Abstract
Gut microbiota has been well recognized in regulation of intestinal homeostasis and pathogenesis of inflammatory bowel diseases. However, the mechanisms involved are still not completely understood. Further, the components of the microbiota which are critically responsible for such effects are also largely unknown. Accumulating evidence suggests that, in addition to pathogen-associated molecular patterns (PAMP), nutrition and bacterial metabolites might greatly impact the immune response in the gut and beyond. Short-chain fatty acids (SCFA), which are metabolized by gut bacteria from otherwise indigestible fiber-rich diets, have been shown to ameliorate diseases in animal models of inflammatory bowel diseases (IBD) and allergic asthma. Although the exact mechanisms for the action of SCFA are still not completely clear, most notable among the SCFA targets is the mammalian G protein-coupled receptor pair of GPR41 and GPR43. In addition to the well-documented inhibition of histone deacetylases (HDAC) activity mainly by butyrate and propionate, which causes anti-inflammatory activities on IEC, macrophages, and dendritic cells, SCFA has recently been implicated in promoting development of Treg cells and possibly other T cells. In addition to animal models, the beneficial effects have also been reported from the clinical studies that used SCFA therapeutically in controlled trial settings in inflammatory disease, in that application of SCFA improved indices of IBD and therapeutic efficacy was demonstrated in acute radiation proctitis. In this review article, we will summarize recent progresses of SCFA in regulation of intestinal homeostasis as well as in pathogenesis of IBD.
Keywords: Microbiota, short chain fatty acids, GCPR, IBD
Introduction
The crucial role of gut microbiota has been well-established in regulation of the intestinal homeostasis and inflammatory bowel disease (IBD). However, the components of the microbiota which are critically responsible for such effects are still largely unknown. Emerging evidence suggests that the host immune system can sense gut bacterial metabolites in addition to pathogen-associated molecular patterns (PAMP) and that recognition of these small molecules can influence the host immune response in the context of disease and inflammation in the gut and beyond [1–3]. Of particular interest are short-chain fatty acids (SCFA), such as acetate, n-propionate, and n-butyrate, which are solely metabolized by gut bacteria from otherwise indigestible carbohydrates, i.e., from fiber-rich diets [4], and have been shown to ameliorate disease in animal models of colitis and allergic asthma [5, 6]. Furthermore, SCFA are associated with reduced risk of various diseases, including IBD, and dysbiosis in IBD patients has been associated with altered SCFA fermentative pathways [6, 7]. In this review, we will focus on SCFA regulation of host immune responses and pathogenesis of IBD.
Formation of SCFA
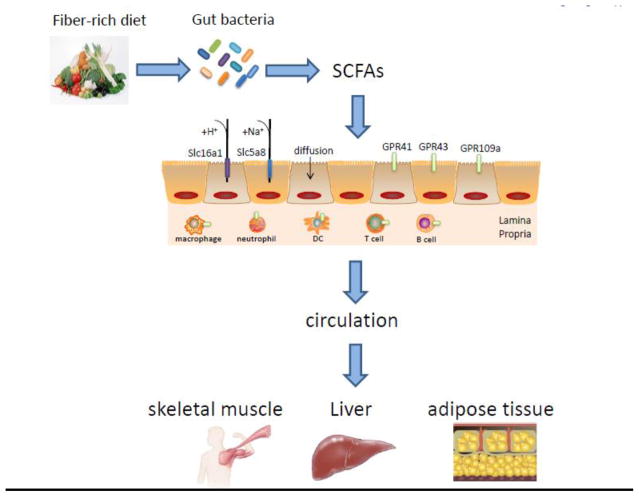
Mammalian GI tract harbors huge amounts of diverse microbes, comprising more than 1000 strains [8]. This commensal microbiota not only contributes to the regulation of host immune response and homeostasis, but also participates in the breakdown of food and energy metabolism [9, 10]. Gut bacteria have enzymes that host cells lack for breaking down carbs, turning them into different useful metabolites. In recent years, microbiota derived metabolites including SCFA, phenolic acids, tryptophan, bile acids, have drawn greater attention [11, 12]. Usually undigested dietary fibers, as well as proteins and peptides, can be fermented in the cecum and colon by gut bacteria. The major products of these fermentative reactions are SCFA, which are defined as the groups of fatty acids with fewer than six carbons, including formic acid (C1), acetic acid (C2), propionic acid (C3), butyric acid (C4) and valeric acid (C5). The major SCFA in gut are C2, C3 and C4, which account for more than 95% of the whole SCFA. In general, C2 can be formed from pyruvate via acetyl-CoA or Wood-Ljungdahl pathway [13]. C3 is mainly produced from succinate via the succinate pathway or from lactate via the acrylate pathway. C4 is formed from acetyl-CoA and butyryl-CoA, as well as acetate and lactate [14]. The concentration of SCFA varies from cecum to colon. It is estimated that the total amount in the proximal colon ranges from 70 to 140mM and it falls to 20 to 70mM in the distal colon. After being absorbed, SCFA are utilized in the colonocytes or transported to blood circulation and other organs. In general, SCFA enter cells through several different ways, the first one as passive diffusion, the second one as carrier-mediated transportation by SMCT1/Slc5a8 and MCT1/Slc16a1, the third one as activating G-protein-coupled cell surface receptors (GCPR). Slc5a8, which is a Na+-coupled high-affinity transporter especially for butyrate, has been shown to protect against colitis and colon cancer under low-fiber dietary conditions. It regulates butyrate-induced expression of IDO1 and Aldh1A2 in DCs as well as development of Treg cells [15, 16]. Slc16a1 transports SCFA depending on the net chemical gradients for H+ [17]. GPR41, GPR43 and GPR109a are the receptors which could be activated by SCFA (Figure 1). SCFA-GPR pathways have been demonstrated to play vital roles in regulation of immune responses [18, 19].
Figure 1. Formation, absorption and transportation of SCFA.
Indigestible dietary fibers can be fermented in the cecum and colon by gut microbes to form SCFA, which usually are utilized in the enterocytes through different ways: passive diffusion; carrier-mediated transportation by Slc5a8 and Slc16a1; and binding GPR41, GPR43 and GPR109a. After being absorbed, SCFAs are transported into portal vein via superior mesenteric vein and inferior mesenteric vein depending on the absorption sites, and dispersed to peripheral tissues as skeletal muscle, liver and adipose tissue to take effects.
SCFA in gut and circulation
SCFA are the most abundant products derived from commensal bacterial fermentation of indigestible dietary fibers in intestines [14]. The principal SCFA in the gut are acetate, propionate and butyrate which constitute more than 95% of all the SCFA content [4]. The concentrations of them in the gut are typically found in a ratio of 3:1:1 [20]. Interestingly, different SCFA vary in different sites throughout the whole intestines: acetate and propionate are found in both small and large intestines, while butyrate is found mainly in the colon and cecum [21]. It has been shown that about 400–800 mmol SCFA are produced with a high-fiber diet per day, equaling that 10g of dietary fiber fermentation. Multiple factors, including diet, specific diversity of gut microbiota and certain amount of commensal bacteria, play a vital role in the production of SCFA [22]. After production, SCFA can be absorbed into colonic epithelial cells through several ways: non-ionic diffusion; via the carrier-mediated transportation; and exchange with bicarbonate [23]. Moreover, SCFA which are absorbed in the cecum, ascending colon and transverse colon, enter the superior mesenteric vein, while SCFA which are absorbed in the descending colon and sigmoid are transported into the inferior mesenteric vein. Then both of them drain into the portal vein and liver [24]. Apart from this pathway, SCFA absorbed in the rectum could drain into the inferior vena cava through the pelvic plexus and play roles in the circulation. After entering the circulation, SCFA have been demonstrated to affect metabolism and the function of peripheral tissues such as modulating adipose tissue, skeletal muscle and liver as well (Figure 1) [22].
Gut SCFA-producing bacteria
According to the formation of three main SCFA, acetate can be produced from pyruvate by two different ways: via acetyl-CoA by enteric bacteria and via Wood-Ljungdahl by acetogens, for instance, Blautia hydrogenotrophica. Butyrate is produced from Acetyl-CoA by several Firmucutes. Propionate is produced by two different pathways: the succinate pathway by Bacteroidetes; the lactate pathway by Firmicutes [11]. The microbial conversions of undigested fibers to SCFA are mediated by specific members of gut bacteria. Recent advances in technology using pyrosequencing analyses of 16S rRNA genes have made great progress in characterization of bacteria responsible for SCFA production. Propionate and butyrate production pathways appear more conserved and substrate specific, while pathways for acetate production are widely distributed among bacterial groups. Although distributed across a number of phyla, propionate production is dominated by relatively few bacterial genera [25]. Deoxy-sugars, such as fucose and rhamnose, are particularly propiogenic in select organisms [25]. A small number of gut bacteria, including Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii and Ruminococcus bromii, are making the majority of butyrate production [26]. Fermentation of resistant starch greatly contributes to butyrate production in the colon, which is dominated by Ruminococcus bromii [27].
SCFA regulation of immune response
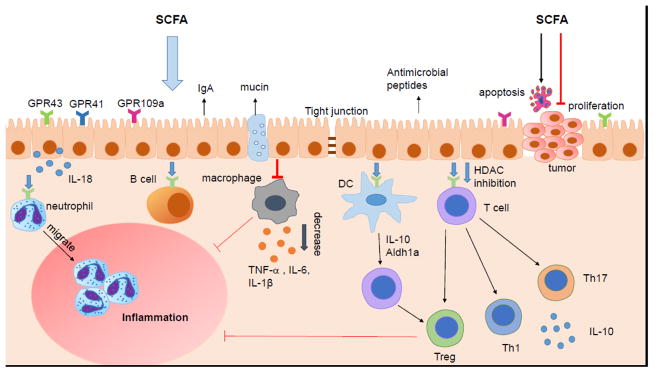
Gut microbiota were recently found to participate in regulation of several systems of the body through their metabolites, principally SCFA produced in colon and also absorbed in blood circulation to reach other organs. Therefore, SCFA modulate functions of different systems, such as gut, nervous, endocrine and blood, serving as a key factor to regulate metabolic disorders and immunity primarily through the inhibition of histone deacetylases and the activation of G-protein coupled receptors such as GPR41, GPR43 and GPR109a [28]. The effects of SCFA have been demonstrated in influencing systemic autoimmune responses and participating in different steps of inflammation process. SCFA are found to regulate the functions of almost every type of immune cells, altering their gene expression, differentiation, chemotaxis, proliferation and apoptosis (Figure 2).
Figure 2. SCFA regulation of intestinal immunity.
SCFA regulate the intestinal mucosal immunity through exerting their effects on various immune cells. SCFA regulate intestinal barrier integrity by inducing intestinal epithelial cell secretion of IL-18, antimicrobial peptides, mucin, and upregulating the expression of tight junction. SCFA induce neutrophils migration to inflammatory site and enhance their ability of phagocytosis. SCFA regulate the T cell function not only through GPCR pathway but also inhibition of HDAC. The differentiation of T cell is mediated both by SCFA regulation of DC and the direct act of SCFA on T cells. SCFA regulate the generation of Th1, Th17 and Treg in different cytokine milieu. SCFA also inhibit intestinal macrophage production of proinflamamtory cytokines through inhibition of HDAC, and possibly induce intestinal IgA production of B cells. Moreover, SCFAs inhibit the carcinogenesis through promoting apoptosis and suppressing proliferation of tumor cells.
During innate immune responses at mucosal sites, the microbial products are recognized by pattern recognition receptors (PRR), such as toll-like receptors (TLRs). SCFA affect pro-inflammatory cytokines production (e.g. IL-6, IL-8, IL-1β and TNFα) through enhancing NF-κB activation in TLR ligand-responses in epithelial cells [29]. During inflammation, SCFA stimulate the migration of neutrophils by activating GPR43 [30] and modulate their production of reactive oxygen species and phagocytosis [31]. Furthermore, SCFA could also inhibit pro-inflammatory cytokine production such as TNFα in neutrophils [32]. SCFA regulate the functions of DCs, which regulate immune response depending not only on secretion of cytokines but also on their ability to interact with T cells. Butyrate and propionate inhibit activation of BMDC via suppressing the LPS-induced expression of co-stimulatory molecule CD40 and secretion of IL-6 and IL-12p40 [33]. A recent study demonstrated that DCs exposed to butyrate could facilitate naïve T-cells differentiation into FoxP3+ regulatory T-cells (Tregs) and inhibit the differentiation of naïve T cells into interferon (IFN)-γ -producing cells through butyrate induced expression of immunosuppressive enzymes indoleamine 2,3-dioxygenase 1 (IDO1) and aldehyde dehydrogenase 1A2 (Aldh1A2) [15]. Furthermore, DCs from mice treated with propionate, characterized by reduced expression of CD40, PD-L2 and CD86, exhibited the impaired ability to initiate Th2 effector function and further to promote Th2-mediated allergic airway inflammation [5]. Butyrate has also been shown to modulate the function of intestinal macrophages. Treatment of macrophages with butyrate inhibits LPS-induced proinflammatory mediators, including nitric oxide (NO), IL-6, and IL-12, but does not affect production of TNF-α or MCP-1. Interestingly, these effects are independent of TLR signaling and activation of GPRs, but dependent of the inhibition of HDAC by butyrate [34].
SCFA also regulate adaptive immune responses as well. Mice provided with SCFA had increased number of extrathymic Foxp3+ Treg cells [35] and SCFA promoted conversion of naïve T cells toward Treg under Treg-cell polarization condition [36]. Butyrate upregulated the histone H3 acetylation of Foxp3 and promoted the differentiation of Treg, which acts as a key anti-inflammatory effector [35]. These data suggest that SCFA might influence histone acetylation whose transcription of genes involved in differentiation of T cells. SCFA also enhance Foxp3 expression of colonic T cells via activation of GPR43 on T cells [3]. A recent study further reported that SCFA increase the IL-10 production in T cell, including Th1, Th17 and Treg cells [37]. However, it should be noted that, in this study, SCFA were also found to directly facilitate the conversion of naïve T cells into Th1 or Th17 depending on cytokine milieu. This effect of SCFA is primarily dependent on HDAC inhibitor activity but independent of GPR43 [37]. It has also been shown that cycloinulooligosaccharides dietary increased SCFA and IgA production in mice, indirectly indicating that SCFA might promote the secretion of IgA by B cells [38].
GPCR in SCFA regulation of immune responses
SCFA-sensing G-protein-coupled receptors (GPCRs), which are also called free fatty acid receptors (FFARs), include GPR41 (FFAR3), GPR43 (FFAR2) and GPR109 (hydroxycarboxylic acid receptor 2 or HCA2). GPR41 and GPR43 can be efficiently activated by acetate, propionate, butyrate and other SCFAs [39], while GPR109a can be activated mainly by butyrate and niacin [40, 41]. GPR41, GPR43 and GPR109a are coupled with Gi/0 and their activation by SCFA inhibits cAMP production [42]. SCFA could regulate the immune responses through activating GPCR which is expressed on almost all the immune cells, such as epithelial cells, neutrophils and macrophages [39]. Early works on GPCR revealed that GPCR-deficient mice exhibit severe and unrecovered inflammation in models of DSS-induced colitis, arthritis and asthma [43]. GPR41 signaling is essential to the protection of propionate in allergic airway inflammation (AAI). Propionate-treated GPR41−/− mice after house dust mites exposure did not exhibit the amelioration of AAI compared with pretreated WT and GPR43−/− mice [5]. Dimethyl fumarate (DMF) was found to protect against multiple sclerosis (MS) through its metabolite which active GPR109a. DMF could reduce neutrophil infiltration to improve EAE, a mouse model of MS, in WT mice, but not GPR109a−/− mice, suggesting a critical role of GPR109a in suppression of MS [44]. Following these discoveries, other studies demonstrated that GPR43 could regulate immune responses in a myriad of different ways. GPR43 on intestinal epithelial cells activates the NLRP3 inflammasome and enhances the production of IL-18, which is critical for maintaining epithelial integrity and intestinal homeostasis [19]. Recent studies showed that activation of GPR43 pathway protected against colon cancer through inducing the cancer apoptosis and inhibiting cancer cells proliferation [45]. GPR43 on neutrophil promotes PMNs recruitment to inflammatory site, probably in protein kinase p38α-dependent manner [30]. A protection of GPR43−/− mice from inflammatory tissue destruction in chronic DSS colitis was reported by diminished intestinal migration of PMNs [46]. In the monosodium urate monohydrate (MSU)-induced gout model, GPR43−/− mice showed decreased neutrophil recruitment resulted from lower production of IL-1β in the course of inflammatory response [47]. Furthermore, acetate could inhibit LPS-induced TNFα secretion from both mice and human PBMCs by GPR43 pathway [48]. GPR43 on colonic T cells induces the differentiation and enhances the suppressive function of Foxp3+ Tregs through epigenetic modifications [3]. Taken together, these data suggest that SCFA-sensing GPCRs play an important role in regulation of immunity and inflammation.
SCFA and GPCR regulation of IBD
The mammalian gastrointestinal tract harbors trillions of bacteria. The metabolites of bacteria such as SCFA reach a high concentration in the gut. The gut is the primary site where SCFA mediate their effect on either intestinal epithelial integrity or mucosal immune responses. The disorder of gut microbiota leading to decreased SCFA is associated with colonic diseases, including IBD. In the often-quoted study, the fecal microbiota in European children which are susceptible to develop IBD, showed lower bacterial richness and an absence of bacteria efficient at fiber digestion and SCFA production compared with African children [49]. Moreover, western diet caused microbiome perturbation, SCFA reduction and high risk of colitis. Particularly in this study, expression of GPR43 was found to decrease in CD patients as well as mice fed with high fat and high sugar diet [50]. There is considerable evidence that the concentration of SCFA was decreased in the colonic lumen of UC [51]. Furthermore, a recent study suggested that the dybiosis, characterized by a decrease of the butyrate-producing species Roseburia hominis and Eaecalibacterium prausnitizii, was defined in patients with UC [7].
SCFA-sensing GPCRs protect against the intestinal inflammation not only through intestinal epithelial barrier maintenance but immune regulation. Both GPR43 and GPR109a are important for regulation of gut immunity. It was found that GPR43−/− and GPR109a−/− mice suffered from more severe DSS-induced colitis [43, 52]. Several groups have found that SCFA could promote the generation and suppressive function of colonic Treg in antibiotic treated mice or GF mice. GPR43 on colonic T cells potentially induce the Treg differentiation when binding SCFA. Importantly, propionate or SCFA mix-treated Rag−/− mice injected with naïve T cells and Treg had lower level of colitis than mice received water [3]. Butyrate binding to GPR109a endows the colonic DCs and macrophages with the ability to induce Treg generation by increasing their production of IL-10 and Aldh1a [52]. The Intestinal epithelial barrier plays a critical role in preventing the intestinal inflammation of IBD. SCFA affect intestinal epithelial cells which highly expressed GPR43, functioning as regulators of physical barrier and secretion of mucin, antimicrobial peptides, chemokines and cytokines. SCFA might activate NALP6 through GPCR pathway, further promoting gut goblet cells to secret mucus, which is an important barrier separate the bacteria and epithelial cells [53, 54]. Several recent studies also suggested that butyrate could upregulate the tight junction and regulate epithelial permeability [55]. Chronic hypoxia in intestinal mucosa of IBD results in several alterations of the gut environment. Stabilization of hypoxia inducible factors (HIFs) has been revealed to protect against the inflammation and maintain the intestinal homeostasis [56, 57]. A recent study has shown that SCFA were highly produced in low O2 condition and then regulated O2 metabolism in intestinal epithelial cells and further to maintain epithelial integrity and protect barrier via increase HIF stabilization [58]. In contrast, GPR43−/− and GPR41−/− mice were found to have reduced colonic inflammation after administration of ethanol and 6-trinitrobenzene sulfonic-acid (TNBS); or infection with Citrobacter rodentium, possibly resulting from decreased secretion of proinflammatory cytokines and chemokines in intestinal epithelial cells by promotion of SCFA [18]. Overall, SCFA are involved in both anti- and pro-inflammatory processes of IBD.
Chronic intestinal inflammation such as IBD increases the risk of colorectal carcinogenesis, which is called colitis-associated cancer (CAC). Gut microbiota are associated with the process of inflammation and tumorigenesis. Thus their metabolites SCFA were observed to confer protection in the development of colon cancer, partially in the GPCR-dependent manner. Moreover, the expression of GPR43 is reduced in human colon cancer. Propionate and butyrate induce the cancer apoptosis and inhibit cancer cells proliferation depending on GPR43 pathway [45]. GPR109a is the receptor for butyrate in colon, which is expressed on colonic epithelial cells and immune cells. GPR109a deficiency has been reported to promote inflammation-induced colon carcinogenesis. In addition, GPR109a−/− mice suffered from exacerbated colitis and carcinogenesis compared to WT mice treated with AOM+DSS, being associated with decreased production of IL-10 and IL-18 and increased production of IL-17 [52].
The beneficial effects of SCFA have also been reported in patients with IBD. It has been proposed long time ago to treat patients with IBD by administration of SCFA or prebiotics that are known to enhance SCFA production [59–62]. Treatment of SCFA on UC patients has been demonstrated to be effective to ameliorate colitis [63]. SCFA mixture (sodium acetate, sodium propionate and sodium butyrate) enemas, severing as an adjuvant therapy, enhanced the efficacy of classic IBD treatments such as 5-aminosalicylic acid and corticosteroid therapy [61]. Collectively, SCFA have profound effects in regulation of gut immunity and the pathogenesis of IBD. However, due to partial restricted indications or patient compliance, these treatments have not been established as a standard of care.
Conclusions
The significance of interactions between dietary intake and gut microbiota has been well recognized in human health. The discovery that SCFA are the natural ligands for GPR41, GPR43, and GPR109a, which are expressed on a wide range of cell types, has led to re-emerged interests in the role of SCFA in human health, especially in regulation of inflammatory bowel diseases. With the accumulating data indicating regulatory function of SCFA in wide range of immune cells, including both innate and adaptive cells, they represent a new frontier in manipulation and prevention of IBD. However, many questions remain to be investigated. For examples, how diets with high-fiber regulate gut microbiota composition and function, and the role of SCFA in such process? While most studies are currently focusing on SCFA regulation of innate immune responses, how they regulate gut adaptive immune responses, i.e. do they affect gut B cell development and antibody responses to microbiota as well as to food antigens? Or do they regulate gut T cell responses in addition to promote Treg cells? Can SCFA through high-fiber diets prevent as well as treat intestinal inflammation in chronic animal models and eventually in patients with IBD? Or do they function differently in patients with CD and UC? Understanding such questions will surely provide great insights into development of new therapeutic avenues to treating IBD patients through dietary manipulation.
Acknowledgments
This work was supported by NIH grants DK098370 and DK105585.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2015;43(4):817–29. doi: 10.1016/j.immuni.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. 2012;245(1):164–76. doi: 10.1111/j.1600-065X.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28(10):1221–7. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 6.Eeckhaut V, Machiels K, Perrier C, Romero C, Maes S, Flahou B, et al. Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut. 2013;62(12):1745–52. doi: 10.1136/gutjnl-2012-303611. [DOI] [PubMed] [Google Scholar]
- 7.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 8.Sommer F, Backhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science. 2012;336(6086):1262–7. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 10.Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–52. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 12.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragsdale SW, Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim Biophys Acta. 2008;1784(12):1873–98. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–45. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V. Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem J. 2015;469(2):267–78. doi: 10.1042/BJ20150242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J Biol Chem. 2004;279(14):13293–6. doi: 10.1074/jbc.C400059200. [DOI] [PubMed] [Google Scholar]
- 17.Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT. Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol. 1997;80(3A):17A–25A. doi: 10.1016/s0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145(2):396–406. e1–10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 19.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi: 10.1038/nutd.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt SL, Pena-Diaz J, Finlay BB. Chemical communication in the gut: Effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens. Anaerobe. 2015;34:106–15. doi: 10.1016/j.anaerobe.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 23.Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG., Jr Absorption of short-chain fatty acids by the colon. Gastroenterology. 1980;78(6):1500–7. [PubMed] [Google Scholar]
- 24.Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28(6):657–61. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 2014;8(6):1323–35. doi: 10.1038/ismej.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12(2):304–14. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 27.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6(8):1535–43. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhutia YD, Ganapathy V. Short, but Smart: SCFAs Train T Cells in the Gut to Fight Autoimmunity in the Brain. Immunity. 2015;43(4):629–31. doi: 10.1016/j.immuni.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin MY, de Zoete MR, van Putten JP, Strijbis K. Redirection of Epithelial Immune Responses by Short-Chain Fatty Acids through Inhibition of Histone Deacetylases. Front Immunol. 2015;6:554. doi: 10.3389/fimmu.2015.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH, Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci (Lond) 2009;117(9):331–8. doi: 10.1042/CS20080642. [DOI] [PubMed] [Google Scholar]
- 31.Vinolo MA, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct. 2009;27(1):48–55. doi: 10.1002/cbf.1533. [DOI] [PubMed] [Google Scholar]
- 32.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849–55. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep. 2015;5:16148. doi: 10.1038/srep16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA. 2014;111(6):2247–52. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 36.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity. 2016;44(4):951–3. doi: 10.1016/j.immuni.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8(1):80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishikawa T, Nanjo F. Dietary cycloinulooligosaccharides enhance intestinal immunoglobulin A production in mice. Biosci Biotechnol Biochem. 2009;73(3):677–82. doi: 10.1271/bbb.80733. [DOI] [PubMed] [Google Scholar]
- 39.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 40.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280(29):26649–52. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 41.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121(3):1163–73. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 43.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest. 2014;124(5):2188–92. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128(4):847–56. doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- 46.Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183(11):7514–22. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 47.Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, et al. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015;67(6):1646–56. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- 48.Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, et al. G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis. 2013;19(13):2848–56. doi: 10.1097/01.MIB.0000435444.14860.ea. [DOI] [PubMed] [Google Scholar]
- 49.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–6. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agus A, Denizot J, Thevenot J, Martinez-Medina M, Massier S, Sauvanet P, et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6:19032. doi: 10.1038/srep19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernia P, Gnaedinger A, Hauck W, Breuer RI. Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci. 1988;33(11):1353–8. doi: 10.1007/BF01536987. [DOI] [PubMed] [Google Scholar]
- 52.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40(1):128–39. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156(5):1045–59. doi: 10.1016/j.cell.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–42. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tambuwala MM, Cummins EP, Lenihan CR, Kiss J, Stauch M, Scholz CC, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139(6):2093–101. doi: 10.1053/j.gastro.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 57.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114(8):1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe. 2015;17(5):662–71. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Breuer RI, Soergel KH, Lashner BA, Christ ML, Hanauer SB, Vanagunas A, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40(4):485–91. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheppach W. Treatment of distal ulcerative colitis with short-chain fatty acid enemas. A placebo-controlled trial. German-Austrian SCFA Study Group. Dig Dis Sci. 1996;41(11):2254–9. doi: 10.1007/BF02071409. [DOI] [PubMed] [Google Scholar]
- 61.Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, et al. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9(3):309–13. doi: 10.1111/j.1365-2036.1995.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 62.Vernia P, Annese V, Bresci G, d’Albasio G, D’Inca R, Giaccari S, et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest. 2003;33(3):244–8. doi: 10.1046/j.1365-2362.2003.01130.x. [DOI] [PubMed] [Google Scholar]
- 63.Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103(1):51–6. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]