Abstract
Major depressive disorder (MDD) is one of the leading forms of psychiatric disorders, characterized by aversion to mobility, neurotransmitters deficiency and energy metabolic decline. Low-level laser therapy (LLLT) has been investigated in a variety of neurodegenerative disorders associated with mitochondrial dysfunction and functional impairments. The goal of this study was to examine the effect of LLLT on depression-like behaviors, and to explore the potential mechanism by detecting mitochondrial function following LLLT. Depression models in space restriction mice and Abelson helper integrationsite-1 (Ahi1) KO mice were employed in this work. Our results revealed that LLLT effectively improved depression-like behaviors, in the two depression mice models, by decreasing immobility duration in behavioral despair tests. In addition, ATP biosynthesis and the level of mitochondrial complex IV expression and activity were significantly elevated in prefrontal cortex (PFC) following LLLT. Intriguingly, LLLT has no effects on ATP content and mitochondrial complex I-IV levels in other tested brain regions, hippocampus and hypothalamus. As a whole, these findings shed light on a novel strategy of transcranial LLLT on depression improvement by ameliorating neurotransmitter abnormalities and promoting mitochondrial function in PFC. The present work provides concrete groundwork for further investigation of LLLT for depression treatment.
Keywords: Low-level laser therapy, depression, neurotransmitters deficiency, mitochondria
Introduction
Depression is a prevalent psychiatric disorder which is characterized by irritability, depressed mood, fatigue, attention deficits, and diminished capacity for enjoyment [1]. It affects about 16% of the world’s population with high risk of suicide, increased susceptibility to develop atherosclerosis, type 2 diabetes and other chronic medical conditions [2–4]. In addition, depression substantially contributes to the global burden of public health, and leads to a detrimental effect on family well-being [5].
A line of research has demonstrated that the pathogenesis of depression is linked to the imbalance of monoamine neurotransmitters in the brain with regard to serotonin and dopamine. Distinct depression symptoms correspond to specific neurotransmitter deficiencies, suggesting the pathology of depression is neurotransmitter-dependent, which has been targeted by specific anti-depressant drug treatment [6]. Secreted by serotonergic neurons, serotonin is involved in the regulation of a variety of important physiological functions, including sleep, aggression, eating and mood. Current research suggests that a decrease in serotonin production by these neurons will lead to depression, and cause remarkably elevated suicidal tendency. Dopamine is another important neurotransmitter associated with depression, which plays a crucial role in regulating the drive to seek out rewards, as well as the ability to obtain a sense of pleasure. There are accumulating studies suggest that people who are vulnerable to depression may have a dopamine deficit with difficulty in handling the effects of stress efficiently.
Characterized by lack of energy, concentration difficulty and fatigue, depression is believed to be closely associated with mitochondrial dysfunction which is manifested by a resultant decline of ATP biosynthesis [7]. As the main source of cellular energy, ATP is generated via the electron transport chain (ETC) which consists of several compounds from mitochondrial complex I to IV, transferring electrons from electron donors to electron acceptors via redox reactions, and converting the conserved proton motive force to a final ATP form. Studies on mitochondria associated chronic depression models have found that the levels of several mitochondrial complexes were reduced in the brain cortex area [8,9]. Neuroimaging studies in humans detecting metabolic rates in different brain regions, also suggested an impaired cerebral energy metabolism in depression patients [10,11].
To date, large-scale studies have been involved in depression treatment mainly by pharmacological interventions [12]. However, due to their nonspecific interactions with multiple receptors and the so-called treatment-resistance that happens in about 41% of patients with depression during drug administration, antidepressants still show limited success and are associated with a number of adverse effects [13,14]. Low level laser treatment (LLLT) has been reported to modulate a variety of biological processes, like anti-oxidation, anti-inflammation, cell proliferation and angiogenesis [15–17]. Compelling evidences have demonstrated that LLLT can preserve mitochondrial function by increasing cytochrome c oxidase activity and ATP synthesis [18,19]. In addition, Lapchak et al. demonstrated the efficacy of LLLT in facilitating ATP synthesis in cerebral cortex [20], suggesting its potential improvement on neurodegenerative disorders. The present study was designed to investigate the effect of LLLT on depression-like behaviors in a mouse model by assessing the behavior performances and typical neurotransmitter alterations. In particular, we examined the changes of mitochondrial complexes levels and ATP contents in different brain regions following LLLT.
Materials and Methods
Animals and experimental groups
Male adult ICR mice were purchased from SLAC Company (Shanghai, China). Ahi1 knockout (KO) mice were generated as described previously [21]. The mice were housed in plastic cages and maintained at an ambient temperature of 20–22 °C and moisture of 50–60% under a 12-hour light: 12-hour dark cycle, and allowed free access to food and water. Mice were acclimated to their environment for 30 minutes prior to behavioral tests. All animal procedures involving the use of animals in this study were approved by the University Committee on Animal Care of Soochow University and conducted in accordance with the guidelines of Animal Use and Care of the National Institutes of Health. Both space restriction mice and Ahi1 KO mice were randomly divided into 3 groups: control group, without depressive disorder and laser treatment (Con); LLLT-treated group, subjected to depressive disorder and laser treatment (LLLT-treated); sham-operated group, subjected to depressive disorder and underwent identical procedures in LLLT treatment except that the laser power was not turned on (Sham-treated). Mice used in this case met the minimal number requirement to achieve statistically meaningful results. After behavior tests, all the animals were sacrificed under deep anesthesia, and the brains were collected for further biochemical analyses.
Spatial restraint stress
To develop a depression phenotype, mice were individually and gently placed into a modified, well-ventilated 50 ml centrifuge tube for 2 hours every day for 2 weeks as previously described [22]. Mice movements were restricted and were unable to move forward or backward. Control mice remained undisturbed in their original cages without water and food as stress mice. After spatial restraint stress, mice were returned to their original cages.
Low-level laser therapy (LLLT) treatments
LLLT treatment was performed using a diode laser with continuous wave of 808nm and maximal power output of 30 mW equipped with quartz-silica fiber (FU808AD500-BD10, Shenzhen Fuzhe Technology co., Ltd.). For laser treatment, mice were positioned on a plastic plate and and the head were covered by an aluminum sheet with a 1cm diameter hole centered cerebral cortex. After mice were fixed, LLLT was initiated with a power output density of 23 mW/cm2 by focusing the distal tip of the fiber optics on the top of the mice head with shaved scalp. The duration of laser irradiation was 30 min per day for 28 days.
Forced Swimming Test
The forced swimming test (FST) was performed as previously described with minor modification [21,23]. Briefly, mice were individually placed in a 2 L glass beaker filled with 1.5L of water at 25 ± 1°C, and allowed to swim freely for 6 minutes. Immobility time was recorded as the lack of all movements except that required to keep the mouse afloat. Mice were then returned to home cages under a heating lamp to warm gently for 20 minutes.
Tail Suspension Test
The tail suspension test (TST) was performed as previously described [21,24]. Mouse tail was attached to a wood rod by adhesive tape that kept the mouse to be suspended at 35 cm above the ground. During 6 minutes’ testing period, immobility time was recorded by a stopwatch. After the trial, mice were returned to their home cages.
Measurement of serotonin and dopamine levels
The test was performed as previously described [22,21]. Brain regions including prefrontal cortex (PFC), hippocampus (Hip) and hypothalamus (Hy) were dissected with a small plastic plate on ice pad after decapitation. All brain regions were homogenized after adding 200 µl of perchloric acid (0.4 M). The homogenates were centrifuged at 10,000 g for 15 min. Then perchloric acid (0.4 M) was added to a final volume of 1 ml and injected into an HPLC system. The turnover of serotonin was assessed by the ratio of 5-HIAA/serotonin. Data were analyzed and expressed as ng/g (serotonin) and µg/g (dopamine) respectively.
Measurement of mitochondrial complex I~ IV content and ATP level
As previously described [25], ATP concentration was determined using a mouse ATP Elisa kit (68CV-ATP-S1000, Rapidbio; America) following vendor’s instructions. Briefly, brain regions of PFC, Hip, and Hy were dissected and homogenized. 30 µg of sample proteins from total protein fractions were suspended in 100 µl of reconstituted rL/L reagent buffer containing luciferase, D-luciferin, Tris-acetate buffer (pH 7.75), ethylenediaminetetraacetic acid (EDTA), magnesium acetate, bovine serum albumin (BSA) and dithiothreitol (DTT). Light emission from the reaction was measured in a standard microplate luminometer (PE Applied Biosystems). Values of ATP levels were determined using an ATP standard curve. Data were analyzed and expressed as ng/mg. Mitochondrial complexes content were measured using Mouse MRCC I~IV Elisa kits (68CV-MRCCI-S1000, 68CV-MRCCII-T500, 68CV-MRCCIII-2R781, 68CV-MRCCIV-2R800, Rapidbio; America) in accordance with manufacturer’s instructions. Final values were determined by light absorbance at 450 nm using a microplate reader (Tecan infinite M200 PRO, Austria).
Measurement of mitochondrial complex IV activity
PFC region was collected and homogenized with cold assay buffer from a Mitochondrial complex IV Activity Assay kit (Suzhou Comin Biotechnology Co., Ltd.). Mitochondrial complex IV Activity analysis was measured according to the manufacturer’s instructions. Equivalent volumes of each sample (10µL) and 200µL working buffer were mixed in the 96 well plates. The absorbance at 550 nm was immediately recorded as A1; the absorbance at 550 nm was recorded as A2 at 1 min later. The difference of the absorbances (ΔA) was calculated (A2-A1). Mitochondrial complex IV activity (nmol/min/g tissue) was calculated via the formula: 444×ΔA/tissue weight (g).
Statistical Analysis
All data were expressed as mean ± SEM. Mean differences between groups were determined with a Student t test at p < 0.05 and the differences among groups were performed with analyses of variance (ANOVAs) followed by Turkey’s multiple-comparison test. P<0.05 was considered statistically significant. Statistical analyses were conducted with Graph Pad Prism 5.0 (San Diego, California).
Results
Spatial restraint stress caused depressive phenotypes in mice
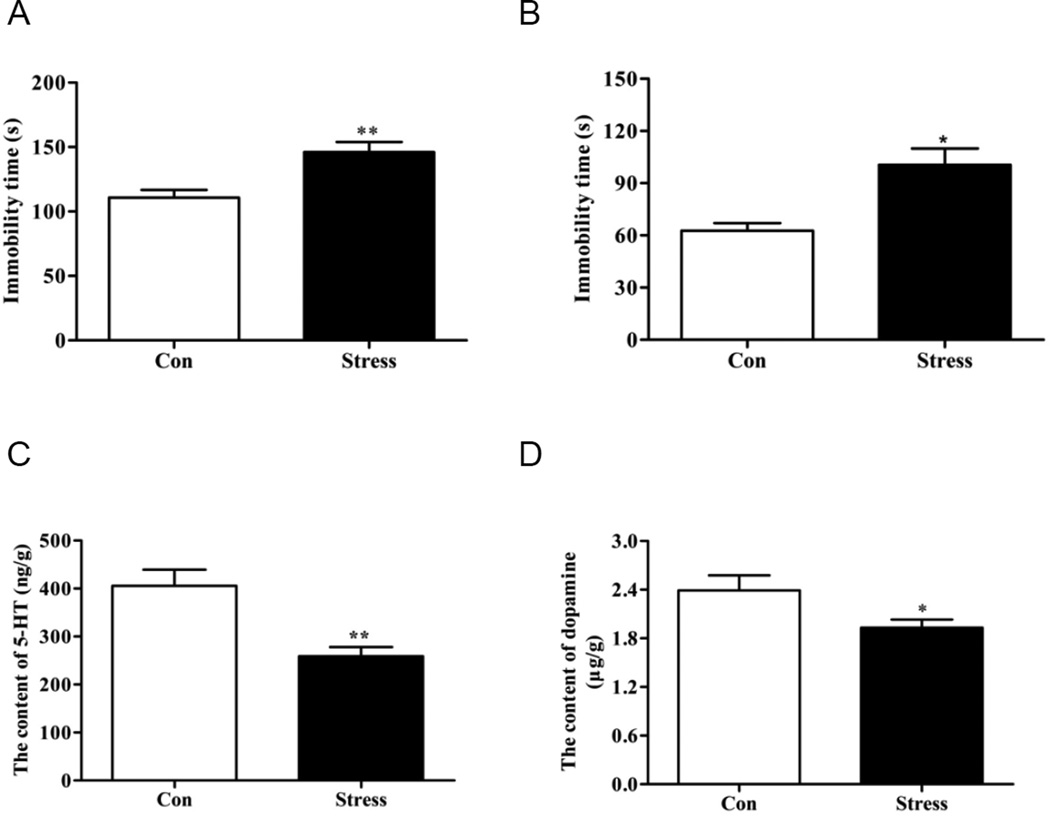
Repeated spatial restraint stress causes depressive phenotypes and has been widely used in the establishment of depression models [26,27]. FST and TST are two commonly used screening tests for antidepressants in rodent models of depression [28]. In the present study, both FST (Fig. 1A) and TST (Fig. 1B) were employed, showing that the stressed mice exhibited significantly greater immobility and less motor activity than control mice, indicating a representative depressive-like phenotype after repeated spatial restraint stress operations. Moreover, since both animals and patients with depression-like behaviors are characterized by neurotransmitters deficiency, represented by serotonin (5-HT) and dopamine [21], levels of 5-HT and dopamine in hippocampus were subsequently examined by HPLC to further verify the establishment of our depression model. Results revealed that both 5-HT (Fig. 1C) and dopamine (Fig. 1D) levels in the hippocampus were significantly decreased in stress mice compared with control mice. Together with the demonstrated neurotransmitters decline and motor activity decrease, our data suggested that depressive phenotype has been successfully developed in mice subjected to spatial restraint stress.
Figure 1. Spatial restraint stress caused depressive phenotype with significant decrease in neurotransmitters.
(A) Immobility time of depressive mice was significantly elevated compared with control group in FST. (B) Immobility time of TST in stress mice and control group. Note that a depressive-like phenotype has been developed after spatial restraint stress. Levels of 5-HT (C) and dopamine (D) in hippocampus were subsequently examined by HPLC, showing the resultant declines of these depression-associated neurotransmitters. *P<0.05, **P<0.01 versus control group. N = 9–10.
LLLT attenuated depression-like behaviors in space restraint mice
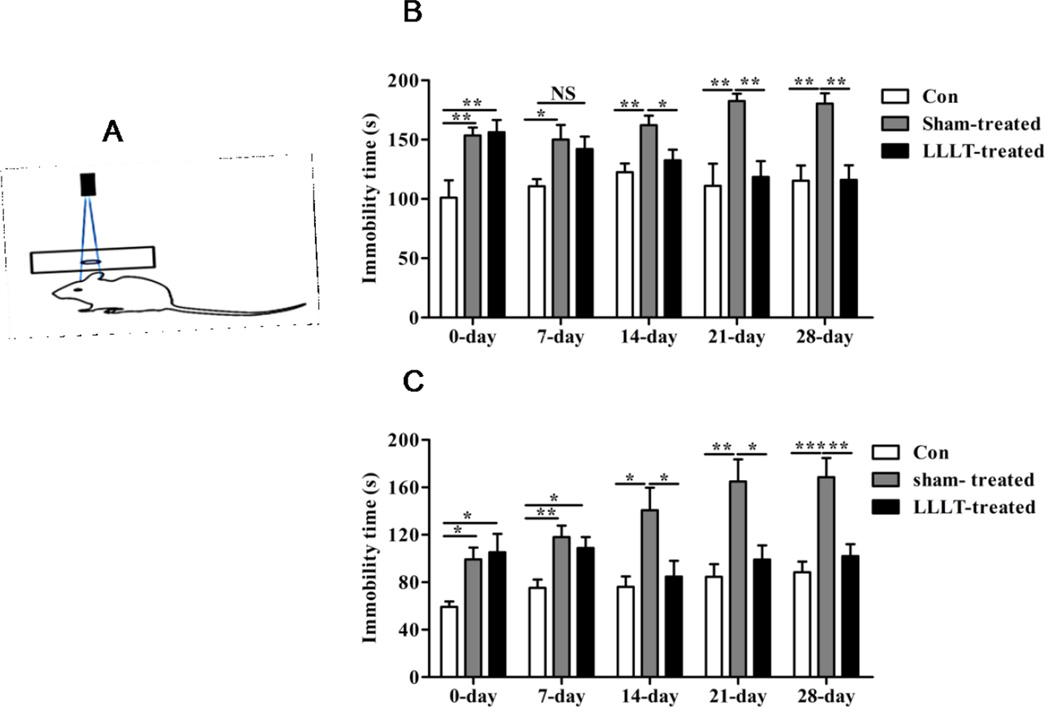
To investigate the effect of LLLT treatment on depression-like behaviors, LLLT with a wavelength of 808 nm was immediately performed after spatial restraint operation for 30 minutes every day for a duration of 28 days, with detailed protocol described in Method part (and see diagram in Figure 2A). Meanwhile, behavior tests of FST and TST were carried out with immobility time recorded to examine the effects of LLLT on depressive behaviors at different time points. As shown in Fig. 2B and Fig. 2C, significant difference in immobility time between sham-treated and LLLT-treated groups was not observed until 14 days after space restraint operation. Afterwards, immobility time in mice subjected to laser irradiation was significantly reduced compared with depression group, and interestingly these differences were maintained at a relatively stable level from the 21st day on. These findings indicate that LLLT of 808nm exerts efficacious depressive attenuation on depression-like behaviors by decreasing the immobility and promoting motor activity.
Figure 2. LLLT rescued depressive behaviors in stress mice.
(A) Schematic diagram showing laser treatment presentation. Immobility time in FST (B) and TST (C) of each group was recorded at different time point following LLLT. Results suggested that LLLT significantly improved the depressive behaviors by decreasing the immobility time from the 14th day after the initiation of laser treatment. *P<0.05, **P<0.01, ***P<0.001. N = 9–10.
LLLT attenuated depression-like behaviors in Ahi1 KO mice
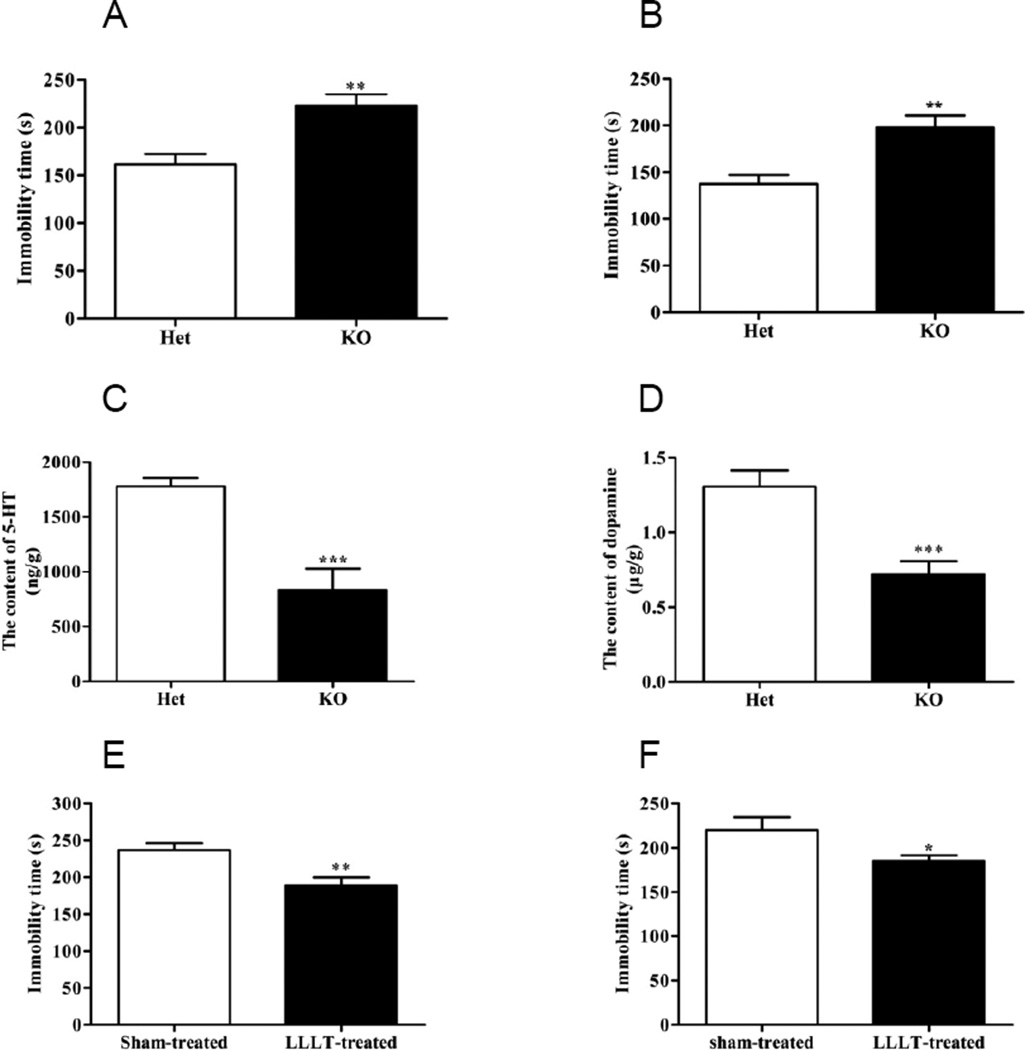
Increasing evidences have shown that Abelson helper integrationsite-1 (Ahi1) mutation is associated with a series of psychiatric diseases [29,30]. In addition, our previous studies also demonstrated that Ahi1 KO causes depression-like behaviors in mice [31,21], suggesting Ahi1 can be effectively targeted to develop a depression model. To further verify the effect of LLLT on depression disorder from a genetic mutation model, Ahi1 KO mice were used in the current study. In both tests of FST (Fig. 3A) and TST (Fig. 3B), Ahi1 KO mice exhibited greater immobility than control (Het) mice, indicating a representative depressive-like phenotype. Serotonin and dopamine levels were successively detected by HPLC to further confirm the formation of depression pathology. Results showed that both serotonin (Fig. 3C) and dopamine (Fig. 3D) contents in hippocampus were significantly down-regulated in Ahi1 KO mice, noting that Ahi1 KO mice have successfully developed depressive disorder, which is consistent with our previous results [21]. To examine the effect of LLLT on Ahi1 KO induced depression, Ahi1 KO mice were randomly divided into 2 groups, with one group received LLLT treatment and the other treated as sham-control. Our result showed that LLLT for consecutive 28 days significantly decreased the immobility time in FST (Fig. 3E) and TST (Fig. 3F) tests compared with sham-treated group, suggesting an efficacious alleviation of LLLT on depression-like behaviors in Ahi1 KO mice.
Figure 3. LLLT attenuated Ahi1 KO-induced depression-like behaviors and neurotransmitters decrease in mice.
Immobility time in FST (A) and TST (B) was shown in control and Ahi1 KO mice. Levels of neurotransmitter serotonin (C) and dopamine (D) in hippocampus were subsequently examined by HPLC. **P<0.01, ***P<0.001 versus control group. After 28 days’ laser treatment, FST (E) and TST (F) were performed in Sham-treated and LLLT-treated mice with immobility time recorded. *P<0.05, **P<0.01 versus sham-treated mice. N = 9–10.
LLLT promoted ATP biosynthesis in mice PFC
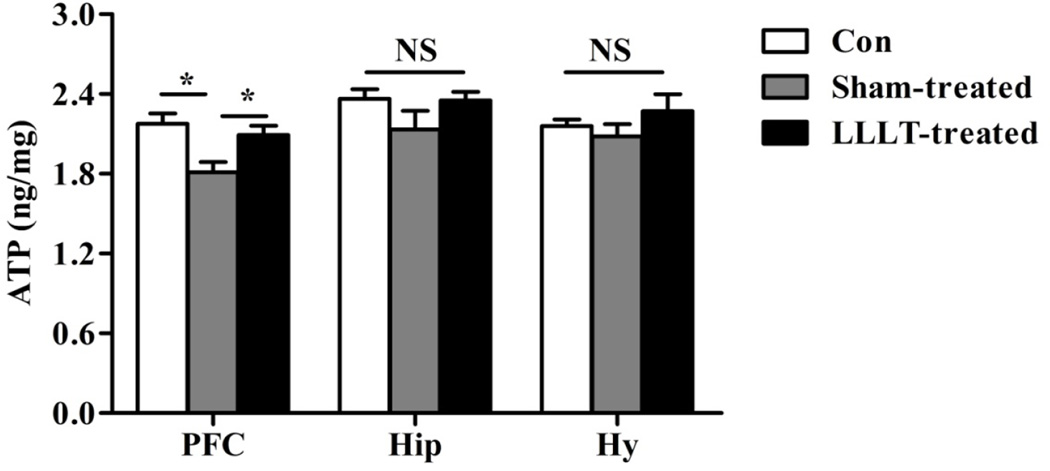
It has been reported that dysregulation of genes involved in ATP biosynthesis and utilization in PFC exists in depressive behavior [32]. To examine whether LLLT can rescue depression-like behaviors in mice by elevating ATP level in PFC, as well as in other brain regions, ATP contents in prefrontal cortex (PFC), hippocampus (Hip) and hypothalamus (Hy) were detected by ELISA analysis. Our results showed that ATP level in stress group was significantly decreased compared with control mice in PFC. By contrast, LLLT significantly increased ATP biosynthesis compared with sham group, which unveils a underlying mechanism of LLLT as an efficacious anti-depression strategy. Interestingly, no significant alterations of ATP levels following LLLT were observed in other brain regions, such as Hip and Hy (Fig. 4). This observation is reasonable as previous studies show that PFC, a brain region required for the adaptive orchestration of behavioral responses, is implicated in chronic stress-related psychiatric diseases including major depression [33].
Figure 4. LLLT promoted ATP biosynthesis in the PFC of stress mice.
ATP levels of each group from Prefrontal cortex (PFC), hippocampus (Hip) and hypothalamus (Hy) were determined by ELISA assay. Result showed that significant increased ATP synthesis following LLLT in stress mice was only observed in PFC region, while there were no significant ATP changes in Hip and Hy. N= 9–10. *P<0.05. NS: not significant difference.
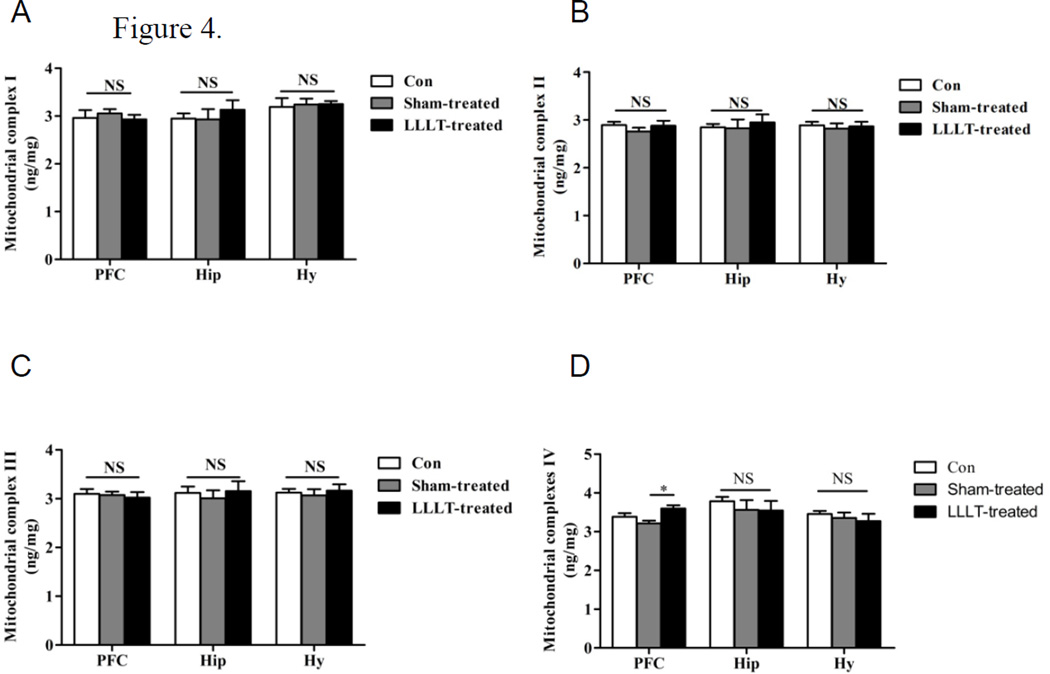
LLLT elevated the expression level and activity of mitochondrial complex IV in mice PFC
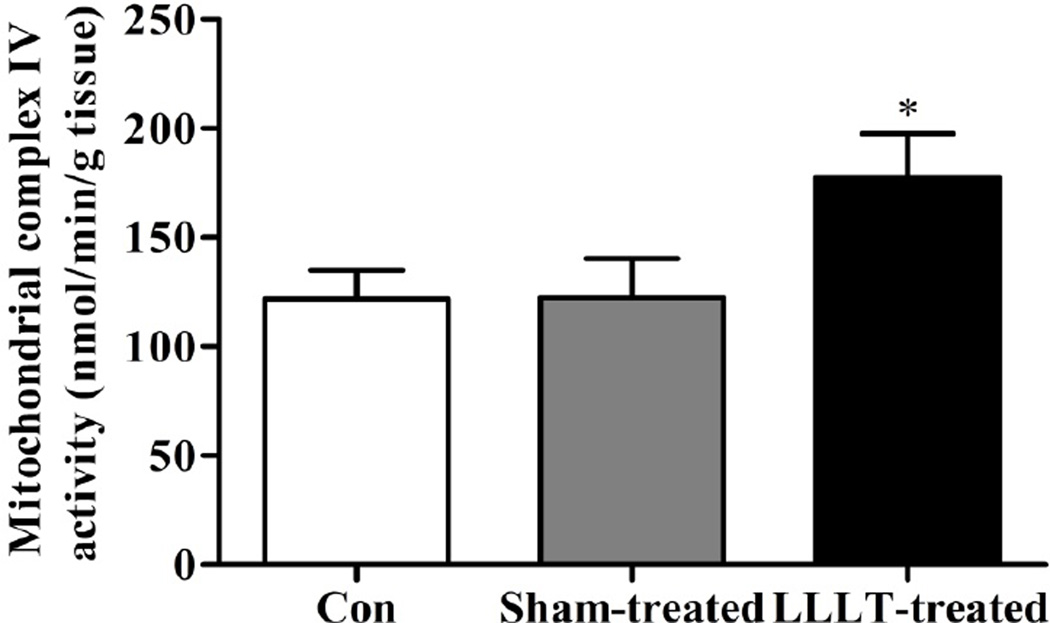
Since ATP is generated by oxidative phosphorylation via electron transport chain (ETC) within mitochondrial inner membrane, elevated ATP synthesis should be based on the preservation of mitochondrial ETC function, manifested by the increase in mitochondrial complexes contents. To verify this hypothesis, levels of ETC components, including complex I–IV from each group were further examined in stress model groups. As shown in Fig. 5A–5C, there were no significant alterations of mitochondrial complex I-III content in PFC, Hy and Hip regions among control, Sham-LLLT and LLT groups. However, as shown in Fig. 5D, the expression level of mitochondrial complex IV in PFC was significantly increased in the LLLT-treated animals compared with LLLT-untreated groups. In addition, complex IV level was not changed in Hy and Hip between groups. To confirm this, we determined the activity of complex IV in the PFC region. Our results showed that LLLT treatment significantly increased the activity of complex IV in the PFC region compared with LLLT-untreated group and control group (P < 0.05, Figure 6). These data suggest that the pathology of major depression disorder is PFC-dependent, and the elevation in ATP generation following LLLT was not based on the preservation of the whole ETC, but only by modulation of mitochondrial complex IV expression and activity.
Figure 5. LLLT increased mitochondrial IV level in the PFC of stress mice.
Biochemical indicators of mitochondrial complex I–IV levels from the brain regions of PFC, Hip and Hy were detected by ELISA assay in each group. Consistent with the results in ATP analysis, data revealed that LLLT only elevated the mitochondrial IV level in PFC, while no significant alterations of mitochondrial complex I-III were observed in the other tested brain regions. N= 9–10. *P<0.05 versus sham-treated group. NS: not significant difference.
Figure 6. LLLT increased mitochondrial IV activity in the PFC of stress mice.
Mitochondrial IV activity from PFC in each group from was determined. Result showed that significant increased Mitochondrial IV activity following LLLT in stress mice was observed in PFC region. N= 9–10. *P<0.05.
Discussion
Major depression disorder (MDD) is believed to be closely associated with mitochondrial dysfunction manifested by significant energy metabolic deficit and subsequent ATP decline, features further confirmed in this study [34–36]. LLLT has been widely applied in certain animal models with its properties of anti-oxidation, anti-inflammation and cell proliferation promotion, and a novel effect on the modulation of mitochondrial function [37–39]. Using two depression models, the current study provides compelling evidence, for the first time, that LLLT was able to attenuate depressive disorder by rescuing depressive-like behaviors and the associated neurotransmitter deficiency. A further mechanism was investigated by examining the effects of LLLT on depression-induced mitochondrial dysfunction with regard to significantly decreased mitochondrial ATP synthesis in prefrontal cortex. Our results provide concrete evidences supporting that mitochondrial dysfunction assumes an increasingly important role in depression disorder, while LLLT treatment can effectively improve depressive-like behaviors by elevating mitochondrial complex IV content and activity, subsequently stimulating ATP biosynthesis.
Several hypotheses involving depression pathogenesis have been proposed, among which the monoamine hypothesis about depression-associated neurotransmitter deficiency is most prevalent during the past few decades. It suggests that depressive disorder is closely associated with the imbalance of monoamine neurotransmitters such as serotonin and dopamine. Apparent decreases of serotonin and dopamine have been found in a variety of depressive animal models [40]. Additionally, clinical studies also suggested that specific neurotransmitter decline leads to the corresponding depressive symptoms [6]. For example, serotonin deficiency is subjected to a higher suicide tendency, while patients with dopamine decline tend to cause the inability to regulate their stress effect [41]. The fact that either early antidepressants, such as tricyclics and monoamine oxidase inhibitors or recent selective serotonin reuptake inhibitors (SSRIs) have analogical effect on enhancing monoamine function further supported this hypothesis [42,43]. In our present study, decreased serotonin and dopamine were also confirmed in both stress mice and Ahi1 KO mice by HPLC analysis, suggesting depressive disorder is indeed linked to low-level neurotransmitter expression.
Our work demonstrated remarkable efficacy of LLLT on the improvement of depression disorder by promoting mitochondrial energy biosynthesis. It’s well known that mitochondria are involved in a series of intracellular processes coupled to energy metabolism, signal transduction, neuron survival and plasticity. Impairment on mitochondrial function is manifested by damaged bioenergetics, decreased ATP production, impaired calcium homeostasis, increased free radicals accumulation, and consequently related to a variety of psychiatric and neurodegenerative diseases, like MDD and anxiety [44–46]. As a mental disorder resulting from metabolic system failure, depression is closely related to mitochondrial dysfunction which is believed to play a key role in depressive pathophysiology [47]. As the major form of intracellular energy storage, ATP is mainly generated on electron transport chain composed of I-IV complexes that transfer electrons, pump protons outwardly to form proton gradient, and drive the final ATP synthesis. Growing data have shown that mitochondrial complex IV (cytochrome c oxidase) is the key component which reduces the terminal O2 to H2O, and retains the partially reduced intermediates until full reduction is completed [48]. Also, complex IV was suggested as a pivotal endogenous metabolic marker for neuronal activity [49]. A line of reports have demonstrated the effect of LLLT on mitochondrial functional modulation by stimulating mitochondrial oxidative phosphorylation and ATP production in models both in vivo and in vitro [18,50,39]. Correspondingly, results in present study indicated that LLLT significantly attenuated the energy decline by elevating mitochondrial complex IV level and ATP production in depressive mice, indicating an important role of LLLT in facilitating mitochondrial energetic metabolism. The present study also suggest that ATP is a key factor involved in modulation of depressive-like behavior, and that LLLT improves depressive behavior through its facilitation on PFC-specific ATP generation and mitochondrial complex IV activation.
Due to the limited success of antidepressants and their associated side effect profile, there is a demonstrable need for innovative treatment options [13,14]. LLLT treatment is currently being integrated into mainstream medicine alongside ongoing research to ascertain demonstrable efficacy [51]. Our data showed that LLLT improves depressive behavior by increasing mitochondrial complex IV oxidase activity and ATP synthesis. These results indicate a role for LLLT in not only depression, but a host of other neurological disorders where metabolic failure is implicated as a primary or secondary pathological role, such as stroke, dementia, Alzheimer’s, or neonatal hypoxic ischemic encephalopathy [citations needed]. The key caveat is that the effects of LLLT seem to be relegated to particular wavelengths and power densities. Our future studies will work to isolate the efficacious wavelengths and dosages to determine the best course of clinical treatment in patients with depression.
In summary, the current study is the first to report the protective effects of LLLT against depression-induced behavioral impairments and neurotransmitters decrease in both space restraint and Ahi1 KO mice depression models. Notably, it provides concrete mechanism investigation supporting the novel effect of LLLT towards depression-associated energy metabolic deficit by elucidating the alterations of mitochondrial complexes content and resultant ATP synthesis. This study points LLLT as a promising physical intervention on depressive disorder, and provides important findings for future studies of laser treatment in clinical application.
Acknowledgments
This study was supported by the grants from National Natural Science Foundation of China (81071095, 81120108011, and 81200893), Jiangsu Province Science and Technology Project (BK20151197), Suzhou Science and Technology Project (SYS201372 and LCZX201316), the Priority Academic Program Development of Jiangsu Higher Education Institutions of China, and a Research Grant NS086929 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health USA. The funders have no role in study design and data collection.
References
- 1.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 4.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P, Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biological psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 6.Nutt DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. The Journal of clinical psychiatry. 2008;69(Suppl E1):4–7. [PubMed] [Google Scholar]
- 7.Karabatsiakis A, Bock C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE, Kolassa IT. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Translational psychiatry. 2014;4:e397. doi: 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;24(4):420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 9.Rezin GT, Cardoso MR, Goncalves CL, Scaini G, Fraga DB, Riegel RE, Comim CM, Quevedo J, Streck EL. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochemistry international. 2008;53(6–8):395–400. doi: 10.1016/j.neuint.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. The American journal of psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 11.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 12.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 13.Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355(9207):911–918. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- 14.Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. The Journal of clinical psychiatry. 1998;59(Suppl 18):23–29. [PubMed] [Google Scholar]
- 15.Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. Journal of clinical laser medicine & surgery. 2003;21(2):67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- 16.Albertini R, Villaverde AB, Aimbire F, Salgado MA, Bjordal JM, Alves LP, Munin E, Costa MS. Anti-inflammatory effects of low-level laser therapy (LLLT) with two different red wavelengths (660 nm and 684 nm) in carrageenan-induced rat paw edema. Journal of photochemistry and photobiology B, Biology. 2007;89(1):50–55. doi: 10.1016/j.jphotobiol.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Rojas JC, Lee J, John JM, Gonzalez-Lima F. Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(50):13511–13521. doi: 10.1523/JNEUROSCI.3457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraresi C, Kaippert B, Avci P, Huang YY, de Sousa MV, Bagnato VS, Parizotto NA, Hamblin MR. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3–6 h. Photochemistry and photobiology. 2015;91(2):411–416. doi: 10.1111/php.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilden L, Karthein R. Import of radiation phenomena of electrons and therapeutic low-level laser in regard to the mitochondrial energy transfer. Journal of clinical laser medicine & surgery. 1998;16(3):159–165. doi: 10.1089/clm.1998.16.159. [DOI] [PubMed] [Google Scholar]
- 20.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5'-triphosphate (ATP) content following embolic strokes in rabbits. Brain research. 2010;1306:100–105. doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Ren L, Qian X, Zhai L, Sun M, Miao Z, Li J, Xu X. Loss of Ahi1 impairs neurotransmitter release and causes depressive behaviors in mice. PloS one. 2014;9(4):e93640. doi: 10.1371/journal.pone.0093640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Chen L, Yang L, Hua X, Zhou B, Miao Z, Li J, Hu H, Namaka M, Kong J, Xu X. Combined use of spatial restraint stress and middle cerebral artery occlusion is a novel model of post-stroke depression in mice. Scientific reports. 2015;5:16751. doi: 10.1038/srep16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 24.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 25.Lu Q, Tucker D, Dong Y, Zhao N, Zhang Q. Neuroprotective and Functional Improvement Effects of Methylene Blue in Global Cerebral Ischemia. Molecular neurobiology. 2015 doi: 10.1007/s12035-015-9455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abush H, Akirav I. Cannabinoids ameliorate impairments induced by chronic stress to synaptic plasticity and short-term memory. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(8):1521–1534. doi: 10.1038/npp.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2011;Chapter 8(Unit 8 10A) doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- 29.Porcelli S, Pae CU, Han C, Lee SJ, Patkar AA, Masand PS, Balzarro B, Alberti S, De Ronchi D, Serretti A. Abelson helper integration site-1 gene variants on major depressive disorder and bipolar disorder. Psychiatry investigation. 2014;11(4):481–486. doi: 10.4306/pi.2014.11.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elsayed SM, Phillips JB, Heller R, Thoenes M, Elsobky E, Nurnberg G, Nurnberg P, Seland S, Ebermann I, Altmuller J, Thiele H, Toliat M, Korber F, Hu XJ, Wu YD, Zaki MS, Abdel-Salam G, Gleeson J, Boltshauser E, Westerfield M, Bolz HJ. Non-manifesting AHI1 truncations indicate localized loss-of-function tolerance in a severe Mendelian disease gene. Human molecular genetics. 2015;24(9):2594–2603. doi: 10.1093/hmg/ddv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Yang H, Lin YF, Li X, Cape A, Ressler KJ, Li S, Li XJ. Neuronal Abelson helper integration site-1 (Ahi1) deficiency in mice alters TrkB signaling with a depressive phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(44):19126–19131. doi: 10.1073/pnas.1013032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klempan TA, Sequeira A, Canetti L, Lalovic A, Ernst C, ffrench-Mullen J, Turecki G. Altered expression of genes involved in ATP biosynthesis and GABAergic neurotransmission in the ventral prefrontal cortex of suicides with and without major depression. Molecular psychiatry. 2009;14(2):175–189. doi: 10.1038/sj.mp.4002110. [DOI] [PubMed] [Google Scholar]
- 33.Negron-Oyarzo I, Aboitiz F, Fuentealba P. Impaired Functional Connectivity in the Prefrontal Cortex: A Mechanism for Chronic Stress-Induced Neuropsychiatric Disorders. Neural plasticity. 2016;2016:7539065. doi: 10.1155/2016/7539065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Molecular psychiatry. 2005;10(10):900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 35.Kato T. Role of mitochondrial DNA in calcium signaling abnormality in bipolar disorder. Cell calcium. 2008;44(1):92–102. doi: 10.1016/j.ceca.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Quiroz JA, Gray NA, Kato T, Manji HK. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(11):2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- 37.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Annals of biomedical engineering. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silveira PC, Silva LA, Fraga DB, Freitas TP, Streck EL, Pinho R. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. Journal of photochemistry and photobiology B, Biology. 2009;95(2):89–92. doi: 10.1016/j.jphotobiol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Souza NH, Ferrari RA, Silva DF, Nunes FD, Bussadori SK, Fernandes KP. Effect of low-level laser therapy on the modulation of the mitochondrial activity of macrophages. Brazilian journal of physical therapy. 2014;18(4):308–314. doi: 10.1590/bjpt-rbf.2014.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of general psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 41.Shaw DM, Camps FE, Eccleston EG. 5-Hydroxytryptamine in the hind-brain of depressive suicides. The British journal of psychiatry : the journal of mental science. 1967;113(505):1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- 42.Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression and anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 43.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. The American journal of psychiatry. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 44.Toker L, Agam G. Mitochondrial dysfunction in psychiatric morbidity: current evidence and therapeutic prospects. Neuropsychiatric disease and treatment. 2015;11:2441–2447. doi: 10.2147/NDT.S70346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orth M, Schapira AH. Mitochondria and degenerative disorders. American journal of medical genetics. 2001;106(1):27–36. doi: 10.1002/ajmg.1425. [DOI] [PubMed] [Google Scholar]
- 46.Schapira AH. Mitochondrial diseases. Lancet. 2012;379(9828):1825–1834. doi: 10.1016/S0140-6736(11)61305-6. [DOI] [PubMed] [Google Scholar]
- 47.Bansal Y, Kuhad A. Mitochondrial Dysfunction in Depression. Current neuropharmacology. 2016 doi: 10.2174/1570159X14666160229114755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends in neurosciences. 1989;12(3):94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- 50.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. Journal of lasers in medical sciences. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 51.Brosseau L, Robinson V, Wells G, Debie R, Gam A, Harman K, Morin M, Shea B, Tugwell P. Low level laser therapy (Classes I, II and III) for treating rheumatoid arthritis. The Cochrane database of systematic reviews. 2005;(4):CD002049. doi: 10.1002/14651858.CD002049.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]