillo EM, Breier JI, Sarkari S, Pataraia E, et al. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg. 2004;100(5):867–876. doi: 10.3171/jns.2004.100.5.0867. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Billingsley-Marshall RL, Castillo EM, Breier JI, Simos PG, Sarkari S, et al. Organization of receptive language-specific cortex before and after left temporal lobectomy. Neurology. 2005;64(3):481–487. doi: 10.1212/01.WNL.0000150900.71773.E6. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Lindinger G, Deecke L, Mayer D, Baumgartner C. Combined MEG/EEG analysis of the interictal spike complex in mesial temporal lobe epilepsy. Neuroimage. 2005;24(3):607–614. doi: 10.1016/j.neuroimage.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Pataraia E, Simos PG, Castillo EM, Billingsley RL, Sarkari S, Wheless JW, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62(6):943–948. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- Pedersen M, Omidvarnia AH, Walz JM, Jackson GD. Increased segregation of brain networks in focal epilepsy: An fMRI graph theory finding. Neuroimage Clin. 2015;8:536–542. doi: 10.1016/j.nicl.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, et al. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: evidence from resting state fMRI. BMC Neurosci. 2010;11:66. doi: 10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirmoradi M, Beland R, Nguyen DK, Bacon BA, Lassonde M. Language tasks used for the presurgical assessment of epileptic patients with MEG. Epileptic Disord. 2010;12(2):97–108. doi: 10.1684/epd.2010.0314. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36(1):209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, et al. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. Neuroimage. 2006;32(1):388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Protzner AB, McAndrews MP. Network alterations supporting word retrieval in patients with medial temporal lobe epilepsy. J Cogn Neurosci. 2011;23(9):2605–2619. doi: 10.1162/jocn.2010.21599. [DOI] [PubMed] [Google Scholar]
- Pustina D, Doucet G, Evans J, Sharan A, Sperling M, Skidmore C, et al. Distinct types of white matter changes are observed after anterior temporal lobectomy in epilepsy. PLoS One. 2014;9(8):e104211. doi: 10.1371/journal.pone.0104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie R, Narayana S, Schiller K, Birg L, Wheless JW, Boop FA, et al. Assessment of hemispheric dominance for receptive language in pediatric patients under sedation using magnetoencephalography. Front Hum Neurosci. 2014;8:657. doi: 10.3389/fnhum.2014.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Hodel J, de Vanssay A, Baudoin-Chial S, et al. Language lateralization in temporal lobe epilepsy using functional MRI and probabilistic tractography. Epilepsia. 2008;49(8):1367–1376. doi: 10.1111/j.1528-1167.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- Ruff IM, Petrovich Brennan NM, Peck KK, Hou BL, Tabar V, Brennan CW, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol. 2008;29(3):528–535. doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, Alpherts WC, van Veelen CW. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. Neuroimage. 2002;17(1):447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- Sabbah P, Chassoux F, Leveque C, Landre E, Baudoin-Chial S, Devaux B, et al. Functional MR imaging in assessment of language dominance in epileptic patients. Neuroimage. 2003;18(2):460–467. doi: 10.1016/s1053-8119(03)00025-9. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Swanson SJ, Hammeke TA, Spanaki MV, Possing ET, Morris GL, 3rd, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Sawrie SM, Martin RC, Gilliam FG, Faught RE, Maton B, Hugg JW, et al. Visual confrontation naming and hippocampal function: A neural network study using quantitative (1)H magnetic resonance spectroscopy. Brain. 2000;123(Pt 4):770–780. doi: 10.1093/brain/123.4.770. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Resor SR, Jr, De La Paz R, Goodman RR. Functional magnetic resonance imaging localization of ictal onset to a dysplastic cleft with simultaneous sensorimotor mapping: intraoperative electrophysiological confirmation and postoperative follow-up: technical note. Neurosurgery. 1998;43(3):639–644. doi: 10.1097/00006123-199809000-00150. discussion 644-635. [DOI] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A. Group analysis and the subject factor in functional magnetic resonance imaging: analysis of fifty right-handed healthy subjects in a semantic language task. Hum Brain Mapp. 2008;29(4):461–477. doi: 10.1002/hbm.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Geary E, Hermann B. Investigating temporal lobe contribution to confrontation naming using MRI quantitative volumetrics. J Int Neuropsychol Soc. 2005;11(4):358–366. [PubMed] [Google Scholar]
- Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Jr, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12(2):303–316. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007;38(2):306–320. doi: 10.1016/j.neuroimage.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Maggio WW, Gormley WB, Zouridakis G, Willmore LJ, et al. Atypical temporal lobe language representation: MEG and intraoperative stimulation mapping correlation. Neuroreport. 1999;10(1):139–142. doi: 10.1097/00001756-199901180-00026. [DOI] [PubMed] [Google Scholar]
- Spreer J, Arnold S, Quiske A, Wohlfarth R, Ziyeh S, Altenmuller D, et al. Determination of hemisphere dominance for language: comparison of frontal and temporal fMRI activation with intracarotid amytal testing. Neuroradiology. 2002;44(6):467–474. doi: 10.1007/s00234-002-0782-2. [DOI] [PubMed] [Google Scholar]
- Stafiniak P, Saykin AJ, Sperling MR, Kester DB, Robinson LJ, O'Connor MJ, et al. Acute naming deficits following dominant temporal lobectomy: prediction by age at 1st risk for seizures. Neurology. 1990;40(10):1509–1512. doi: 10.1212/wnl.40.10.1509. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Liu H, Reinsberger C, Madsen JR, Bourgeois BF, Dworetzky BA, et al. Language lateralization represented by spatiotemporal mapping of magnetoencephalography. AJNR Am J Neuroradiol. 2013;34(3):558–563. doi: 10.3174/ajnr.A3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage. 2005;24(3):841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Osipowicz KZ. A conceptual framework for interpreting neuroimaging studies of brain neuroplasticity and cognitive recovery. NeuroRehabilitation. 2011;29(4):331–338. doi: 10.3233/NRE-2011-0709. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I. Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage. 2008;39(1):1–9. doi: 10.1016/j.neuroimage.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JF, Vlooswijk MC, Hofman PA, Majoie HJ, Aldenkamp AP, et al. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex. 2012;22(9):2139–2147. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- Van Poppel M, Wheless JW, Clarke DF, McGregor A, McManis MH, Perkins FF, Jr, et al. Passive language mapping with magnetoencephalography in pediatric patients with epilepsy. J Neurosurg Pediatr. 2012;10(2):96–102. doi: 10.3171/2012.4.PEDS11301. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59(2):335–343. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Lang SY, Hong LU, Lin MA, Yan-ling MA, Yang F. Changes in extratemporal integrity and cognition in temporal lobe epilepsy: a diffusion tensor imaging study. Neurol India. 2010;58(6):891–899. doi: 10.4103/0028-3886.73739. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Castillo E, Maggio V, Kim HL, Breier JI, Simos PG, et al. Magnetoencephalography (MEG) and magnetic source imaging (MSI) Neurologist. 2004;10(3):138–153. doi: 10.1097/01.nrl.0000126589.21840.a1. [DOI] [PubMed] [Google Scholar]
- Wiebe S. Epidemiology of temporal lobe epilepsy. Can J Neurol Sci. 2000;27(1):S6–10. doi: 10.1017/s0317167100000561. discussion S20-11. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness & Efficiency of Surgery for Temporal Lobe Epilepsy Study, G. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Williams BA, Abbott KJ, Manson JI. Cerebral tumors in children presenting with epilepsy. J Child Neurol. 1992;7(3):291–294. doi: 10.1177/088307389200700309. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, et al. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Wong SW, Jong L, Bandur D, Bihari F, Yen YF, Takahashi AM, et al. Cortical reorganization following anterior temporal lobectomy in patients with temporal lobe epilepsy. Neurology. 2009;73(7):518–525. doi: 10.1212/WNL.0b013e3181b2a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington C, Vincent DJ, Bryant AE, Roberts DR, Vera CL, Ross DA, et al. Comparison of functional magnetic resonance imaging for language localization and intracarotid speech amytal testing in presurgical evaluation for intractable epilepsy. Preliminary results. Stereotact Funct Neurosurg. 1997;69(1-4 Pt 2):197–201. doi: 10.1159/000099874. [DOI] [PubMed] [Google Scholar]
- Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, et al. Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol. 1998;19(6):1095–1098. [PMC free article] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, et al. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(Pt 8):2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]


