Abstract
Background
Biomarkers, preferably non-invasive, that predict asthma inception children are lacking.
Objective
Little is known about biomarkers of type 2 inflammation in early life in relation to asthma inception. We evaluated aeroallergen sensitization, peripheral blood eosinophils and serum periostin as potential biomarkers of asthma in children.
Methods
Children enrolled in the Childhood Origins of ASThma (COAST) study were followed prospectively from birth. Blood samples were collected at ages 2, 4, 6, and 11 years, and serum-specific IgE, blood eosionophils and periostin were measured in 244 children. Relationships among these biomarkers, age and asthma were assessed.
Results
Serum periostin levels were approximately 2–3 fold higher in children than previously-observed adult levels. Levels were highest at 2 years (145 ng/mL), and did not change significantly between 4 and 11 years (128 and 130 ng/mL). Age 2 periostin≥150 ng/mL predicted asthma at age 6 [Odds Ratio (OR) 2.3 95%CI (1.3–4.4)]. Eosinophil count ≥300 cells/ul and aeroallergen sensitization at age 2 were each associated with increased risk of asthma at age 6 [OR 3.1(1.7–6.0) and 3.3(1.7–6.3)]. Children with any two of the biomarkers had significantly increased risk of developing asthma by school age [≥2 biomarkers vs. none OR= 6.6 (95%CI 2.7–16.0).
Conclusion
Serum periostin levels are significantly higher in children than adults, likely due to bone turnover, which impairs clinical utility in children. Early life aeroallergen sensitization and elevated blood eosinophils are robust predictors of asthma development. Children with evidence of activation of multiple pathways of type 2 inflammation in early life are at greatest risk for asthma development.
Keywords: biomarkers, children, asthma development, periostin, aeroallergen sensitization, peripheral blood eosinophils
Summary
Asthma biomarkers in children are currently lacking. Serum periostin is elevated in children who develop asthma, although confounding by bone turnover and growth limits the clinical utility of periostin in this age group. Early life eosinophilia and aeroallergen sensitization are predictors of asthma development. Combinations of these early life biomarkers enhance asthma risk.
Introduction
Asthma is one of the most common chronic diseases in children. Recurrent wheezing during the preschool years is frequently the presenting sign of asthma; however, many children who wheeze during early life do not go on to develop childhood asthma.1 Predicting which children will develop asthma remains challenging. Furthermore, once asthma is established, there is significant heterogeneity in response to therapy. The emergence of biomarkers in asthma and allergic airway disease is being utilized for the paradigm of “personalized” medicine and is an area of active research, although this has been primarily in adult patient populations to date. Biomarkers that predict asthma inception and/or response to therapy in children are currently lacking.
Type 2 inflammation is characterized by the production of a unique profile of cytokines by a host of cells including: epithelial cells, mast cells, innate lymphoid cells and T helper-2 (TH2) cells. These pro-inflammatory cytokines include, but are not limited to, IL-4, IL-5, IL-13, IL-25, IL-33, and TSLP, and are thought to play a central role in the pathophysiology of allergic asthma.2 A small number of biomarkers of type 2 inflammation have emerged as potentially useful and relevant for asthma.
Personal atopic history in early life is one of the most important factors determining an individual's risk of persistent asthma.3 In fact, an NIH expert panel on biomarkers in asthma concluded that of all the biomarkers, only multiallergen screening to define atopy is recommended as a core asthma outcome for asthma clinical trials.4 Many studies have demonstrated a significant relationship between inhalant aeroallergen sensitization and asthma development.5–9 Specific patterns of aeroallergen sensitization have identified differential impact on asthma risk, and sensitization to multiple allergens in early life has been strongly associated with an increased asthma risk.10
Peripheral blood eosinophils have been identified as a surrogate marker of Type 2 inflammation, and eosinophils are considered a principle effector cell in the pathophysiology of asthma.11 High levels of peripheral blood eosinophils are recognized as an important biomarker for the eosinophilic asthma phenotype and has been identified as a readily available biomarker that correlates with disease severity and may predict response to asthma therapy, specifically as it relates to immunologic-based interventions.12,13 However, the role of peripheral blood eosinophilia and risk of asthma development is less clear. The pediatric asthma predictive index (API)14,15 identifies peripheral blood eosinophil percentage (greater than or equal to 4% of total WBC) as a minor risk factor; however, existing predictive models for asthma development in children have inadequate accuracy and are, generally, better at excluding asthma than predicting it.16 Small prospective cohorts of infants hospitalized with wheezing illness have demonstrated that elevated eosinophils at convalescence predicted increased asthma risk later in life.17,18 Notably, there is a clear association between peripheral blood eosinophilia and atopy in children, which potentially complicates the utility of peripheral blood eosinophils as an isolated biomarker in children11,19.
Periostin is a matricellular protein whose expression can be induced by Type 2 inflammatory cytokines IL-4 and IL-13, as well as by other stimuli such as TGFβ. Specifically, IL-13 induces the secretion of periostin from bronchial epithelial cells.20 Periostin is secreted basally from airway epithelial cells where it has pleotropic effects on epithelial cell function and on the development of airway fibroblasts, which is thought to promote airway remodeling in patients with asthma, even from a young age.21 Airway epithelial cells from children with allergic asthma produce greater amounts of periostin compared to healthy children.21 In addition to bronchial epithelial cells, there are other tissue sources of periostin including skin, tendon and bone.22 Periostin levels in peripheral blood have been identified as an easily obtained systemic biomarker of Type 2 airway inflammation in adults and may also predict responsiveness to therapy.20,23 While the literature is quickly evolving, there are a paucity of published studies of periostin in childhood, with conflicting results. While one study suggests elevated serum periostin correlates with airway hyper-responsiveness, another found that high periostin was not a predictor of asthma morbidity24,25. There are no published studies of early life serum periostin levels in children, specifically as it relates to asthma inception.
Biomarkers, preferably non-invasive, that predict asthma inception and/or response to therapy in children are desirable, but currently lacking. In this study, we utilized samples from the Childhood Origins of ASThma (COAST) study to assess these biomarkers and their relationship to asthma inception.
Methods
Study population
Two hundred and eighty-nine newborns at high-risk for the development of asthma and allergic disease were recruited from November 1998 through May 2000 into the Childhood Origins of ASThma (COAST) study. Details of the study population and design have been described previously.26 Briefly, to qualify for the study, at least one parent was required to have respiratory allergies (defined as one or more positive aeroallergen skin tests) and/or a history of physician-diagnosed asthma. Of these children, 244 had available serum samples available from age 2 and were included in this study. Informed consent was obtained from the parents, and the Human Subjects Committee at the University of Wisconsin-Madison approved the study (IRB approval number H-2007-0044).10
Measurements
Peripheral blood samples were obtained at annual visits, at ages 2, 4, 6 and 11 years. Serum was stored at −80°C prior to measurement of periostin. The serum samples were analyzed using the clinical trial version of the Elecsys® Periostin assay (Roche Diagnostics, Penzberg, Germany) intended for use on the Cobas e601. The Elecsys® Periostin assay is an automated electrochemiluminescence immunoassay, based on the sandwich principal.27
Peripheral blood eosinophil numbers were measured by standard methods. Total and allergen specific IgE were measured by fluoroenzyme immunoassay (UniCAP® 100, Pharmacia Diagnostics AB, Uppsala, Sweden) as previously described.10
Serum concentrations of specific IgE antibodies for five common inhalant allergens (Dermatophagoides farinae, Dermatophagoides pteronyssinus, cat, dog, and Alternaria alternata) were determined at ages 2, 6, and 11 years. Additionally, allergen-specific IgE to ragweed, silver birch, timothy grass and cockroach were measured at ages 6 and 11 years. The detection limit of the assay with regards to specific IgE was 0.35 kUA/L, and aeroallergen sensitization was defined as at least one value ≥0.35 kUA/L.
Clinical Definitions
Asthma was diagnosed clinically at ages 6 and 11 as previously described.28 Briefly, current asthma was defined based on the documented presence of one or more of the following during the previous year: (1) physician diagnosis of asthma, (2) use of albuterol for coughing or wheezing episodes (prescribed by physician), (3) use of a daily controller medication, (4) use of a step-up plan including use of albuterol or short-term use of inhaled corticosteroids during illness, and (5) use of systemic corticosteroids for asthma exacerbation.
Statistical Analysis
Periostin measurements over the years were assessed using a longitudinal mixed effect model with fixed effect for gender and years. Periostin levels (ages 2, 4, 6 and 11) and Eosinophil counts (ages 2, 6 and 11) were compared with asthma diagnosis at age 6 and 11 using longitudinal mixed effect models with fixed effects for years, gender, asthma status and a random effect for the subject to account for repeated measure. Both outcomes were log-transformed to attain normality. Least square (LS) means on the log scale were back-transformed to geometric means and associated 95% confidence intervals.
The dichotomous outcome of aeroallergen sensitization at years 2, 6 and 11 was compared with asthma status at ages 6, 11 using generalized linear mixed models adjusted for gender. These relationships were summarized using odds ratios (OR) with 95% confidence intervals (CI).
For the purpose of this manuscript, we determined cut points to identify a positive biomarker: peripheral blood eosinophils ≥300 (literature-based),12,13 any aeroallergen sensitization (at least one value ≥0.35 kUA/L) and serum periostin≥150 (approximate median of periostin levels at age 2 in our population). Logistic regressions were used to predict asthma diagnosis at age 6 and 11 with both individual and combinations of these 3 markers. A 2-sided P value of less than 0.05 was regarded as statistically significant. Analyses were performed in SAS statistical software, version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
Demographics
Data were analyzed from 244 children at age 2 years with periostin, peripheral blood eosinophils and/or aeroallergen sensitization assessed. There was no significant difference in the characteristics of the children who had the markers measured at all years compared with those who had missing data. Briefly, 43% of the children were female and 57% male, 29% had asthma at age 6 and 31% had asthma at age 11 and 36% had asthma, either at age 6 or 11. Of those who had asthma at age 6, 47% had mild intermittent asthma, 35% had mild persistent asthma and 18% had moderate-severe persistent asthma. Allergic sensitization to aeroallergens for the entire cohort of patients occurred early (1–2 years) in 23%, later (3–11 years) in 33%, and 44% never developed allergic sensitization.
Developmental levels of periostin with age and gender
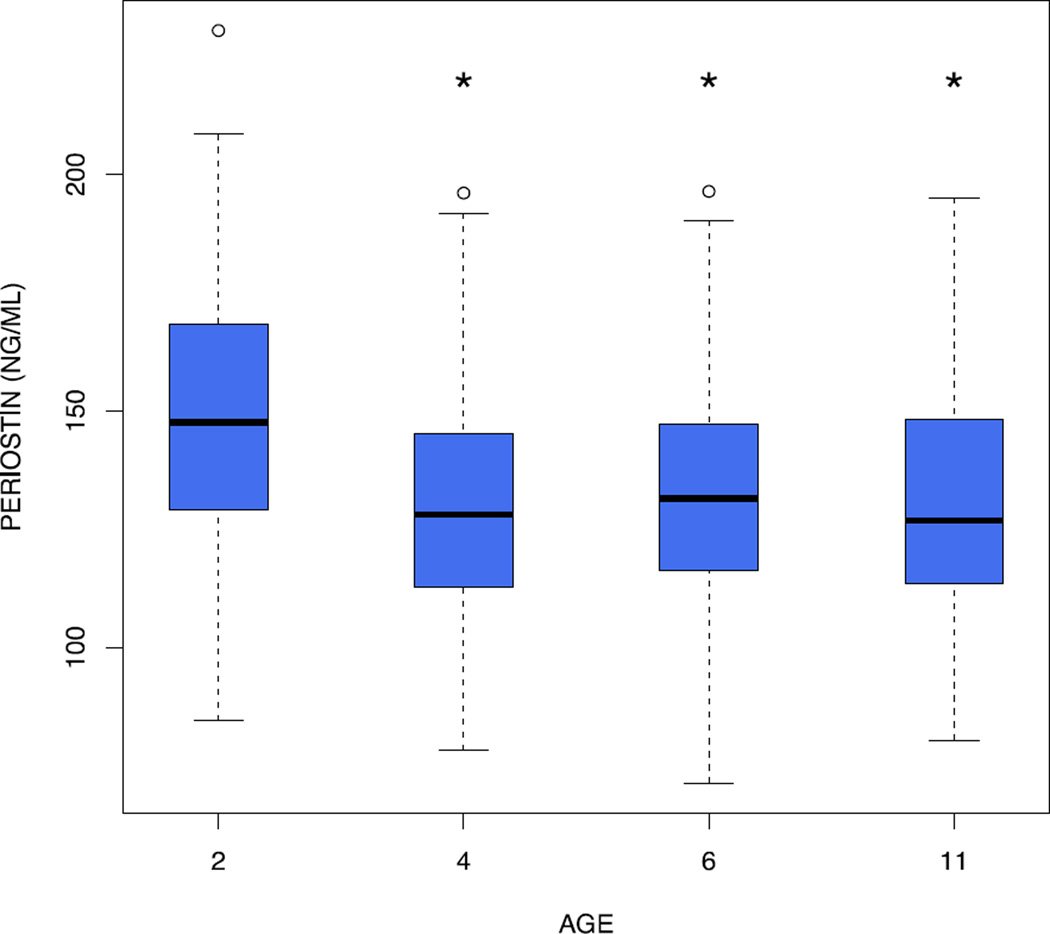
We first assessed the effects of age on serum periostin levels. Periostin was highest at age 2 years (geometric mean: 145 ng/mL, p<0.0001 for age 2 vs. ages 4, 6 and 11 years). Periostin levels did not change from age 4 to 11 (geometric means: 128, 130 and 130 ng/mL at ages 4, 6, and 11 years, respectively) (Figure 1).
Figure 1.
Serum periostin decreases with age. The shaded boxes represent the interquartile range; the black horizontal line represents the median. The whiskers represent the minimum and maximum of all data, with the exception of outliers, which are denoted by a small round circle. The * above age 4, 6 and 11 years denotes a statistically significant difference compared to age 2 years.
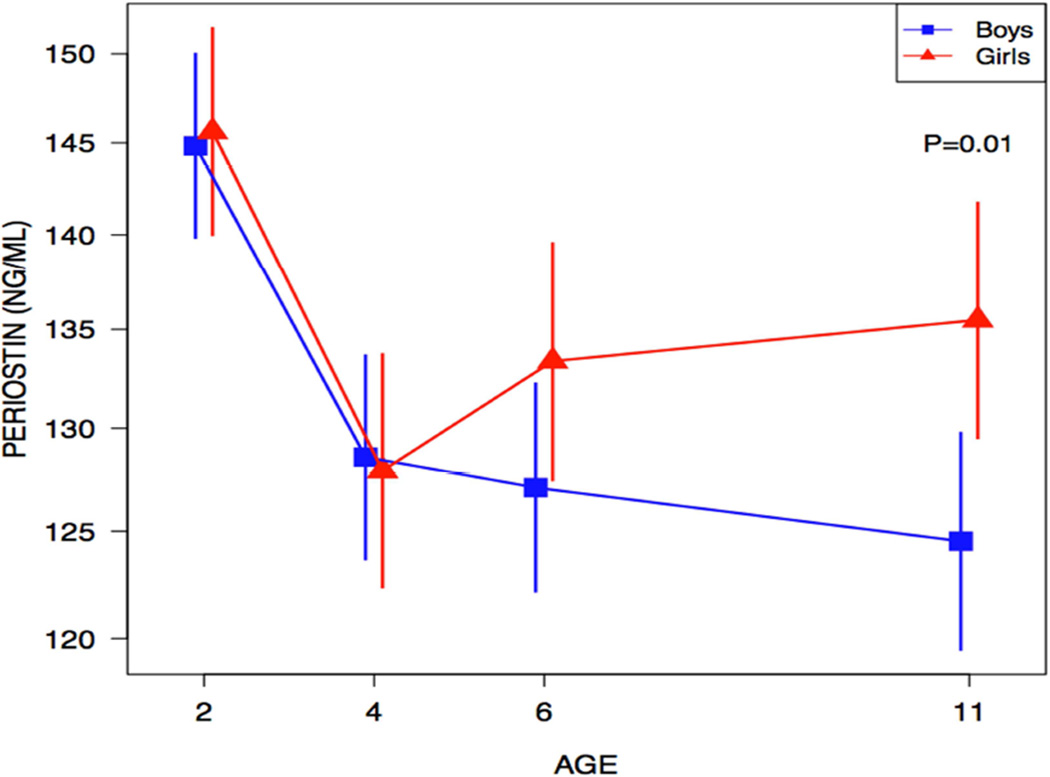
We next examined the relationships of periostin and age stratified by gender. There were no significant differences of periostin levels for males versus females at ages 2, 4 and 6 years (Figure 2). However, levels of serum periostin were significantly higher in girls at age 11 (geometric mean: females 136 vs. males 125 ng/mL, p=0.01).
Figure 2.
Serum periostin is significantly higher in girls at age 11 years. Geometric mean with 95% CI. The vertical lines represent the 95% confidence interval.
Developmental levels of eosinophils and aeroallergen sensitization with age and gender
We next assessed the developmental levels of peripheral blood eosinophils at ages 2 [Geometric mean (GM) (95% confidence interval): 167 cells/ul (149–187)], 6 [GM (95% CI): 148 (131–167)] and 11 [GM (95% CI): 156 (137–179)] of age. Eosinophils levels did not change significantly between 2 and 11 years of age (Age 2 vs. 6, p=0.10; Age 2 vs. 11; p=0.42; Age 6 vs. 11; p=0.48). Peripheral blood eosinophils did not differ between males and females at age 2 (p=0.30), age 6 (p=0.42) or age 11 (p= 0.96).
As anticipated, aeroallergen sensitization increased with age from age 2 to age 11 in our cohort (data not shown). There was no significant difference in rates of aeroallergen sensitization between males versus females at age 2 (p=0.24), age 6 (p=0.49) or age 11 (p=0.76).
Cross-sectional evaluation of serum periostin and peripheral blood eosinophils and relationship to asthma development
Periostin and blood eosinophils were measured and evaluated cross-sectionally in relation to asthma at age 6 and 11 years to assess whether children with asthma at ages 6 and 11 had higher levels of serum periostin and/or eosinophils compared to age-matched individuals without asthma (Table 1). Children with asthma had higher periostin levels compared to children without asthma at age 6 years, although not statistically significant (geometric mean (GM): asthma 135 vs. non-asthma 128 ng/mL, p = 0.12) and 11 years (GM: asthma 136 vs. non-asthma 127 ng/mL, p=0.04).
Table 1.
Early life periostin and peripheral blood eosinophils predict development of asthma and cross-sectional assessment reveals higher periostin and peripheral blood eosinophils in children with asthma compared to age-matched children without asthma. Early life periostin is significantly higher at age 2 and age 4 in individuals who develop asthma by age 6. Similarly, periostin is higher at ages 2, 4 and 6 in individuals who develop by age 11, although not statistically significant. Peripheral blood eosinophil count ≥300 is significantly higher at age 2 in children who develop asthma by age 6 and is also significantly higher at ages 2 and 6 for children who develop asthma by age 11. Cross-sectional assessment of serum periostin and peripheral blood eosinophils demonstrates that children with asthma at ages 6 and 11 have higher levels of periostin and eosinophils when compared to aged-match individuals. Least square means (Lsmean) are summarized by geometric means and its 95% confidence interval (CI). The data was adjusted for sex.
| PERIOSTIN | |||||
|---|---|---|---|---|---|
| Age 6 | |||||
| No asthma | Asthma | ||||
| Age (years) |
N | Geometric Mean (95% CI) |
N | Geometric Mean (95% CI) |
P-value |
| 2 | 141 | 143 (139–148) | 59 | 154 (146–161) | 0.02 |
| 4 | 100 | 125 (121–129) | 45 | 139 (131–146) | 0.002 |
| 6 | 100 | 128 (124–133) | 45 | 135 (128–143) | 0.12 |
| Age 11 | |||||
| No asthma | Asthma | ||||
| Age (years) |
N | Geometric Mean (95% CI) |
N | Geometric Mean (95% CI) |
P-value |
| 2 | 119 | 143 (138–148) | 54 | 151(144–160) | 0.07 |
| 4 | 90 | 126 (122–131) | 44 | 132 (125–139) | 0.23 |
| 6 | 90 | 127(123–132) | 44 | 134(127–141) | 0.15 |
| 11 | 91 | 127 (122–132) | 42 | 136(129–144) | 0.04 |
| EOSINOPHIL COUNT | |||||
|---|---|---|---|---|---|
| Age 6 | |||||
| No asthma | Asthma | ||||
| Age (years) |
N | Geometric Mean (95% CI) |
N | Geometric Mean (95% CI) |
P-value |
| 2 | 161 | 148(130–168) | 69 | 226(185–276) | 0.001 |
| 6 | 149 | 132(115–151)) | 56 | 190(152–238) | 0.006 |
| Age 11 | |||||
| No asthma | Asthma | ||||
| Age (years) |
N | Geometric Mean (95% CI) |
N | Geometric Mean (95% CI) |
P-value |
| 2 | 138 | 142 (123–164) | 63 | 239 (193–295) | <.0001 |
| 6 | 124 | 128(110–149) | 60 | 188(151–234) | 0.005 |
| 11 | 111 | 134 (115–158) | 51 | 223(176–282) | 0.001 |
Statistical significance is P-value <0.05.
Children with asthma had significantly higher peripheral blood eosinophil counts than age-matched children without asthma at both ages 6 years (GM: asthma 190 vs. non-asthma 132 cells/ul, p=0.006) and 11 years (GM: asthma 223 vs. non-asthma 134 cells/ul, p=0.006).
Predictive value of early life serum periostin and peripheral blood eosinophils and relationship to asthma development
We next assessed whether elevated early life periostin and peripheral blood eosinophil levels predict the development of asthma at age 6 and 11 years (Table 1). Children who developed asthma by age 6 years had increased serum periostin at age 2 years, when compared to children who did not develop asthma by age 6 years (GM: asthma 154 vs. non-asthma 143 ng/mL; p=0.02). Similarly, children who developed asthma by age 6 and 11 years had higher eosinophil counts at 2 years, when compared to children who did not develop asthma by age 6 or 11 years (GM age 6: asthma 226 vs. non-asthma 148cells/ul p=0.001; GM age 11: asthma 239 vs. non-asthma 142cells/ul; p<0.001).
Aeroallergen sensitization and asthma development
The relationship of aeroallergen sensitization to asthma development was assessed (Table 2). If a child was sensitized to inhalant aeroallergen at age 2, the risk of asthma at age 6 was 51% [OR 3.3 (95% CI 1.7, 6.3); p=0.0005] and 53% for asthma at age 11 [OR 3.2 (1.6, 6.6); p= 0.001]. If a child was not sensitized to aeroallergen at age 2, the risk of asthma development at age 6 was 24% and 26% for asthma at age 11. Cross-sectional evaluation reveals that aeroallergen sensitization at age 6 is associated with a 40% risk of asthma [OR 2.6 (95% CI 1.4, 4.9); p=0.003] compared to an 18% risk for those children who are not aeroallergen sensitized at age 6. Similarly, cross-sectional evaluation at age 11 demonstrates sensitization at 11 years confers an asthma risk of 41% [OR 3.0 (95% CI 1.5, 6.3); p=0.003] compared to an 18% risk for asthma in those children who do not have aeroallergen sensitization at age 11.
Table 2.
Early life aeroallergen sensitization increases asthma risk and cross-sectional assessment reveals increased asthma risk for children with aeroallergen sensitization. The presence of aeroallergen sensitization at age 2 predicts greater asthma risk at age 6 and 11 compared to age-matched individuals without aeroallergen sensitization. Aeroallergen sensitization at age 6 is associated with increased asthma risk at age 6 and the same is demonstrated at age 11. Odds ratios are reported with 95% CI. The model was adjusted for gender.
| Asthma at Age 6 | |||||
| Age (years) |
Aeroallergen sensitization |
N | Asthma 6 n (%) |
OR (95% CI) |
P value |
| 2 | Yes | 47 | 24 (51%) | 3.3 (1.7, 6.3) | 0.0005 |
| No | 196 | 47 (24%) | |||
| 6 | Yes | 91 | 36 (40%) | 2.6 (1.4, 4.9) | 0.003 |
| No | 111 | 20(18%) | |||
| Asthma at age 11 | |||||
| Age (years) |
Aeroallergen sensitization |
N | Asthma 11 n (%) |
OR (95% CI) |
P value |
| 2 | Yes | 40 | 21(53%) | 3.2 (1.6, 6.6) | 0.001 |
| No | 168 | 43 (26%) | |||
| 6 | Yes | 82 | 38(46%) | 3.2 (1.7, 6.1) | 0.0004 |
| No | 100 | 20 (20%) | |||
| 11 | Yes | 103 | 42(41%) | 3.0(1.5, 6.3) | 0.003 |
| No | 63 | 11(18%) | |||
Statistical significance is P value <0.05.
Predicting asthma development using biomarkers of Type 2 inflammation
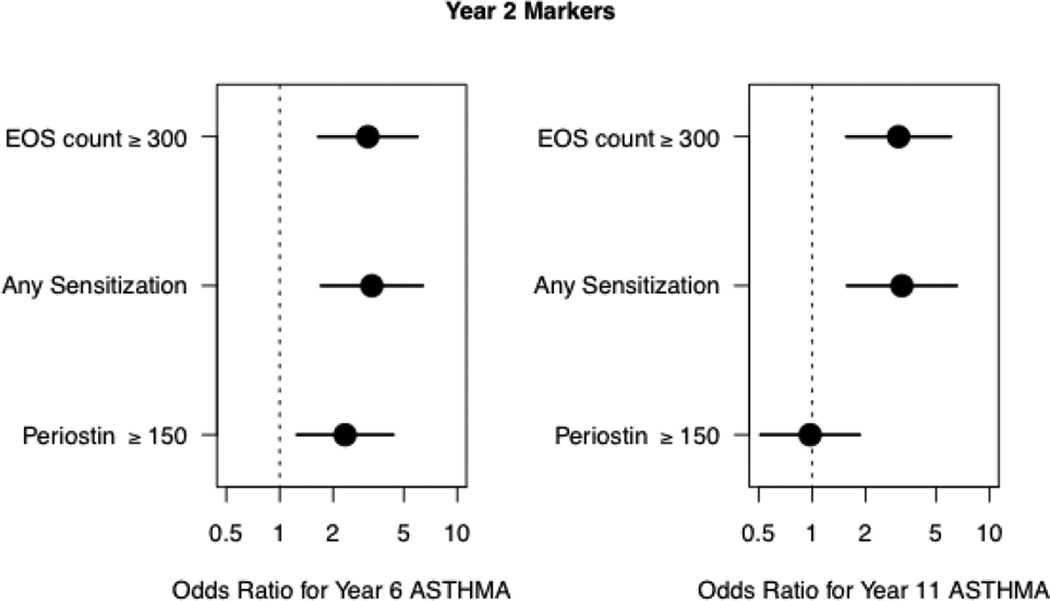
For the purpose of this manuscript, we determined cut points to identify a positive biomarker: peripheral blood eosinophils ≥300cells/ul (literature-based),12,13 any aeroallergen sensitization (at least one value ≥0.35 kUA/L) and serum periostin≥150 ng/ml (approximate median of periostin levels at age 2 in our population). Utilizing the aforementioned cut-points, at age 2, we assessed the risk of asthma at age 6 and 11 (Figure 3). Eosinophil count ≥300 cells/ul and aeroallergen sensitivity at age 2 were each associated with a similar risk of asthma at age 6 [Odds Ratio (OR) 3.1(1.7–6.0) and 3.3(1.7–6.3), respectively]. Similar findings were observed for eosinophil count ≥300 cells/ul and any aeroallergen sensitization at age 2 for asthma at age 11 [OR 3.1 (1.6–6.1) and 3.2(1.6–6.5), respectively]. While periostin≥150 ng/mL at age 2 predicted asthma at age 6 (OR 2.3 (1.3–4.4) the effect was not sustained for predicting asthma at age 11 (OR 1.0 (0.5–1.9).
Figure 3.
Positive biomarkers at age 2 have increased odds ratios for asthma development at ages 6 and 11. Utilizing cut points to identify positive biomarkers we assessed the risk for asthma. The presence of a positive biomarker does not necessarily mean that other biomarkers are not also positive. Odds ratios are depicted with black circle with the horizontal bars representing the 95% CI.
We next aimed to assess whether combining biomarkers could enhance predictive value. (Table 3) For the 196 children who had data for all biomarkers for asthma development at age 6, we found that having only one positive biomarker was not associated with an increased risk of asthma (Odds ratio 1.1(95%CI 0.5, 2.4) for any one compared to none. However, if any two (or all 3) of the biomarkers were present, children had a significantly increased risk of developing asthma by school age [Any two or more vs. none OR= 6.6 (95%CI 2.7, 16.0); any two or more vs. only one OR= 6.0 (95%CI 2.63, 13.7)]. Similarly, for the 170 children who had data for all biomarkers for asthma development at age 11, a similar pattern was identified [Any two or more vs. none OR= 3.2 (95%CI 1.3, 7.9); any two or more vs. only one OR= 3.3 (95%CI 1.4, 7.6)].
Table 3.
Combinations, of two or more positive biomarkers, predict higher risk for asthma development at age 6 and age 11. Utilizing the data from the children in the cohort who had data on all biomarkers, we assessed the utility of combinations of biomarkers. ORs are reported with associated 95% CI comparing none, only one and any two as it relates to development at age 6 and 11.
| Asthma at age 6 | |||||
| Asthma | OR(95% CI) | ||||
| N(%) | 6 | N(%) | vs. None | vs. Only one | |
| None | 68(35%) | Yes | 14(21%) | Ref | |
| No | 54(79%) | ||||
| Only one | 90(46%) | Yes | 20(22%) | 1.1 (0.5, 2.4) | Ref |
| No | 70(78%) | ||||
| Any two or more | 38(19%) | Yes | 24(63%) | 6.6 (2.7, 16.0) | 6.0 (2.6, 13.7) |
| No | 14(37%) | ||||
| Asthma at age 11 | |||||
| Asthma | OR(95% CI) | ||||
| N(%) | 11 | N(%) | vs. None | vs. Only one | |
| None | 58(34%) | Yes | 15(26%) | Ref | |
| No | 43(74%) | ||||
| Only one | 78(46%) | Yes | 20(26%) | 1.0 (0.5, 2.2) | Ref |
| No | 58(74%) | ||||
| Any two or more | 34(19%) | Yes | 18(53%) | 3.2 (1.3, 7.9) | 3.3 (1.4, 7.6) |
| No | 16(47%) | ||||
Discussion
Asthma is a common chronic illness in children and we continue to seek ways to better understand, identify and predict the development of asthma in the pediatric population. One area of active interest is that of biomarker identification. Utilizing our prospective COAST cohort of high-risk children, we were able to evaluate current biomarkers of interest (aeroallergen sensitization, peripheral blood eosinophil count and serum periostin level) and their relationship to pediatric asthma development in this population.
Periostin has been identified as a promising biomarker in adults. To our knowledge, this is the first study to evaluate and report on early life peripheral blood periostin levels in children, specifically as it relates to asthma development. Our findings demonstrated significant effects of age on periostin levels. Serum periostin levels were several-fold higher in children than those previously observed in adults. Using the same periostin assay, the median serum periostin level in healthy adults is about 44 ng/ml with an interquartile range of 36–54 ng/ml, while in moderate-severe adult asthma patients the median serum periostin level is about 50 ng/ml with an IQR of 44–59 ng/ml.29 Children enrolled in our study had serum periostin levels ranging from 120–150 ng/mL. Serum periostin levels were highest at age 2 years, regardless of the subsequent development of asthma. Serum periostin levels at ages 4, 6 and 11 were significantly lower compared to age 2 and there was no significant change in the distribution of serum periostin levels between ages 4–11. This developmental change observed in serum periostin levels may be related to bone turnover and growth, as periostin is a component of the extracellular matrix and regulates type I collagen formation, which is important for skin, tendon and bone development.22 Most healthy infants and children grow in a predictable fashion.30 Height velocity varies with age, with the most rapid period of growth occurring during the first year of life, followed by a gradual slowing of height attainment and a relative plateau of height velocity from age 4 until puberty. There is an expected deceleration of height velocity prior to the pubertal growth spurt. Thus, developmental changes in serum periostin in this study appear to parallel changes in growth velocity.
Interestingly, there were no sex-related effects on periostin until age 11, at which point girls had significantly higher serum periostin than boys. This observation also fits with the concept that periostin is higher during times of growth, specifically linear growth. On average, females enter puberty at age 9 years and reach peak growth about 2.5 years into puberty.31 On the contrary, males tend to enter puberty slightly later compared to females at around age 11, and peak growth occurs about 3 years later. Thus, if serum periostin levels reflect rates of bone growth it is likely that gender effects on serum periostin may change during adolescence. It is unclear when the periostin levels decrease to expected, and much lower, adult levels, but we suspect this occurs sometime after puberty and once adult height has been attained.
We demonstrated that periostin is elevated in children with asthma, cross291 sectionally, and that it is also modestly predictive of asthma development from measurement as early as 2 years of age. However, the ability to predict asthma development decreases with increasing age, which is somewhat counter to what one might expect given that periostin has been shown to have predictive capacity in adults, at least with regards to response to immunologic-based therapy6,13. Our study did not assess whether serum periostin levels in children with asthma predict response to treatment, which would be an important topic for future research. However, due to the varying levels by age and gender, it will be challenging to establish a universal cut-off for the pediatric population.
Many studies, including previously reported COAST cohort data, have demonstrated a significant relationship between aeroallergen sensitization and asthma development.10 Similarly, peripheral blood eosinophils are also a key biomarker for allergic asthma, at least in regards to response to therapy in adult patients. Our data analysis confirms these relationships with asthma development. In early life, the presence of aeroallergen sensitization and/or peripheral eosinophil count ≥300 cells/ul signifies an increased risk for developing asthma. Interestingly, the data from this study suggests that peripheral eosinophilia may be as robust of a predictor as aeroallergen sensitization for asthma development. Historically, the role of peripheral blood eosinophilia and risk of asthma development has not been clear. As alluded to previously, the API identifies peripheral eosinophil percentage greater than or equal to 4% as a minor risk factor, but our data suggest that absolute eosinophil counts ≥300 cells/ul should be considered a worthy biomarker for asthma inception risk in children. While periostin (≥150 ng/mL) has modest predictive value for asthma development at age 6, its predictive effect wanes for asthma by age 11.
These data analyses do suggest that combinations of positive biomarkers (any two or more) at age 2 can provide additional predictive information for asthma inception. The presence of only one positive biomarker did not add significant predictive value, but any combination of two or more was associated with an increased risk, which could have clinical utility. This suggests that children with evidence for activation of multiple pathways of type 2 inflammation are at greatest risk of asthma development. Furthermore, it is important to recognize that the predictive value, of the aforementioned biomarkers, is irrespective of personal wheezing history.
The strengths of this study are the prospective design and longitudinal follow up with high-retention rates. Additionally, the children are carefully characterized with respect to allergy and asthma phenotypes. One limitation to this study is that an enrollment criterion included only patients at higher risk for the development of asthma based on parental history of atopy or asthma. In addition, the COAST cohort is comprised primarily of Caucasian children so little information regarding potential effects of race on biomarkers in children, including serum periostin levels, is available from this study. Also, periostin assays are currently clinically unavailable for the pediatric population, further limiting its clinical utility in practice at this time.
In conclusion, our study found that early life aeroallergen sensitization and peripheral blood eosinophilia are both strong predictors of asthma inception. Serum periostin levels are significantly higher in children compared to published adult values and change developmentally, which may be due to bone turnover. While serum periostin may have modest predictive value for asthma development at age 6, the confounding by growth limits its clinical utility in children. Notably, children with evidence of activation of multiple pathways of type 2 inflammation in early life are at greatest risk for the subsequent development of asthma.
Clinical Implications.
Children with evidence of activation of multiple pathways of type 2 inflammation in early life are at greatest risk for the subsequent development of asthma.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Klein Wolterink RG, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Curr Allergy Asthma Rep. 2013;13(3):271–280. doi: 10.1007/s11882-013-0346-z. [DOI] [PubMed] [Google Scholar]
- 3.Sly PD, Boner AL, Bjorksten B, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–1106. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J. Allergy Clin. Immunol. 2012;129(3 Suppl):S9–S23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brockow I, Zutavern A, Hoffmann U, et al. Early allergic sensitizations and their relevance to atopic diseases in children aged 6 years: results of the GINI study. J. Investig. Allergol. Clin. Immunol. 2009;19(3):180–187. [PubMed] [Google Scholar]
- 6.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N. Engl. J. Med. 1990;323(8):502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 7.Gaffin JM, Phipatanakul W. The role of indoor allergens in the development of asthma. Curr. Opin. Allergy Clin. Immunol. 2009;9(2):128–135. doi: 10.1097/aci.0b013e32832678b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 9.Kusel MM, de Klerk NH, Kebadze T, et al. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J. Allergy Clin. Immunol. 2007;119(5):1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoltz DJ, Jackson DJ, Evans MD, et al. Specific patterns of allergic sensitization in early childhood and asthma & rhinitis risk. Clin. Exp. Allergy. 2013;43(2):233–241. doi: 10.1111/cea.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbes SJ, Jr, Calatroni A, Mitchell HE, Gergen PJ. Age-dependent interaction between atopy and eosinophils in asthma cases: results from NHANES 2005–2006. Clin. Exp. Allergy. 2013;43(5):544–551. doi: 10.1111/cea.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N. Engl. J. Med. 2013;368(26):2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 13.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 14.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am. J. Respir. Crit. Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 15.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N. Engl. J. Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Nkoy FL, Stone BL, Schmick D, Johnson MD. A systematic review of predictive models for asthma development in children. BMC Med. Inform. Decis. Mak. 2015;15:99. doi: 10.1186/s12911-015-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backman K, Nuolivirta K, Ollikainen H, Korppi M, Piippo-Savolainen E. Low eosinophils during bronchiolitis in infancy are associated with lower risk of adulthood asthma. Pediatr. Allergy Immunol. 2015;26(7):668–673. doi: 10.1111/pai.12448. [DOI] [PubMed] [Google Scholar]
- 18.Piippo-Savolainen E, Remes S, Korppi M. Does blood eosinophilia in wheezing infants predict later asthma? A prospective 18–20-year follow-up. Allergy Asthma Proc. 2007;28(2):163–169. doi: 10.2500/app.2007.28.2946. [DOI] [PubMed] [Google Scholar]
- 19.Genuneit J. Interaction between atopy and blood eosinophils in the development of childhood wheeze. J. Allergy Clin. Immunol. 2013;132(5):1237.e1235–1239.e1235. doi: 10.1016/j.jaci.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 2011;365(12):1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J. Allergy Clin. Immunol. 2012;129(4):990.e996–997.e996. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell. Mol. Life Sci. 2011;68(19):3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J. Allergy Clin. Immunol. 2012;130(3):647.e610–654.e610. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JS, You JS, Jeong SI, et al. Serum periostin levels correlate with airway hyper-responsiveness to methacholine and mannitol in children with asthma. Allergy. 2015;70(6):674–681. doi: 10.1111/all.12599. [DOI] [PubMed] [Google Scholar]
- 25.Konradsen JR, Skantz E, Nordlund B, et al. Predicting asthma morbidity in children using proposed markers of Th2-type inflammation. Pediatr. Allergy Immunol. 2015;26(8):772–779. doi: 10.1111/pai.12457. [DOI] [PubMed] [Google Scholar]
- 26.Lemanske RF. The childhood origins of asthma (COAST) study. Pediatr. Allergy Immunol. 2002;13 Suppl(15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 27.Sherman J, Holweg C, Kincaid H, et al. The Elecsys Periostin assay as a companion diagnostic for the novel asthma drug lebrikizumab. Clinical Chemistry. 2014;60(10):S25–S26. [Google Scholar]
- 28.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arron JR, Choy DF, Scheerens H, Matthews JG. Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Annals of the American Thoracic Society. 2013;10(Suppl):S206–S213. doi: 10.1513/AnnalsATS.201303-047AW. [DOI] [PubMed] [Google Scholar]
- 30.Boom J. Normal growth patterns in infants and prepubertal children.: UpToDate. 2013 [Google Scholar]
- 31.Abbassi V. Growth and normal puberty. Pediatrics. 1998;102(2 Pt 3):507–511. [PubMed] [Google Scholar]