Abstract
Background
The extraction of bioactive compounds from herbal materials requires optimization in order to recover the highest active dose. Response surface methodology was used to optimize variables affecting the microwave extraction of zerumbone from Zingiber zerumbet using the Box–Behnken design. The influence of variables, such as ethanol concentration (X1), microwave power (X2), irradiation time (X3), and liquid-to-solid ratio (X4), on the extraction of zerumbone was modeled using a second-order regression equation. The antiproliferative activity of optimized and non-optimized extracts was evaluated against the HeLa cancer cell line using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
Results
Two linear parameters, X1 and X4, and their quadratic parameters were highly significant at the P < 0.01 level. Two interaction parameters, X1X4 and X2X3 were significant, whereas interactions of X1X2, X1X3, X2X4 and X3X4 were insignificant (P > 0.05). The optimum microwave extraction conditions were as follows: ethanol concentration, 44%; microwave power, 518 W; irradiation time, 38.5 s; and liquid-to-solid ratio, 38 mL/g. Under these conditions, the maximum zerumbone yield was 5.88 mg/g DM, which was similar to the predicted value (5.946 mg/g DM). Optimized and non-optimized Z. zerumbet rhizome extracts exhibited significant antiproliferative activity against HeLa cancer cells, with half-maximal inhibitory concentration (IC50) values of 4.3 and 7.8 μg/mL, respectively, compared with 1.68 μg/mL for the anticancer drug cisplatin. When the extract concentration increased from 4.3 to 16.0 μg/mL, the inhibition of cancer cell growth increased from 50.0 to 79.5%.
Conclusions
In this study, the optimized microwave protocol developed for extracting zerumbone from Z. zerumbet was faster and consumed less solvent than previous methods, while improving and enhancing the antiproliferative activity.
Keywords: Response surface methodology, Zerumbone, Antiproliferative activity, HeLa cancer, Microwave extraction
Background
Herbs and spices produce a large number of phytochemicals, and have been used as food preservatives, flavorings, and traditional medicines for thousands of years. To date, a large number of studies in the medical industry have appraised phytochemicals to identify any biological activities with potential benefits for human health. The Zingiberaceae family of plants produces various compounds that are useful in the food industry, as seasonings and flavoring agents, and the pharmaceutical industry, as antimicrobial and antioxidant agents [1]. Zingiber zerumbet (L.) Roscoe ex Sm., known locally in Malaysia as “lempuyang,” or as “shampoo ginger” in English, is a plant that belongs to the Zingiberaceae family. Z. zerumbet is a perennial, tuberous root plant that grows naturally in damp and shaded parts of lowlands or hill slopes as scattered plants or thickets, and is native to India and the Malaysian Peninsula. Z. zerumbet is a traditional folk remedy and reportedly possesses various phytochemicals and secondary metabolites with antitumor [2], antioxidant [3], antipyretic, analgesic, [4], antibacterial [5], anti-inflammatory, antiallergic [6], and antihypersensitive [7] activities. Extracts of Z. zerumbet rhizomes have been reported to contain zerumbone, humulene, and camphene [8]. The main component of the essential oil of Z. zerumbet herb is zerumbone ((2E,6E,10E)-2,6,9,9-tetramethylcycloundeca-2,6,10-trien-1-one), a monocyclic sesquiterpene containing a cross-conjugated dienone moiety [9]. Zerumbone has a wide range of pharmaceutical activity, such as antioxidant activity [10], anti-inflammation activity [11, 12] anticancer activity [13, 14], antibacterial and antimutagenic activity [15], antimicrobial activity [16] and antidiabetic activity [17]. With a lot of medicinal benefit of zerumbone it seems that optimization of extraction process of this compound from Zingiber zerumbet L. (the only natural source of zerumbone) is necessary.
In industrial process control, response surface methodology (RSM) is usually used to determine the levels of input variables that optimize a particular response [18–20]. RSM is a technique that consists of: (a) designing experiments to deliver adequate and reliable response measurements, (b) developing a mathematical model with the best fit to the data obtained from the experimental design, and (c) determining the optimal value of the independent variables that produce the maximum or minimum values of the response. From an industrial perspective, mathematical models of the microwave extraction of bioactive compounds from plant material are required to optimize and predict the process in order for it to replace conventional extraction methods. Appropriate and optimized processing conditions, such as extraction, are required for efficient recovery and cost effectiveness when used on an industrial scale. The aim of this study is to consider the effect of different extraction methods and solvents on the zerumbone content extracted from Z. zerumbet rhizome, and optimize the selected extraction method using response surface methodology. The antiproliferative activities of the optimized extracts against the HeLa cancer cell line will also be considered.
Methods
Plant sampling
Rhizomes of Z. zerumbet were collected from the Universiti Putra Malaysia (UPM) glasshouse complex, where they had been grown using a plantation method described in our previous report [1]. Samples were submitted to the Institute of Bioscience, UPM, and were identified as Z. zerumbet and voucher specimens were deposited at the herbarium of the Institute of Bioscience, Universiti Putra Malaysia.
Extraction
Microwave extraction
Briefly, 10 mL of the selected solvents (ethanol, methanol, n-hexane and chloroform) was added to Z. zerumbet powder (2.0 g) in a 50-mL double-necked flask attached to a cooling system. The resultant mixture then underwent microwave extraction, after which the flask was removed and the solution was water-cooled to room temperature. The mixture was vacuum filtered through Whatman No. 1 filter paper and collected in a volumetric flask. The residue was freeze-dried and stored at −20 °C for future analysis.
Reflux extraction
Lyophilized rhizome samples (2.0 g) were extracted with the 10 mL of selected solvents (ethanol, methanol, n-hexane and chloroform). Extraction was performed by refluxing at 65 °C for 35 min, after which the solution was cooled to room temperature and filtered through Whatman No. 1 filter. paper. Excess solvent was removed using a rotary evaporator and the resultant residue was freeze-dried and stored at −2.0 °C for future analysis.
Sonication
Lyophilized rhizome samples (2.0 g) were extracted with the 10 mL of selected solvent (ethanol, methanol, n-hexane and chloroform). The resultant mixture was sonicated at 65 °C for 35 min, after which the solution was cooled at room temperature and filtered through Whatman No. 1 filter paper. Excess solvent was removed using a rotary evaporator and the resultant residue was freeze-dried and stored at −2.0 °C for future analysis.
Isolation and identification of zerumbone
The chromatographic isolation of zerumbone was achieved using ultra-high performance liquid chromatography (UHPLC; Agilent, Model 1200) with an Agilent C18 reversed-phase column (5 μm particle size, 4.6 × 150 mm). A gradient elution with a mobile phase comprising 0.1% formic acid in water (A) and methanol (B) was used as follows: 0 min, 60% B; 16 min, 60% B; 16.1 min, 100% B; 20 min, 100% B; 20.1 min, 60% B; and 25 min, 60% B. The detector wavelengths were set at 270 nm. The flow rate and injection volume were 1.2 mL/min and 10 μL, respectively. The column temperature was set at 40 °C. Zerumbone was identified by comparing retention time of extract with retention time of standard, UV spectra, and UV absorbance ratios after co-injection of samples and standards. Zerumbone standard (CAS Number 471-05-6, ≥98%) was purchased from Sigma-Aldrich (M) Sdn Bhd, Selangor, Malaysia. Zerumbone was dissolved in methanol HPLC grade to make different concentration (100, 200, 400, 800 and 1600 µg/mL). Linear regression equations were calculated using Y = aX ± b, where X is the concentration of the zerumbone and Y the peak area of the zerumbone obtained from UHPLC. The linearity was established by the coefficient of determination (R2).
Optimization of zerumbone extraction process
Zingiber zerumbet powder (2.0 g) was stirred in aqueous ethanol in preparation for extraction using the microwave extraction system. Range of variables were chosen based on higher content of zerumbone from the previous preliminary experiment (single factor method). The microwave extraction parameters were microwave power (400–600 W), extraction time (30–90 s), liquid-to-solid ratio (20–40 mL/g), and ethanol proportion (20–60%). Each extraction process was carried out in triplicate. After extraction, mixtures were vacuum filtered through Whatman No. 1 filter paper and collected in a volumetric flask. The residue was then freeze-dried and stored at −20 °C for future analysis.
Experimental design and statistical analyses
RSM was conducted to determine the microwave optimized extraction process variables for the maximum recovery of zerumbone using a Box–Behnken design (Minitab, version 8.0.7.1, USA). Three replicates were used to evaluate the pure error. Experimental design software (DOE, Minitab) was used for regression analysis of the data to fit a second-order polynomial equation (quadratic model), according to the following general equation which was then used to predict optimum conditions for the extraction process.
where Y is the response function (zerumbone yield in this case); B 0 is a constant coefficient; Bi, Bii, and Bij are the coefficients of the linear, quadratic, and interactive terms, respectively; and Xi and Xj are the coded independent variables. The regression coefficients of individual linear, quadratic, and interaction terms were determined according to the analysis of variance (ANOVA). In order to visualize the relationship between the response and experimental levels of each factor, and to deduce the optimum conditions, the regression coefficients were used to generate 3-D surface plots and contour plots from the fitted polynomial equation. The factor levels were coded as −1 (low), 0 (central point or middle), and +1 (high), respectively. The variables were coded according to the following equation:
where Xi is the (dimensionless) coded value of the variable X i; X 0 is the value of X at the center point, and ΔX is the step change.
Determination of antiproliferative activity
Cell culture and treatment
HeLa cells and normal human mammary epithelial cells (MCF-10A) were cultured in RPMI 1640 Medium (Roswell Park Memorial Institute) containing 10% fetal bovine serum (FBS). The cells were incubated overnight at 37 °C in 5% CO2 to allow attachment to the culture plates.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
The MTT assay was conducted as follows: Cells were seeded in 96-well plates at a density of 1 × 104 cells/well in 100 μL of RPMI medium. After 24 h, the medium was removed and the cells were incubated for 3 days with RPMI medium in the presence or absence of Z. zerumbet rhizome extracts at various concentrations. The extract concentrations used ranged from 1 to 16 μg/mL. After incubation, 20 μL of MTT reagent was added to each well. The plate was then incubated again in a CO2 incubator at 37 °C for 4 h. The reduced formazan products were quantified by measuring the absorbance at 570 nm by using an enzyme-linked immunosorbent assay (ELISA) reader. Each point represents the mean of triplicate experiments. Cell viability was determined using the following formula:
Results and discussion
Effect of different solvents and extraction methods on zerumbone content recovery
Many steps are involved in obtaining phytochemicals from plants, including milling, grinding, homogenization, and extraction. Among these steps, extraction has the greatest effect on the recovery and isolation of phytochemicals from plant material. Extraction efficiency is affected by the chemical nature of the phytochemicals, extraction method, sample particle size, extraction solvent, and the presence of interfering substances. Impact of different extraction methods (reflux, microwave, soxhlet and sonication) and solvents (ethanol, methanol, n-hexane and chloroform) on zerumbone content from the Z. zerumbet rhizomes was evaluated (Table 1). Significant differences was observed among different extraction methods and solvents. The lowest concentration of zerumbone (2.66 mg/g DM) was detected form chloroform solvent and soxhlet extraction method, while, the highest concentration (4.82 mg/g DM) was observed in ethanol solvent and microwave extraction method. Of all the solvent systems tested, ethanol proved to be the most efficient solvent for zerumbone extraction (Table 1). The highest zerumbone content was observed using ethanol solvent in microwave extraction, followed by sonication extraction. A similar result was reported previously in the microwave-assisted solvent extraction of effective constituents from Herba epimedii [21].
Table 1.
Effect of different extraction solvents and methods on zerumbone content of Z. zerumbet
| Extraction methods | Ethanol | Methanol | n-Hexane | Chloroform |
|---|---|---|---|---|
| Reflux | 3.76 ± 0.03c | 3.24 ± 0.08f | 3.26 ± 0.07f | 2.81 ± 0.01i |
| Microwave | 4.82 ± 0.07a | 4.06 ± 0.05b | 4.07 ± 0.05b | 3.36 ± 0.02e |
| Soxhlet | 3.70 ± 0.06c | 3.12 ± 0.02h | 3.18 ± 0.04g | 2.66 ± 0.01j |
| Sonication | 4.11 ± 0.10b | 3.55 ± 0.04d | 3.25 ± 0.06f | 3.1 ± 0.03h |
All analyses are the mean of triplicate measurements ± standard deviation. Means not sharing a common letter were significantly different at P < 0.05. Unit is: mg/g DM
Analysis of single factor method
Influence of ethanol concentration
The recovery of zerumbone from the Z. zerumbet rhizome with respect to ethanol concentration followed a parabolic curve from 20 to 60% ethanol (Table 2). The zerumbone content increased with increasing ethanol concentration in the extraction medium, up to 40%. A lower ethanol concentration in water can access the cells easily, but a high ethanol concentration can cause protein denaturation, preventing the dissolution of zerumbone and influencing the extraction rate. Ethanol is less polar while, water is strong polar solvent, and its suggested that they can blended together in any proportion [22]. It was provided that with decreasing of ethanol volume in ethanol/water solution the polarity of the solvent mixture will increase continuously [23]. Zerumbone is also a polar molecule, thus the zerumbone content in the extract increased with increasing water content, according to the “like dissolves like” principle [24]. In current study, when the ratio of ethanol/water decreased from 100 to 40%, the zerumbone recovery increased significantly from 3.25 to 4.87 mg/g DM. This might be attributed to the different dielectric properties of the solvents towards microwave heating, which play an important role in microwave extraction by facilitating heat distribution throughout the sample. In this study, 40% ethanol absorbed microwave energy relatively well, and was a good extraction solvent. The proportion of ethanol in the extraction solvent was examined at levels of 20–60% during optimization.
Table 2.
Effect of single-factors on extraction of zerumbone from Z. zerumbet rhizome using microwave extraction method
| Single-factor experiments | |||||||
|---|---|---|---|---|---|---|---|
| Ethanol/water (% v/v) | Irradiation time (s) | MW Power (W) | Liquid-to-solid ratio (mL/g) | ||||
| (% v/v) | Zerumbone (mg/g DM) | (s) | Zerumbone (mg/g DM) | (W) | Zerumbone (mg/g DM) | Ratio (mL/g) | Zerumbone (mg/g DM) |
| 20 | 4.11 ± 0.17b | 30 | 4.15 ± 0.10d | 300 | 3.78 ± 0.09c | 10 | 3.26 ± 0.08e |
| 40 | 4.87 ± 0.13a | 60 | 5.1 ± 0.18a | 400 | 4.32 ± 0.12b | 20 | 3.88 ± 0.09d |
| 60 | 3.66 ± 0.08c | 90 | 4.86 ± 0.15b | 500 | 4.65 ± 0.10a | 25 | 4.21 ± 0.16c |
| 80 | 3.43 ± 0.14d | 120 | 4.45 ± 0.16c | 600 | 3.55 ± 0.08d | 30 | 5.08 ± 0.18a |
| 100 | 3.25 ± 0.09e | 180 | 4.30 ± 0.17c | 700 | 3.21 ± 0.08e | 40 | 4.76 ± 0.15b |
| 210 | 3.52 ± 0.11e | 800 | 2.91 ± 0.06f | ||||
All analyses are the mean of triplicate measurements ± standard deviation. Means not sharing a common letter in each column were significantly different at P < 0.05
Influence of microwave power
The effect of microwave power on the zerumbone recovered from Z. zerumbet rhizome was investigated in the range 300–800 W using a fixed solvent concentration (40% ethanol), an irradiation time of 2 min, and a liquid-to-solid ratio of 20 mL/g. A significant increase in the zerumbone recovery, from 4.32 to 4.65 mg/g DM, was observed at power levels of 400–500 W (Table 2). However, the recovery reduced significantly beyond 600 W, with the lowest recovery observed at 800 W (2.91 mg/g DM). The lower zerumbone recoveries above 600 W might be due to thermal degradation of the phytochemicals at higher microwave power levels. The heat generated by microwaves, with volumetric heating, in the plant cells could be strong enough to breakdown the phytochemicals, resulting in them not being recovered at higher power levels. These observations demonstrated that extractions at higher microwave output power levels, usually more than 800 W, did not ensure a better recovery of zerumbone than extractions at medium power. Based on our observations, microwave power levels of 400, 500, and 600 W were selected as the lower, middle, and upper levels, respectively, applied in RSM optimization.
Influence of irradiation time
In this study, zerumbone recovery was examined at different irradiation times (30–210 s) with three other fixed variables: solvent, 40% ethanol; microwave power level, 500 W; and liquid-to-solid ratio, 20 mL/g (Table 2). The results indicated that the zerumbone recovery increased with increasing microwave irradiation time at the beginning of extraction, with a maximum of 5.1 mg/g DM achieved in 60 s, followed by a decrease after 90 s. According to these results, an irradiation time of 30–90 s was used for further microwave extractions for RSM optimization.
Influence of extraction liquid-to-solid ratio
As in other extraction techniques, the liquid-to-solid ratio is an important parameter influencing zerumbone recovery. For example, in an industrial extraction process, it is important to maximize the extraction yield and minimize solvent consumption [25]. In the current study, the zerumbone recovery from Z. zerumbet rhizome increased with increasing liquid-to-solid ratio during extraction (Table 2). The maximum zerumbone recovery (5.08 mg/g DM) was observed at a liquid-to-solid ratio of 30 mL/g. A ratio of 20–40 mL/g was used during further optimization of process parameters for microwave extraction.
Optimization of microwave extraction conditions
Modeling and model fitting using RSM
The regression coefficients of the intercept, linear, quadratic and interaction terms of the model for optimization of zerumbone extraction were calculated using the least square technique and are given in Table 3. The regression coefficients of the intercept, linear, quadratic, and interaction terms of the model were calculated using the least square technique, and are given in Table 3. The two linear parameters, ethanol concentration (X1) and liquid-to-solid ratio (X4), and their quadratic parameters were shown to be highly significant, at the level of P < 0.01, whereas interactions of X1X2, X1X3, X2X4 and X3X4 were insignificant (P > 0.05). The interactions X1X4 and X2X3 were also significant (P < 0.01). Discounting the non-significant parameters (P > 0.05), the following final predictive equation was obtained:
Table 3.
Analysis of variance for the experimental results of zerumbone content from Z. zerumbet rhizomes
| Parameter | Estimated coefficient | Standard error | Degree of freedom | Sum of squares | F value | Prob > F |
|---|---|---|---|---|---|---|
| Model intercept B0 | 5.51 | 0.084 | 14 | 3.95 | 13.38 | 0.0001 |
| Linear | ||||||
| X1 | 0.14 | 0.042 | 1 | 0.25 | 11.74 | 0.005 |
| X2 | 0.017 | 0.042 | 1 | 0.00343 | 0.16 | 0.693 |
| X3 | −0.055 | 0.042 | 1 | 0.037 | 1.77 | 0.211 |
| X4 | 0.27 | 0.042 | 1 | 0.085 | 40.46 | 0.0001 |
| Quadratic | ||||||
| −0.41 | 0.063 | 1 | 0.89 | 42.02 | 0.0001 | |
| −0.096 | 0.063 | 1 | 0.05 | 2.35 | 0.1513 | |
| −0.085 | 0.063 | 1 | 0.039 | 1.84 | 0.2001 | |
| −0.39 | 0.063 | 1 | 0.081 | 38.52 | 0.0001 | |
| Interaction | ||||||
| X1X2 | 0.12 | 0.073 | 1 | 0.057 | 2.71 | 0.1259 |
| X1X3 | 0.14 | 0.073 | 1 | 0.079 | 3.74 | 0.077 |
| X1X4 | −0.31 | 0.073 | 1 | 0.039 | 18.45 | 0.001 |
| X2X3 | 0.45 | 0.073 | 1 | 0.08 | 37.78 | 0.0001 |
| X2X4 | −0.13 | 0.073 | 1 | 0.072 | 3.42 | 0.0894 |
| X3X4 | −0.061 | 0.073 | 1 | 0.015 | 0.69 | 0.4212 |
| Lack of fit | 10 | 0.23 | 2.15 | 0.3596 | ||
| Pure error | 2 | 0.022 | ||||
| Residual | 12 | 0.025 | ||||
| R2 adjusted | 0.875 | |||||
| R2 | 0.939 | |||||
| C.V.% | 2.86 | |||||
| Corr. total | 26 | 4.21 | ||||
X1, ethanol concentration; X2, microwave power; X3, irradiation time; X4, liquid-to-solid ratio
The ANOVA for the experimental results given in Table 3 shows that the model is significant, with an F value of 13.38. There is only a 0.01% chance that a “model F value” this large could occur due to noise. The determination coefficient (R2) was 0.9390, which implied that the sample variations of 93.9% for the microwave extraction efficiency of zerumbone from Z. zerumbet rhizomes were attributed to independent variables, with only 6.1% of the total variations not explained by the model. However, a large value of R2 does not always indicate that the regression model is sound [26]. In a good statistical model, the adjusted R2 (R2 adj.) should be close to R2. As shown in Table 2, the R2 and R2 adj. values for the model were close together. The “lack of fit F value” of 2.15 implied that the lack of fit was not significant relative to pure error (P > 0.0.5), confirming the validity of the model. The coefficient of variation (CV) value of 2.86% and the adequate precision ratio of 16.84 suggested that the model was reliable. In general, the results indicated that the model could work well for the prediction of zerumbone extracted from Z. zerumbet rhizomes.
Response surfaces analysis
To investigate the interactive effects of the independent variables and their mutual interaction on zerumbone extraction recovery, 3-D response surface profiles of multiple non-linear regression models were plotted (Fig. 1). The plots were generated by plotting the response on the z-axis against two independent variables, ethanol concentration (X1) and microwave power (X2), while keeping the other two independent variables [irradiation time (X3) and liquid-to-solid ratio (X4)] at their zero level.
Fig. 1.
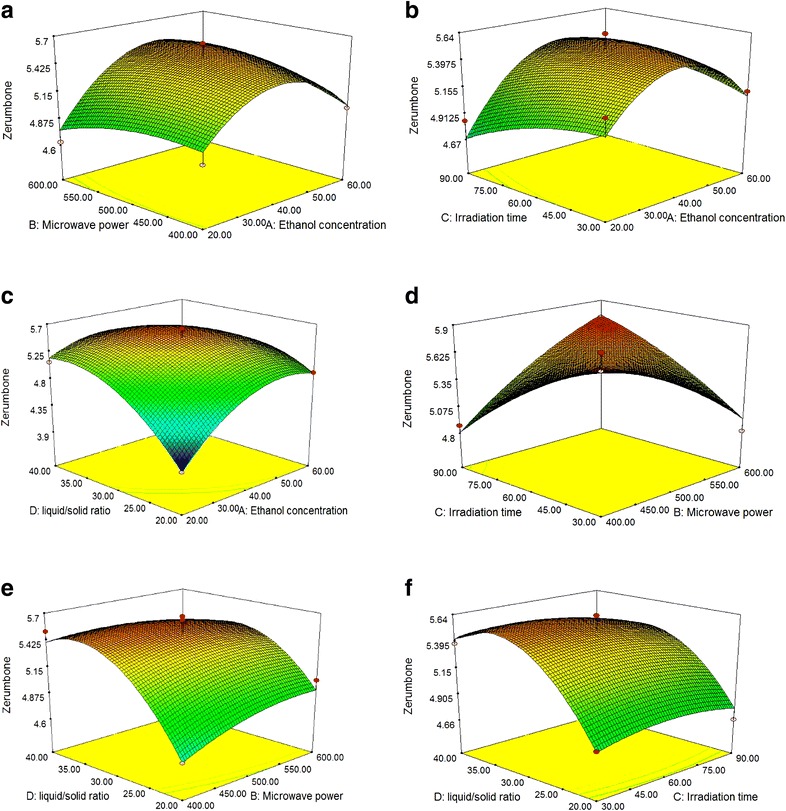
Response surface analysis for the zerumbone content from Z. zerumbet rhizome with microwave extraction with respect to ethanol concentration and microwave power (a); ethanol concentration and extraction/irradiation time (b); ethanol concentration and solvent-to-solid ratio (c); extraction/irradiation time and microwave power (d); microwave power and solvent-to-solid ratio (e); extraction/irradiation time and solvent-to-solid ratio (f)
Figure 1a depicts the relationship between ethanol concentration and each of the three other factors (MW power, irradiation time, and liquid-to-solid ratio) on zerumbone recovery. The zerumbone recovery from Z. zerumbet rhizome increased from 4.83 to 5.46 mg/g DW with increasing ethanol concentration (from 20 to 40%) and extraction power (from 400 to 500 W), nearly reaching a peak at 40% ethanol. Furthermore, the recovery was negatively affected by ethanol concentrations higher than 40% and additional extraction power. The best balance of ethanol concentration and extraction power should be sought to achieve the maximum zerumbone extraction rate. As shown in Table 3, the zerumbone recovery mainly depended on ethanol concentration, because its quadratic and linear effects were highly significant (P < 0.01), resulting in a curvilinear increase in zerumbone yield for all extraction powers and times tested (Fig. 1a, b).
The increase in zerumbone recovery suggested that zerumbone was more soluble in 45% ethanol/water, confirming the results of the single-factor experiments. Ethanol could facilitate an increase in extraction yield and water could enhance the swelling of cell material, favorably increasing the contact surface area between plant matrix and solvent, and resulting in an increased extraction yield [27]. Figure 1c shows the increase in zerumbone recovery with increasing ethanol concentration and extraction liquid-to-solid ratio at the beginning, followed by a decrease at medium values. The data suggested that the extraction liquid-to-solid ratio had a quadratic and linear effect (P < 0.01) on the zerumbone yield (Table 3). The zerumbone yield was maximized using a liquid-to-solid ratio of 30 over a range of the other operational factors (microwave power and irradiation time).
Increased microwave power with a longer irradiation time resulted in a continuous higher temperature in the extraction system. This combination of temperature and time was able to enhance the solubility of zerumbone and decrease the viscosity of the extraction solvent, thus accelerating the release and dissolution of zerumbone (Fig. 1d). Increasing the temperature could also aid the degradation of zerumbone to other compounds. The interaction effect of irradiation time (X2) and microwave power (X3) had a significant influence on the acquired ratio of zerumbone (P < 0.01). The linear and quadratic effects of these parameters (X2, X3) were insignificant (P > 0.05), while the synergistic effect was highly significant (P < 0.01). The zerumbone recovery using microwave energy was found to be a function of the interaction effect of extraction power and time.
In microwave extraction, microwave power was a key variable affecting the release of phytochemicals from different matrices by rupturing cell the wall, and also had the ability to modify equilibrium and mass transfer conditions during extraction. Increasing the microwave power accelerated zerumbone extraction. As shown in Fig. 1e, increasing the microwave power from 400 to 500 W resulted in a gradual increase in extraction yields of zerumbone, followed by a decline with a further increase in liquid-to-solid ratio (beyond 30 mL/g). This zerumbone yield trend could be attributed to the increase in liquid-to-solid ratio decelerating mass transfer, due to the lower heating efficiency under microwave conditions and zerumbone solubility. As shown in Fig. 1f, when the 3-D response surface plot was developed for zerumbone recovery with varying irradiation times and liquid-to-solid ratios, the best recovery of zerumbone was obtained using an extraction time of 60 s and liquid-to-solid ratio of 30 mL/g.
Validation and verification of predictive model
The stationary point, denoting the maximum microwave extraction efficiency of zerumbone, was obtained using an experiment with the following critical values: ethanol concentration, 44%; microwave power, 518 W; irradiation time, 38.5 s; and liquid-to-solid ratio, 38 mL/g. The appropriateness of the model equation for predicting the optimum response values was tested using the above selected optimal conditions. The predicted extraction yield of zerumbone was 5.946 mg/g DM, which was consistent with the experimental yield of 5.88 mg/g DM. The predicted values were in close agreement with experimental values and were found to not be significantly different (P > 0.05) using a paired t test. The predicted response values deviated slightly from the experimental data.
Antiproliferative activity
Optimized extract (using RSM methodology) and non-optimized extract (primary extract, using ethanol as a solvent and microwave as a extraction method, Table 1) of Z. zerumbet rhizome were evaluated for their antiproliferative activity against the HeLa cancer cell line (Fig. 2). Significant differences in antiproliferative activity against the HeLa cancer cell line were observed between optimized and non-optimized extracts. Preliminary screening showed that the optimized and non-optimized extracts of Z. zerumbet rhizome exhibited significant antiproliferative activity against HeLa cancer cells, with half-maximal inhibitory concentration (IC50) values of 4.3 and 7.8 μg/mL, respectively. For comparison, the IC50 value for the anticancer drug cisplatin was 1.68 μg/mL. Lower IC50 values represent stronger cancer growth inhibition, at low concentrations. By optimizing the extraction process, the IC50 for HeLa cancer growth decreased from 7.8 to 4.3 μg/mL. For the optimized extract, increasing the concentration from 4.3 to 16 μg/mL increased the inhibition of cancer growth cells from 50 to 79.5%. Abdul et al. [28] showed that a purified zerumbone crystal from Z. zerumbet exhibited anticancer activity against the HeLa cancer cell line with an IC50 value of 2.50 μg/mL. In another study, zerumbone inhibited the growth of murine lymphoid tumor (P-388D) cells, induced DNA fragmentation in culture, and significantly prolonged the life of P-388D (1)-bearing CDF (1) mice (ILS% = 120.5) at a dosage of 2 mg/kg [29]. As shown in Fig. 3, normal cells treated with the optimized extract of Z. zerumbet rhizome at concentrations of 1–16 μg/mL showed a viability ranging from 96.4 to 78.1%. The antiproliferative activity of Z. zerumbet against a breast cancer cell line (MCF-7) has been reported, with an IC50 of 2.81 μg/mL [30]. Based on the Cytotoxicity Screening Index from the National Cancer Institute [31], IC50 value < 30 μg/mL is considered significant, while < 8 μg/mL is consider very significant. In this study, an IC50 value of 4.3 μg/mL was found for the optimized extract of Z. zerumbet rhizome, which was less than 8 μg/mL and considered very significant. In an herbal supplement, one ingredient may provide the desired therapeutic benefits while others may have toxic effects for humans. For example, many Malaysian herbs and spices might also contain toxic components that have been poorly investigated. According to the obtained results, optimized Z. zerumbet rhizome extracts showed nontoxic effects at the tested concentrations. Normal cells treated with Z. zerumbet extract, at concentrations ranging from 1 to 16 μg/mL, showed cell viabilities ranging from 96.5 to 78.1%. The antioxidant and anticancer activities of the herbal extracts are directly related to the phytochemicals or secondary metabolites of the extract [32–34].
Fig. 2.
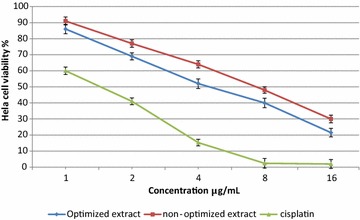
Antiproliferative activity of optimized and non-optimized extract of Z. zerumbet rhizome against HeLa cancer cell line. Bars represent standard errors of the means
Fig. 3.

Cytotoxicity effect of optimized extract of Z. zerumbet rhizome against normal cell line. Bars represent standard errors of the means
Conclusion
The results of this study indicated that zerumbone recovery of Z. zerumbet rhizome using microwave extraction was 17.9% higher than with sonication extraction, 28.1% higher than with reflux extraction, and 30.2% higher with than Soxhlet extraction. From an industrial perspective, the application of a microwave extraction method to the extraction of bioactive compounds from plant material requires a mathematical model to optimize and predict the process in order for it to replace conventional extraction methods. Appropriate and optimized processing conditions, such as extraction, are required for efficient recovery and cost effectiveness when used on an industrial scale. The present work established an improved and optimized procedure for extracting zerumbone from Z. zerumbet rhizome using a microwave extraction method. The following conditions were appropriate for the extraction of zerumbone from Z. zerumbet rhizome: Ethanol concentration, 44%; irradiation time, 38.5 s; microwave power, 518 W; and liquid-to solid ratio, 38 mL/g. The optimized extract showed significant antiproliferative activity against HeLa cancer cell lines without toxicity to normal cells.
Authors’ contributions
Study design and experimental work was by AG and HJ. AR was participated in statistical analysis and antiproliferative experiment. MKS was participated in extraction process and statistical analysis. The first draft of the paper was written by AG and reviewed by all authors. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge all the staff of the Laboratory, of Nutrition, Department of Nutrition and Dietetics, Faculty of Medicine, and Health Sciences, Universiti Putra Malaysia (UPM) for all the help and guidance provided in order to accomplish this work.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We have presented all our main data in the form of tables and figures. The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- DMSO
dimethyl sulfoxide
- FBS
fetal bovine serum
- IC50
half-maximal inhibitory concentration
- MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
- UHPLC
ultra-high performance liquid chromatography
Contributor Information
Ali Ghasemzadeh, Phone: +60-17-291-7679, Email: alighasemzadeh@upm.edu.my.
Hawa Z. E. Jaafar, Email: hawazej@upm.edu.my
Asmah Rahmat, Email: profasmah@gmail.com.
Mallappa Kumara Swamy, Email: swamy.bio@gmail.com.
References
- 1.Ghasemzadeh A, Jaafar HZ, Ashkani S, Rahmat A, Juraimi AS, Puteh A, Mohamed MTM. Variation in secondary metabolite production as well as antioxidant and antibacterial activities of Zingiber zerumbet (L.) at different stages of growth. BMC Complement Altern Med. 2016;16(1):1. doi: 10.1186/s12906-016-1072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nag A, Bandyopadhyay M, Mukherjee A. Antioxidant activities and cytotoxicity of Zingiber zerumbet (L.) Smith rhizome. J Pharmacogn Phytochem. 2013;2:102–108. [Google Scholar]
- 3.Yob N, Jofrry SM, Affandi M, Teh L, Salleh M, Zakaria Z. Zingiber zerumbet (L.) Smith: a review of its ethnomedicinal, chemical, and pharmacological uses. Evid Based Complement Altern Med. 2011;2011:543216. doi: 10.1155/2011/543216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somchit M, Shukriyah M, Bustamam A, Zuraini A. Anti-pyretic and analgesic activity of Zingiber zerumbet. Int J Pharmacol. 2005;1(3):277–280. doi: 10.3923/ijp.2005.277.280. [DOI] [Google Scholar]
- 5.Kader G, Nikkon F, Rashid MA, Yeasmin T. Antimicrobial activities of the rhizome extract of Zingiber zerumbet Linn. Asian Pac J Trop Biomed. 2011;1(5):409–412. doi: 10.1016/S2221-1691(11)60090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zakaria ZA, Mohamad A, Chear C, Wong Y, Israf D, Sulaiman M. Antiinflammatory and antinociceptive activities of Zingiber zerumbet methanol extract in experimental model systems. Med Princ Pract. 2010;19(4):287–294. doi: 10.1159/000312715. [DOI] [PubMed] [Google Scholar]
- 7.Chaung H-C, Ho C-T, Huang T-C. Anti-hypersensitive and anti-inflammatory activities of water extract of Zingiber zerumbet (L.) Smith. Food Agric Immunol. 2008;19(2):117–129. doi: 10.1080/09540100802047783. [DOI] [Google Scholar]
- 8.Nhareet SM, Nur SM. Anti inflammatory property of ethanol and water extracts of Zingiber zerumbet. Indian J Pharmacol. 2003;35(3):181. [Google Scholar]
- 9.Kitayama T, Nagao R, Masuda T, Hill RK, Morita M, Takatani M, Sawada S, Okamoto T. The chemistry of zerumbone IV: asymmetric synthesis of Zerumbol. J Mol Catal B Enzym. 2002;17(2):75–79. doi: 10.1016/S1381-1177(02)00009-7. [DOI] [Google Scholar]
- 10.Sidahmed HMA, Hashim NM, Abdulla MA, Ali HM, Mohan S, Abdelwahab SI, Taha MME, Fai LM, Vadivelu J. Antisecretory, gastroprotective, antioxidant and anti-helicobcter pylori activity of zerumbone from Zingiber Zerumbet (L.) Smith. PLoS ONE. 2015;10(3):e0121060. doi: 10.1371/journal.pone.0121060. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Sulaiman MR, Perimal EK, Akhtar MN, Mohamad A, Khalid MH, Tasrip NA, Mokhtar F, Zakaria ZA, Lajis N, Israf D. Anti-inflammatory effect of zerumbone on acute and chronic inflammation models in mice. Fitoterapia. 2010;81(7):855–858. doi: 10.1016/j.fitote.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Murakami A, Miyamoto M, Ohigashi H. Zerumbone, an anti-inflammatory phytochemical, induces expression of proinflammatory cytokine genes in human colon adenocarcinoma cell lines. Biofactors. 2004;21(1–4):95–101. doi: 10.1002/biof.552210118. [DOI] [PubMed] [Google Scholar]
- 13.Murakami A, Takahashi D, Kinoshita T, Koshimizu K, Kim HW, Yoshihiro A, Nakamura Y, Jiwajinda S, Terao J, Ohigashi H. Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: the α, β-unsaturated carbonyl group is a prerequisite. Carcinogenesis. 2002;23(5):795–802. doi: 10.1093/carcin/23.5.795. [DOI] [PubMed] [Google Scholar]
- 14.Kirana C, McIntosh GH, Record IR, Jones GP. Antitumor activity of extract of Zingiber aromaticum and its bioactive sesquiterpenoid zerumbone. Nutr Cancer. 2003;45(2):218–225. doi: 10.1207/S15327914NC4502_12. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SS, Srinivas P, Negi P, Bettadaiah BK. Antibacterial and antimutagenic activities of novel zerumbone analogues. Food Chem. 2013;141(2):1097–1103. doi: 10.1016/j.foodchem.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Abdul AB, Abdelwahab SI, Al-Zubairi AS, Elhassan MM, Murali SM. Anticancer and antimicrobial activities of zerumbone from. Int J Pharmacol. 2008;4(4):301–304. doi: 10.3923/ijp.2008.301.304. [DOI] [Google Scholar]
- 17.Tzeng T-F, Liou S-S, Chang CJ, Liu I-M. Zerumbone, a tropical ginger sesquiterpene, ameliorates streptozotocin-induced diabetic nephropathy in rats by reducing the hyperglycemia-induced inflammatory response. Nutr Metab. 2013;10(1):1. doi: 10.1186/1743-7075-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghasemzadeh A, Jaafar HZ. Optimization of reflux conditions for total flavonoid and total phenolic extraction and enhanced antioxidant capacity in pandan (Pandanus amaryllifolius Roxb.) using response surface methodology. Sci World J. 2014;2014:523120. doi: 10.1155/2014/523120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghasemzadeh A, Jaafar HZ, Karimi E, Rahmat A. Optimization of ultrasound-assisted extraction of flavonoid compounds and their pharmaceutical activity from curry leaf (Murraya koenigii L.) using response surface methodology. BMC Complement Altern Med. 2014;14(1):1. doi: 10.1155/2014/873803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghasemzadeh A, Jaafar HZ, Rahmat A. Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med. 2015;15(1):258. doi: 10.1186/s12906-015-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Jin H, Ding L, Zhang H, Li J, Qu C, Zhang H. Dynamic microwave-assisted extraction of flavonoids from Herba epimedii. Sep Purif Technol. 2008;59(1):50–57. doi: 10.1016/j.seppur.2007.05.025. [DOI] [Google Scholar]
- 22.Ghasemzadeh A, Jaafar HZ, Rahmat A. Effects of solvent type on phenolics and flavonoids content and antioxidant activities in two varieties of young ginger (Zingiber officinale Roscoe) extracts. J Med Plants Res. 2011;5(7):1147–1154. [Google Scholar]
- 23.Franks F, Ives D. The structural properties of alcohol–water mixtures. Q Rev Chem Soc. 1966;20(1):1–44. doi: 10.1039/QR9662000001. [DOI] [Google Scholar]
- 24.Schmid R. Recent advances in the description of the structure of water, the hydrophobic effect, and the like-dissolves-like rule. Berlin: Springer; 2002. [Google Scholar]
- 25.Spigno G, De Faveri D. Microwave-assisted extraction of tea phenols: a phenomenological study. J Food Eng. 2009;93(2):210–217. doi: 10.1016/j.jfoodeng.2009.01.006. [DOI] [Google Scholar]
- 26.Karazhiyan H, Razavi SM, Phillips GO. Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocoll. 2011;25(5):915–920. doi: 10.1016/j.foodhyd.2010.08.022. [DOI] [Google Scholar]
- 27.Huang W, Xue A, Niu H, Jia Z, Wang J. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009;114(3):1147–1154. doi: 10.1016/j.foodchem.2008.10.079. [DOI] [Google Scholar]
- 28.Abdul AB, Al-Zubairi AS, Tailan ND, Wahab A, Ibrahim S, Mohd Zain ZN, Ruslay S, Mohan M, Mohan S. Anticancer activity of natural compound (Zerumbone) extracted from Zingiber zerumbet in human HeLa cervical cancer cells. Int J Pharmacol. 2008;4(3):160–168. doi: 10.3923/ijp.2008.160.168. [DOI] [Google Scholar]
- 29.Huang G-C, Chien T-Y, Chen L-G, Wang C-C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005;71(3):219–224. doi: 10.1055/s-2005-837820. [DOI] [PubMed] [Google Scholar]
- 30.Rashid RA, Pihie AL. The antiproliferative effects of Zingiber zerumbet extracts and fractions on the growth of human breast carcinoma cell lines. Malays J Pharm Sci. 2005;3(1):45–52. [Google Scholar]
- 31.Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94(1–2):57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 32.Ghasemzadeh A, Jaafar HZ, Rahmat A, Ashkani S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) RM Sm grown in different locations of Malaysia. BMC Complement Altern Med. 2015;15(1):1. doi: 10.1186/s12906-015-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemzadeh A, Nasiri A, Jaafar HZ, Baghdadi A, Ahmad I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules. 2014;19(11):17632–17648. doi: 10.3390/molecules191117632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javanmardi J, Stushnoff C, Locke E, Vivanco J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83(4):547–550. doi: 10.1016/S0308-8146(03)00151-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have presented all our main data in the form of tables and figures. The datasets supporting the conclusions of this article are included within the article.


