Abstract
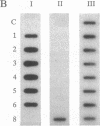
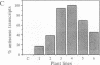
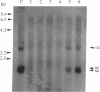
Transgenic tobacco plants carrying a genetic cassette including an antisense DNA sequence of the virally encoded AL1 gene of the geminivirus tomato golden mosaic virus (TGMV) were constructed; AL1 encodes a protein absolutely required for TGMV DNA replication. These genetic cassettes also contained, on the same transcription unit, a gene encoding hygromycin resistance, which allowed selection for concomitant expression of the antisense gene. In transgenic lines, RNA transcripts of the predicted size and strand specificity were detected in antisense plants and sense controls. After infection of plants with TGMV, by agroinoculation, the frequency of symptom development was very significantly reduced in a number of antisense lines and correlated, broadly, with the abundance of antisense RNA transcript and with a reduction in viral DNA harvested from infected leaf tissue. We used an in vitro assay to study viral DNA replication in the absence of cell-to-cell spread; no replication was seen in five of the six antisense lines studied, in contrast to controls.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulcombe D. Strategies for virus resistance in plants. Trends Genet. 1989 Feb;5(2):56–60. doi: 10.1016/0168-9525(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J. S., Brand L., Sunter G., Gardiner W. E., Bisaro D. M., Rogers S. G. Genetic analysis of the tomato golden mosaic virus. II. The product of the AL1 coding sequence is required for replication. Nucleic Acids Res. 1988 Jul 25;16(14B):7043–7060. doi: 10.1093/nar/16.14.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner W. E., Sunter G., Brand L., Elmer J. S., Rogers S. G., Bisaro D. M. Genetic analysis of tomato golden mosaic virus: the coat protein is not required for systemic spread or symptom development. EMBO J. 1988 Apr;7(4):899–904. doi: 10.1002/j.1460-2075.1988.tb02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W. D., Stein V. E., Coutts R. H., Buck K. W. Complete nucleotide sequence of the infectious cloned DNA components of tomato golden mosaic virus: potential coding regions and regulatory sequences. EMBO J. 1984 Sep;3(9):2197–2205. doi: 10.1002/j.1460-2075.1984.tb02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley-Bowdoin L., Elmer J. S., Rogers S. G. Transient expression of heterologous RNAs using tomato golden mosaic virus. Nucleic Acids Res. 1988 Nov 25;16(22):10511–10528. doi: 10.1093/nar/16.22.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Kay R., Chan A., Daly M., McPherson J. Duplication of CaMV 35S Promoter Sequences Creates a Strong Enhancer for Plant Genes. Science. 1987 Jun 5;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of a genomic DNA clone for the small subunit of ribulose bis-phosphate carboxylase-oxygenase from tobacco. Nucleic Acids Res. 1985 Apr 11;13(7):2373–2386. doi: 10.1093/nar/13.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Powell P. A., Stark D. M., Sanders P. R., Beachy R. N. Protection against tobacco mosaic virus in transgenic plants that express tobacco mosaic virus antisense RNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6949–6952. doi: 10.1073/pnas.86.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Bisaro D. M., Horsch R. B., Fraley R. T., Hoffmann N. L., Brand L., Elmer J. S., Lloyd A. M. Tomato golden mosaic virus A component DNA replicates autonomously in transgenic plants. Cell. 1986 May 23;45(4):593–600. doi: 10.1016/0092-8674(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Stanley J., Frischmuth T., Ellwood S. Defective viral DNA ameliorates symptoms of geminivirus infection in transgenic plants. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6291–6295. doi: 10.1073/pnas.87.16.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]
- van den Elzen P. J., Huisman M. J., Willink D. P., Jongedijk E., Hoekema A., Cornelissen B. J. Engineering virus resistance in agricultural crops. Plant Mol Biol. 1989 Sep;13(3):337–346. doi: 10.1007/BF00025322. [DOI] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]