Abstract
Introduction
An ongoing national multicenter survey [Italian Oncologic Pain multiSetting Multicentric Survey (IOPS-MS)] is evaluating the characteristics of breakthrough cancer pain (BTP) in different clinical settings. Preliminary data from the first 1500 cancer patients with BTP enrolled in this study are presented here.
Methods
Thirty-two clinical centers are involved in the survey. A diagnosis of BTP was performed by a standard algorithm. Epidemiological data, Karnofsky index, stage of disease, presence and sites of metastases, ongoing oncologic treatment, and characteristics of background pain and BTP and their treatments were recorded. Background pain and BTP intensity were measured. Patients were also questioned about BTP predictability, BTP onset (≤10 or >10 min), BTP duration, background and BTP medications and their doses, time to meaningful pain relief after BTP medication, and satisfaction with BTP medication. The occurrence of adverse reactions was also assessed, as well as mucosal toxicity.
Results
Background pain was well controlled with opioid treatment (numerical rating scale 3.0 ± 1.1). Patients reported 2.5 ± 1.6 BTP episodes/day with a mean intensity of 7.5 ± 1.4 and duration of 43 ± 40 min; 977 patients (65.1%) reported non-predictable BTP, and 1076 patients (71.7%) reported a rapid onset of BTP (≤10 min). Higher patient satisfaction was reported by patients treated with fast onset opioids.
Conclusions
These preliminary data underline that the standard algorithm used is a valid tool for a proper diagnosis of BTP in cancer patients. Moreover, rapid relief of pain is crucial for patients’ satisfaction. The final IOPS-MS data are necessary to understand relationships between BTP characteristics and other clinical variables in oncologic patients.
Funding
Molteni Farmaceutici, Italy.
Keywords: Breakthrough pain, Cancer pain, Pain assessment, Rapid-onset opioid
Introduction
Pain is common in cancer patients, particularly in the advanced stage of disease when the prevalence is estimated to be more than 70% [1]. Adequate pain control is achieved in most patients with available analgesic therapies [2]. However, despite adequate pain control for most hours of the day, patients may develop transient flares of pain throughout the day. This phenomenon is known as breakthrough cancer pain (BTP) [3]. BTP has been reported to produce a negative impact on quality of life and is associated with a significant physical, psychological, and economic burden [4]. Several studies have assessed the epidemiology of this phenomenon, reporting largely variable data in different settings by using different definitions and methodologies, e.g., without an a priori definition of BTP, without clearly distinguishing background pain intensity and BTP intensity, or without considering the level of opioids used for background analgesia [5–7]. In recent years, BTP has been more meaningfully characterized through a diagnostic algorithm. Moreover, some attempts to better characterize this phenomenon according to a number of variables have been made. Recently, an expert consensus suggested that a BTP subclassification according to the characteristics of BTP may provide tailored treatment [8].
In the previous Italian Oncologic Pain multiSetting (IOPS) study, performed in various settings in a large number of patients, several factors influencing the development and characteristics of BTP were assessed [9]. From this data, the IOPS expert group planned a new multicenter survey, with the aim of providing further information on BTP and the factors influencing its characteristics in a large number of patients, diagnosed according to a specific algorithm. The use of BTP medications and factors interfering with administration of transmucosal opioids, commonly used for the management of BTP because their PK profile fits with BTP onset and duration, were also evaluated [5]. Reported here is a preliminary analysis of data from the first 1500 patients of 4056 patients globally enrolled in this second IOPS study.
Methods
This preliminary analysis included the first 1500 patients recruited in a national, observational, multicenter Italian study. An investigator meeting was held to present and comment on the project with the representatives of each center that participated. Subsequently, each center received an IOPS Multicentric Survey (IOPS-MS) investigator manual.
Thirty-two centers were involved. Each center consecutively enrolled patients for 24 months after obtaining local ethic committee approval and the patients’ informed consent. Patients were recruited in the most common care settings where cancer patients are assessed for pain, including oncology, outpatient pain therapy, palliative care, and radiotherapy settings. The place of assessment was also recorded, including outpatient clinic, day hospital, home care, hospice, and inpatient ward.
Inclusion criteria were age greater than 18 years, cancer diagnosis at any stage, stable background pain in the last week with an intensity of at most 4 on a numerical scale from 0 to 10, and episodes of BTP with an intensity of 5 or more, clearly distinguished from background pain. A standard algorithm to diagnose BTP was followed according to the following definition: BTP is a transitory exacerbation of pain of moderate to severe intensity that occurs spontaneously or predictably [8–11], and is well distinguished from background pain of mild intensity [6, 12]. Exclusion criteria were the absence of a cancer diagnosis, uncontrolled background pain (>4 on a numerical scale of 0 to 10), or no relevant increases in pain intensity (<5) which could be interpreted as BTP episodes. Patients unable to provide information about the data required for the study, as a result of either cognitive failure or terminal disease, were also excluded. Patients meeting the inclusion criteria and assessed at each center were consecutively surveyed.
Epidemiological data, Karnofsky index, stage of disease, presence and sites of metastases, ongoing oncologic treatment, and characteristics regarding background pain and BTP and their treatments were recorded. Type of pain was registered according to routine clinical practice (neuropathic, nociceptive, or coexistent mechanism), and background and BTP intensity were measured on a numerical scale from 0 to 10. Patients were also questioned about BTP predictability, BTP onset (≤10 or >10 min), BTP duration, background and BTP medications and their doses, time to meaningful pain relief after BTP medication, and satisfaction with BTP medication (a four-point scale was used by physicians: very satisfied, satisfied, not satisfied, and neither satisfied nor dissatisfied) [9, 10]. The occurrence of adverse reactions was also assessed, and mucosal toxicity was graded according to the World Health Organization (WHO) criteria [13]. The presence of candidiasis and xerostomia was also recorded. Each patient followed local policy and therapeutic protocols, and no specific treatment for BTP was assigned. To guarantee good quality of the data, these were entered in a web-based clinical report form. Each center had an individual password to enter their data into the system, and the study monitors could check records by local and remote monitoring.
Statistics
Data from the first 1500 patients were preliminarily examined. Continuous variables were summarized as means and standard deviations (SD). Categorical variables were summarized as percentages (absolute numbers). Univariate analysis was performed using the Wilcoxon or Chi square test without correction for continuity for comparison among groups of continuous and categorical variables, respectively. Multivariate analysis was based on generalized linear models, with suitable link function chosen according to the characteristics of the response variable: identity for continuous and logit for binary or proportional-odds ordered categorical variables. All variables considered were entered into the model as they were, without any transformation or cutoff. The nonlinear effect of covariates was modeled by means of a restrictive cubic spline function, and its significance was assessed by means of the χ 2 Wald test. The model strategy was determined by following a backward selection strategy among variables reaching a level of at least 0.25 on univariate analysis. Model fit was considered significantly improved on the basis of the Akaike information criterion (AIC) applied backward for each model at a significance level of 0.05. To avoid inflation in type I error due to multiplicity of testing, subgroup analysis was conducted by introducing interaction terms into the main multivariate model, and its significance assessed by means of AIC. Multivariate models were depicted as nomograms. To evaluate the goodness of fit of the models, cross-validation and bootstrap (1000 runs) techniques were applied by the use of Somer’s D xy. Statistical significance was set at p ≤ 0.05. The R-System statistical package and the Harrell regression modelling strategies libraries were used for analysis.
Compliance with Ethics Guidelines
Each of the 32 centers involved in the study obtained local ethics committee approval. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Results
Patient Characteristics
Of the first 1500 patients recruited in IOPS-MS, most had metastatic disease and were receiving anticancer treatment (Table 1). The most common care settings were oncology and pain therapy, and patients were seen most often in outpatient clinics (37%) and inpatient wards (33%). No differences in gender were found among the different settings (p = 0.989). A lower and a higher Karnofsky index were found in the palliative care and radiotherapy settings, respectively [39.4 ± 10.8 vs 70 ± 18.2; F = 86.7; degrees of freedom (d.f.) = 3.519; p < 0.001]. Finally, older patients (mean ± SD age 73.9 ± 12.5 years) were over-represented in the palliative care setting (F = 27.1; d.f. = 3.519; p < 0.001).
Table 1.
Baseline patient characteristics
| Characteristic | N = 1500 |
|---|---|
| Mean ± SD age, years | 64.8 ± 12.3 |
| Gender, n (%) | |
| Male | 810 (54) |
| Female | 690 (46) |
| Karnofsky index score, mean ± SD | 61.1 ± 18.2 |
| Place of assessment, n (%) | |
| Outpatient clinic | 549 (37) |
| Day hospital | 171 (11) |
| Home care | 232 (15) |
| Hospice | 47 (3) |
| Hospital inpatient ward | 501 (33) |
| Primary tumor site, n (%) | |
| Lung | 352 (22) |
| Urogenital | 254 (17) |
| Gastrointestinal | 276 (18) |
| Breast | 201 (13) |
| Pancreas | 129 (8) |
| Liver | 16 (1) |
| Head and neck | 97 (6) |
| Others | 241 (15) |
| Disease, n (%) | |
| Locoregional | 250 (17) |
| Metastatic | 1250 (83) |
| Previous anticancer treatment, n (%)a | 1154 (79) |
| Care setting, n (%) | |
| Palliative care | 289 (19) |
| Oncology | 672 (45) |
| Pain therapy | 526 (35) |
| Radiotherapy | 13 (1) |
All values are presented as mean ± SD or number of patients (proportion of patients)
SD standard deviation
aData available in 1464 patients
BTP Characteristics
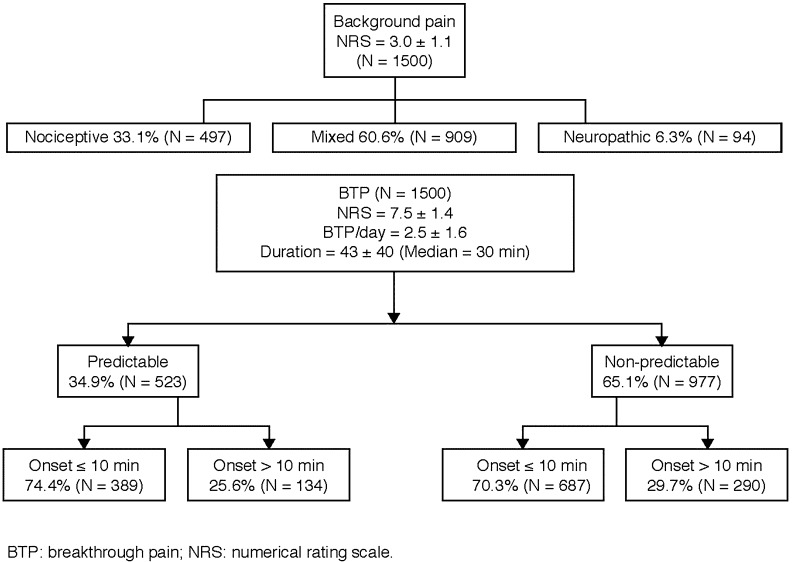
The initial diagnosis of BTP was most often performed by oncologists (n = 616 diagnoses, 41%) and pain physicians (n = 583, 39%), followed by palliative care physicians (n = 241, 16%), nurses (n = 18, 1%), general practitioners (n = 15, 1%), other healthcare providers (n = 15, 1%), and radiotherapists (n = 9, 0.6%). In three cases, data were unavailable. Patients in hospices had a longer time from diagnosis of BTP in comparison with outpatient settings (p = 0.0123). The percentages of patients with baseline pain and the characteristics of BTP are presented in Fig. 1.
Fig. 1.
Percentages of patients with baseline pain and characteristics of BTP
The mean number of BTP episodes/day was 2.5 ± 1.6 (data available for 1499 patients). In patients with higher Karnofsky index and with prostate cancer the number of BTP episodes was significantly higher than in patients with other primary diagnoses (p < 0.001). BTP onset was ≤10 and >10 min in 1076 (71.7%) and 424 (28.3%) patients, respectively.
The mean duration of untreated BTP was 43 ± 40 min (data available for 504 patients). Variables significantly associated with a longer BTP duration were metastatic disease (p = 0.03), head and neck cancer (p = 0.04) and pancreatic cancer, and receiving anticancer therapy (p = 0.05; Table 2). In the multivariate analysis, a significant association with background pain intensity was found, with a linear effect of 10.9 min [95% confidence interval (CI) 9.3–12.5].
Table 2.
Patient characteristics associated with duration of breakthrough pain
| Characteristic | n | Mean duration of BTP, min | SD | p value | |
|---|---|---|---|---|---|
| Disease | Locoregional | 109 | 36.54 | 34.54 | |
| Metastatic | 395 | 44.64 | 41.34 | 0.03 | |
| Primary tumor | Other | 90 | 36.00 | 36.59 | |
| Gastrointestinal/liver | 89 | 42.36 | 38.99 | ||
| Pancreas | 56 | 55.09 | 46.35 | ||
| Lung | 99 | 42.26 | 40.41 | ||
| Breast | 68 | 38.18 | 33.75 | ||
| Head and neck | 16 | 50.38 | 58.09 | 0.04 | |
| Urogenital | 86 | 45.74 | 39.70 | ||
| Anticancer treatment | No | 97 | 37.24 | 32.97 | |
| Yes | 388 | 45.06 | 42.22 | 0.05 |
BTP breakthrough cancer pain, n number of patients, SD standard deviation
The distribution of BTP mechanisms in the different care settings is reported in Table 3. A mixed mechanism of BTP was found to be more represented in oncology and pain therapy settings than in radiotherapy and palliative care settings. Conversely, a nociceptive mechanism was more frequently found in palliative care and radiotherapy settings than in oncology and pain therapy settings.
Table 3.
Frequency of breakthrough pain according to care setting
| Care setting | p value | |||||
|---|---|---|---|---|---|---|
| Palliative care | Oncology | Radiotherapy | Pain therapy | All | ||
| N | 289 | 672 | 13 | 526 | 1500 | |
| Type of BTP experienced, n (%) | ||||||
| Mixed | 113 (39) | 411 (61) | 6 (46) | 364 (69) | 894 (60) | <0.001 |
| Neuropathic | 8 (3) | 63 (9) | 0 (0) | 15 (3) | 86 (6) | |
| Nociceptive | 168 (58) | 198 (29) | 7 (54) | 147 (28) | 520 (35) | |
Predictable BTP
BTP was unpredictable in 977 patients (65.1%) and predictable in 523 patients (34.9%). Predictable BTP was associated with age (p = 0.008), pain mechanism (p < 0.001, lower risk with mixed mechanism), place of assessment (p < 0.001), care setting (p = 0.002), background pain (p = 0.004), diagnosis of prostate cancer (p = 0.030), Karnofsky index (p = 0.046), and oral mucositis (p < 0.001). In the multivariate analysis, lower Karnofsky, lower BTP intensity, and rapid onset of BTP were significantly associated with predictable BTP. The radiotherapy setting was strongly associated with predictable BTP (odds ratio [OR] 9.05). The main trigger for predictable BTP was activity-movement (n = 349, 67%), followed by swallowing (n = 80, 15%), cough (n = 54, 10%), procedure (n = 39, 7%), and bowel movement (n = 31, 6%).
Intensity of Background Pain and BTP
The mean intensity of background pain on assessment and the average pain in the previous week were both 3.0 ± 1.1. The mean doses of oral morphine equivalents (OME) used for background pain were 69.8 ± 139.7 mg/day. The mean intensity of BTP was 7.5 ± 1.4. Rapid-onset BTP and high levels of background pain intensity were associated with a higher BTP intensity. Conversely, a slow-onset BTP was associated with a lower BTP intensity. No differences in BTP intensity among the care settings and triggers of predictable BTP were found. Using mixed pain mechanism as a reference, BTP intensity was higher for neuropathic pain (p = 0.0248) and lower for nociceptive pain (p = 0.0257). BTP was of lower intensity in older patients (p = 0.0002), in patients with higher Karnofsky status (p = 0.0016), and in patients with breast cancer (p = 0.04). Finally, mucositis was associated with higher BTP intensity (p = 0.0083).
BTP Medications
A total of 1263 (84%) patients were receiving opioid drugs for the management of BTP, including fentanyl pectin nasal spray (FPNS, 23%), oral morphine (OM, 17%), fentanyl buccal sublingual tablet (FBST, 15%), fentanyl buccal tablet (FBT, 11%), oral transmucosal fentanyl citrate (OTFC, 5%), subcutaneous morphine (SC-M, 4%), intravenous morphine (IV-M, 3%), and intranasal fentanyl spray (INFS, 1%). The mean ± SD doses of each drug were 178 ± 144 µg (FPNS), 13 ± 11 mg (OM), 227 ± 169 µg (FBST), 261 ± 207 µg (FBT), 490 ± 330 µg (OTFC), 11 ± 5 mg (SC-M), 9 ± 9 mg (IV-M), and 109 ± 59 µg (INFS). No differences in BTP medication according to the characteristics of BTP were found. FPNS was less frequently used in radiotherapy and pain therapy settings (p = 0.008), while SC-M was more frequently used in oncology and palliative care settings (p = 0.004). There was a significant relationship between OME and opioid doses for BTP (correlation 0.42, 95% CI 0.37–0.46).
Time to Meaningful Pain Relief After Drug Administration
The mean time for achieving meaningful pain relief after BTP medication was 17 ± 14 min. In Table 4, the variables associated with the time for meaningful pain relief are presented (data were available for 810 patients). In the multivariate analysis, factors associated with shorter meaningful pain relief were assessment in the inpatient ward (p < 0.001), drug therapy (INFS, FPNS, and IV-M, p = 0.012), and pancreas and head and neck cancers (p = 0.0193).
Table 4.
Time to meaningful pain relief by treatment and other variables
| Mean ± SD time to pain relief, min | p value | |
|---|---|---|
| BTP treatment | ||
| FBST | 16.15 ± 14.3 | |
| FBT | 13.78 ± 11.0 | |
| FPNS | 10.99 ± 8.6 | 0.012 |
| INFS | 10.64 ± 5.2 | 0.012 |
| IV-M | 13.44 ± 8.6 | 0.012 |
| SC-M | 15.36 ± 10.2 | |
| OM | 18.84 ± 12.1 | |
| OTFC | 12.97 ± 5.4 | |
| Other | 27.73 ± 18.1 | |
| Place of assessment | ||
| Outpatient clinic | 23.08 ± 18.0 | |
| Day hospital | 14.95 ± 10.8 | |
| Home | 16.24 ± 13.0 | |
| Hospice | 14.82 ± 8.0 | |
| Inpatient ward | 14.05 ± 11.0 | <0.001 |
| Primary tumor site | ||
| Gastrointestinal–liver | 15.29 ± 11.7 | |
| Pancreas | 13.93 ± 10.8 | 0.0193 |
| Lung | 16.11 ± 16.0 | |
| Breast | 23.02 ± 18.9 | |
| Head and neck | 14.33 ± 10.7 | 0.0193 |
| Urogenital | 19.60 ± 12.9 | |
| Other | 16.87 ± 13.0 | |
BTP breakthrough pain, FBST fentanyl buccal sublingual tablet, FBT fentanyl buccal tablet, FPNS fentanyl pectin nasal spray, INFS intranasal fentanyl spray, IV-M intravenous morphine, OM oral morphine, OTFC oral transmucosal fentanyl citrate, SC-M subcutaneous morphine, SD standard deviation
Satisfaction with BTP Medication
Patients were very satisfied, satisfied, not satisfied, and neither satisfied nor dissatisfied with their BTP medication in 154 (11%), 765 (55%), 262 (19%), and 211 (15%) cases (data available in 1392 patients). The level of satisfaction was significantly associated with the use of FPNS (p = 0.0002). Also, the outpatient clinic (p = 0.04), care in the oncology setting (p = 0.0011), and receiving anticancer treatment (p = 0.0166) were associated with patients’ satisfaction (Table 5).
Table 5.
Multivariate model for dissatisfaction
| OR (95% CI) | p value | |
|---|---|---|
| BTP treatment | 0.0002 | |
| Other vs FPNS | 1.98 (1.42–2.76) | |
| FBST vs FPNS | 1.51 (1.03–2.21) | |
| FBT vs FPNS | 1.31 (0.86–1.99) | |
| INFS vs FPNS | 0.41 (0.14–1.24) | |
| IV-M vs FPNS | 0.47 (0.22–1.00) | |
| SC-M vs FPNS | 0.99 (0.50–1.94) | |
| OM vs FPNS | 1.35 (0.93–1.95) | |
| OTFC vs FPNS | 1.65 (0.95–2.88) | |
| Place of assessment | 0.04 | |
| Day hospital vs outpatient clinic | 0.71 (0.45–1.13) | |
| Home care vs outpatient clinic | 0.29 (0.10–0.85) | |
| Hospice vs outpatient clinic | 0.52 (0.15–1.72) | |
| Inpatient vs outpatient clinic | 0.72 (0.51–1.03) | |
| Previous anticancer treatment vs no previous anticancer treatment | 1.41 (1.06–1.87) | 0.0166 |
| Care setting | 0.0011 | |
| Palliative care vs oncology | 0.84 (0.29–2.44) | |
| Radiotherapy vs oncology | 1.58 (0.54–4.59) | |
| Pain therapy vs oncology | 0.53 (0.38–0.74) |
95% CI 95% confidence interval, BTP breakthrough pain, FBST fentanyl buccal sublingual tablet, FBT fentanyl buccal tablet, FPNS fentanyl pectin nasal spray, INFS intranasal fentanyl spray, IV-M intravenous morphine, OM oral morphine, OR odds ratio, OTFC oral transmucosal fentanyl citrate, SC-M subcutaneous morphine
Adverse Effects of BTP Medications
Adverse reactions attributed to BTP medications were reported in 53 out of 1500 (4%) patients and were constipation (n = 18), dizziness (n = 18), nausea (n = 5), headache (n = 2), vomiting (n = 1), and other unspecified adverse effects (n = 9). The intensity was mild in 46 patients (88%) and moderate in 6 patients (12%). In 38 patients (83%) no specific therapeutic change was required, while in the remaining 8 cases (17%) it was deemed necessary to treat the adverse effects or discontinue the BTP medication. No association was found between adverse reactions and choice and dosage of opioids used for BTP (p = 0.843). Finally, no medication abuse was reported.
Oral Mucositis
Two hundred and twelve patients (14%) presented with different levels of oral mucositis. Of them, 134 patients had oral aching/erythema, 56 had oral erythema/ulcer/solid diet tolerated, 17 patients had oral ulcers/only liquid diet tolerated, and in 5 patients oral feeding was impossible (from level 1 to level 4, respectively). Head and neck cancer was positively associated with the severity of oral mucositis (OR 5.42; 95% CI 2.70–10.86; p < 0.001). Of interest, the grade of mucositis was positively associated with BTP on swallowing (OR 4.85; 95% CI 2.79–8.40). No association was found between levels of oral mucositis and choice of drugs for BTP and their doses. Candidiasis and xerostomia were detected in 90 (6%) and 280 (19%) patients, respectively.
Discussion
Preliminary data for the first 1500 patients of the IOPS-MS survey suggest that, in general, in patients with BTP, older patients and patients with a lower Karnofsky index were most frequently followed in a palliative care setting. This information is consistent with data collected in the previous IOPS survey [9] and in other surveys performed either in oncology or in palliative care settings [14, 15], confirming that the patients’ characteristics differ among the settings of care, particularly in patients with the highest morbidity under the care of palliative care physicians. Data suggest that higher prevalence rates of BTP are reported in studies performed in the hospice setting [9, 16, 17].
Results of this survey suggest that the diagnosis of BTP was performed more frequently by oncologists than by palliative care physicians. Conversely, a longer time for diagnosis of BTP was reported in the hospice setting. Oncologists generally have more opportunities to make an early diagnosis of BTP, as they see patients more often through the course of disease [18], whereas physicians in palliative care see patients later in the course of their disease, which may explain this result. Another explanation could be that oncologists have improved their pain assessment skills in the years since large surveys showed worrying data, suggesting a great need for continuing education programs in pain management among oncologists [19, 20]. However, it is important to note that these findings may not adequately represent the situation, particularly as the differences in the number of patients with BTP in oncology versus palliative care setting may simply be due to the sampling design. Further investigation is warranted.
In this preliminary survey, prostate cancer, a tumor commonly associated with multiple bone metastases, significantly produced more episodes of BTP, potentially representing a risk factor for this phenomenon (see below, predictable BTP). This observation should be confirmed by the complete analysis of the IOPS-MS data. In a European survey, patients had a median of 3 BTP episodes/day. Of interest, patients were included whether they had just 1 episode/month or up to 24 episodes/day [10]. Patients who had a better Karnofsky index were more likely to have more BTP episodes. It is likely that more physical activity may produce more episodes of BTP. Alternately, one can argue that the management of background pain of these patients could be better optimized. This observation confirms previous data, in which very advanced and bedridden patients had fewer BTP episodes with longer onset [9].
The mean duration of untreated BTP was about 40 min, reflecting data from many epidemiological studies that describe a variable duration of 30–60 min [9, 10, 21]. BTP duration has been reported to be longer in spontaneous unpredictable BTP than in patients with incident-type BTP [10]. It should be considered that BTP duration in untreated BTP is more difficult for patients to properly assess, and not all patients are able to do so.
To facilitate the patients’ orientation, a dichotomous measure was chosen for BTP onset (≤10 or >10 min). BTP onset was rapid in 71.7% of patients and slower in 28.3% of patients. Similar values, with a median of 10 min, were found in a multicenter European survey [10] and an Italian survey [9], where they were lower with incident-type BTP.
BTP predictability is an important clinical factor with obvious therapeutic consequences for timing and choice of available BTP medications. Moreover, incident-predictable BTP has been considered to be a negative factor for cancer pain management [17, 22, 23]. This is due to the difficulties in balancing analgesia at rest and pain on movement, which often results in attempts to improve basal analgesia with a possible occurrence of opioid-induced adverse effects. Predictable BTP has a faster onset, typically observed in patients with bone metastases, triggered by physical activity or movement. In this survey, about 35% of patients had predictable BTP, and physical activity was the most frequent trigger.
Some factors were independently associated with predictable BTP and included lower Karnofsky index, lower BTP intensity, and faster BTP onset. Predictable BTP has been previously found to be associated with a faster onset of BTP [9, 10]. Pain induced by movement in patients with bone metastases occurs rapidly and is clearly predictable. A worse performance status was associated with predictable BTP. This is in contrast to a previous finding and probably due to the different care setting distribution in the first IOPS study [9]. It is reasonable to hypothesize that patients with a lower Karnofsky index have lower background pain intensity at rest for most daytime hours, but develop predictable BTP on movement. These data should be confirmed in a larger number of patients with complete analysis the IOPS-MS study. Furthermore, the relationship between predictable BTP and BTP intensity is complex. Patients with a higher BTP intensity had less predictable BTP. This could be explained by patients’ attitudes in limiting a sustaining trigger that induced a predictable BTP, thus avoiding a higher peak of pain intensity.
Of interest, predictable BTP was more frequently observed in the radiotherapy setting, which could be explained by the fact that patients are commonly referred to these specialists for the treatment of bone metastases.
Among the other trigger factors for predictable BTP, swallowing was associated with oral mucositis. Thus, mucosal damage, commonly reported in patients who have received or are still receiving toxic agents [24], is more likely to produce a predictable BTP on swallowing. As expected, mucositis was associated with head and neck cancer, possibly due to previous anticancer treatment. The presence of mucosal damage was also associated with higher levels of BTP intensity. Mucositis is a typical example of BTP occurring with swallowing only. Moreover, the presence of oral mucositis has obvious clinical consequences in terms of route of administration when considering the possible use of transmucosal agents such as rapid-onset opioids, prejudicing reliable absorption of oral transmucosal agents [5]. This suggests that physicians should pay more attention to the diagnosis of mucositis, but also to xerostomia and candidiasis, for optimal selection of BTP therapy.
The relationship between background analgesia and BTP intensity is fundamental in describing the phenomenon of BTP, particularly from a therapeutic perspective. It has been reported that a meaningful cutoff of these levels of pain intensity, as reported in the real world by patients instructed in BTP, is about double [12]. In this survey these levels were maintained on average (3 and 7.5 for background pain and BTP intensity, respectively), suggesting that the standard algorithm used in this study allows an appropriate diagnosis of BTP in cancer patients. Of interest, younger patients, higher background pain intensity, a short BTP onset, the level of mucositis, and neuropathic mechanisms were also independently related to BTP pain intensity. These aspects are worthy of further evaluation with the complete data.
The relationship between background pain and BTP intensity is problematic. Some patients, for example, avoid taking a medication because BTP intensity is not considered high enough. On the other hand, in a recent Delphi survey, experts in the field of BTP suggested that transient pain exacerbations can occur independently of background pain level and ongoing pain medication, and the phenomenon includes several subgroups of BTP types [8].
In our survey, a large number of patients were receiving opioids for the management of BTP, particularly transmucosal fentanyl, in relatively similar or proportional doses, according to the fentanyl availability of different delivery systems. Of interest, a highly significant relationship between the doses of BTP opioid medications and opioid doses for background pain was found. This finding reflects the growing evidence suggesting that a dose proportional to the basal opioid regimen is both safe and effective [25–28], regardless of recommendations suggesting titrating the dose against the effect [29]. Moreover, adverse reactions attributed to BTP medications were limited and of mild intensity in most cases, and were independent of the drug and dose used. This observation confirms that opioid medications given in doses proportional to background opioid dose are relatively safe [25, 26]. This aspect deserves further analysis.
Nasal administration of fentanyl provided faster analgesia relative to other fentanyl products [30]. Patient-reported satisfaction with pain treatment is an important outcome measure when assessing both background pain and BTP [8]. Of interest, the use of FPNS and IV-M, home care assessment, pain therapy setting, and the absence of anticancer treatment were associated with the highest level of satisfaction. Therefore, faster analgesia and patients’ satisfaction should be strongly considered in order to prescribe optimal treatment. These aspects deserve further research and will be better explored with the complete data of IOPS-MS.
There are some limitations to this survey, mainly due to the inherit nature of the study design. Firstly, caution must be taken when interpreting some of the outcomes because of the retrospective nature of the survey. Furthermore, for some outcomes, data are missing.
Conclusions
Overall, the number of patients allows a preliminary analysis only. Although preliminary, these data provide interesting information that will be developed with the complete IOPS-MS survey. BTP diagnosis was performed according to strict criteria, including stable background analgesia achieved with analgesics given around the clock. BTP intensity was clearly distinguished from basal pain, confirming the validity of the algorithm used for the diagnosis of BTP. These aspects allow us to better evaluate the BTP phenomenon. The characteristics of BTP, including the number of episodes, predictability, onset, intensity, duration, and time from diagnosis, were influenced by the many variables taken into consideration. From a therapeutic point of view, opioids, particularly fentanyl products, were largely given for BTP management. The analgesic effect of BTP medications was dependent on a number of variables. Satisfaction with BTP medications was relatively good, particularly in specific settings and with fentanyl preparations. Tolerability was acceptable in most cases, independently of the medication used. Despite the presence of oral mucositis, there was no association with specific drugs or delivery systems. Further data from IOPS-MS should provide a more complete picture of BTP in patients with different cancer types receiving various anticancer treatments, to finally understand this “phenomenon”.
The IOPS MS study group: Amanda Caruselli, Giovanna Prestia, Raffaele Giusti, Andrea Costanzi, Silvia Angelini, Daniela Iacono, Federica Mazzuca, Alessia Carnevale, Paolo Bonome, Luca Nicosia, Claudia Scaringi, Adelaide Montalto, Gennaro Russo, Cira Antonietta Forte, Gennaro Esposito, Paola Bracchi, Ernesto Zecca, Tiziana Campa, Silvia Grecchi, Alessandra Pigni, Annunziata Sammaro, Lucia Dodaro, Giovanna Ballerini, Renato Vellucci, Clarissa Caldarulo, Elisa Palombo, Marco Diviza, Valentina Gianfelice, Claudia Silvestri, Simona Finocchi, Viviana Contu, Gianluca Fora, Fulvia Pedani, Massimiliano Icardi, Elisa Bellini, Alfredo Celano, Roberto Berardo, Oliviero Ostellino, Katya Sartori, Paola Demartini, Raffaella Scroccaro, Giorgia Boscolo, Donata Sartori, Francesco Rosetti, Grazia Artioli, Lucia Borgato, Giovanni Bertoldero, Barbara Veronese, Ilaria Vallini, Alessandro Tuzi, Elena Bolzacchini, Graziella Pinotti, Massimiliano Alù, Antonella Usset, Carmela Arcuri, Agata Laudani, Alessio Pepe, Sarah Scagliarini, Massimiliano Sgarlata, Maria Savio, Massimo Raimondi, Salvatore Maria Giovanni Valenti, Alberto Bucceri, Alma Kasa, Paola Budel, Anna Caramellino, Paola Ballarino, Emanuela Donelli, Stefania Silvestro, Rossella Tonetti, Samuela Bozzoni, Angela Cocquio, Carmela Romano, Anna Nappi, Lucrezia Silvestro, Chiara De Divitiis, Silvia Ghidoni, Rosalba Cortinovis, Roberta Marchesi, Michele Fortis, Loredana Palermo, Giovanni Maria Pisanu, Maura Carboni, Francesca Meloni, Daniele Barillari, Luca Imperatori, Gianni Grilli, Gianluca Laici, Sara Diacciati, Paolo Petreni, Boaz Samolsky Dekel, Federica Marsigli, Miriam Manfreda, Silvia Ghedini, Emanuela Bruno, Emanuele Salvini, Davide Gerboni, Serena Saulle, Roberta Carella, Gaetano Pascoletti, Silvia Bolzonello, Marta Bonotto, Silvia Ellero, Gianpiero Fasola, Elena Ongaro, Cristina Borghesi, Lucia Germani, Edoardo de Ruvo, Federica Marchetti, Milena Pasquale, Lucia Masu, Dario Tammaro, Elisabetta Saracco, Maria Teresa Di Dato, Maurizio Ferrara, Andrea Pironti, Pietro Buonavolontà, Laura Ginocchi, Gianna Musettini, Andrea Antonuzzo, Chiara Caparello, Maurizio Lucchesi, Azzurra Farnesi, Mari Zampieri, Manuela Pisciotta, Iacopo Fioroni, Raffaele Ratta, Maria Concetta Cursano, Daniele Santini, Cinzia Potestà, Gloria Gallo, Benhaz Saber, Giovanni Cuccu, Antonio Cocchirella, Gabriella Maria Monasterolo, Maria Rita Zappoli.
Acknowledgements
Sponsorship for this study was funded by an unrestricted grant from Molteni Farmaceutici, Italy. However, data were independently analyzed under the responsibility of the IOPS scientific committee. Article processing charges and the open access charge for this study were funded by Molteni Farmaceutici, Italy.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis and the work as a whole, and have given final approval for the version to be published.
English-language editing and journal styling prior to submission was provided by Ray Hill, an independent medical writer, and was funded by Molteni Farmaceutici, Italy. English-language editing after peer review was provided by Simone Boniface of Springer Healthcare and was funded by Molteni Farmaceutici, Italy.
Disclosures
Sebastiano Mercadante, Paolo Marchetti, Arturo Cuomo, Augusto Caraceni, Rocco Domenico Mediati, Massimo Mammucari, Silvia Natoli, Marzia Lazzari, Mario Dauri, Mario Airoldi, Giuseppe Azzarello, Mauro Bandera, Livio Blasi, Giacomo Cartenì, Bruno Chiurazzi, Benedetta Veruska Pierpaola Costanzo, Daniela Degiovanni, Flavio Fusco, Vittorio Guardamagna, Vincenzo Iaffaioli, Simeone Liguori, Vito Lorusso, Sergio Mameli, Rodolfo Mattioli, Teresita Mazzei, Rita Maria Melotti, Valentino Menardo, Danilo Miotti, Stefano Moroso, Stefano De Santis, Remo Orsetti, Alfonso Papa, Sergio Ricci, Alessandro Fabrizio Sabato, Elvira Scelzi, Michele Sofia, Giuseppe Tonini, Federica Aielli and Alessandro Valle have nothing to disclose.
Compliance with Ethics Guidelines
Each of the 32 centers involved in the study obtained local ethics committee approval. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D517F0605B74E7B8.
Contributor Information
Sebastiano Mercadante, Email: terapiadeldolore@lamaddalenanet.it.
On behalf of the IOPS MS study group:
Amanda Caruselli, Giovanna Prestia, Raffaele Giusti, Andrea Costanzi, Silvia Angelini, Daniela Iacono, Federica Mazzuca, Alessia Carnevale, Paolo Bonome, Luca Nicosia, Claudia Scaringi, Adelaide Montalto, Gennaro Russo, Cira Antonietta Forte, Gennaro Esposito, Paola Bracchi, Ernesto Zecca, Tiziana Campa, Silvia Grecchi, Alessandra Pigni, Annunziata Sammaro, Lucia Dodaro, Giovanna Ballerini, Renato Vellucci, Clarissa Caldarulo, Elisa Palombo, Marco Diviza, Valentina Gianfelice, Claudia Silvestri, Simona Finocchi, Viviana Contu, Gianluca Fora, Fulvia Pedani, Massimiliano Icardi, Elisa Bellini, Alfredo Celano, Roberto Berardo, Oliviero Ostellino, Kayta Sartori, Paola Demartini, Raffaella Scroccaro, Giorgia Boscolo, Donata Sartori, Francesco Rosetti, Grazia Artioli, Lucia Borgato, Giovanni Bertoldero, Barbara Veronese, Ilaria Vallini, Alessandro Tuzi, Elena Bolzacchini, Graziella Pinotti, Massimiliano Alù, Antonella Usset, Carmela Arcuri, Agata Laudani, Alessio Pepe, Sarah Scagliarini, Massimiliano Savio Maria Sgarlata, Massimo Raimondi, Salvatore Maria Giovanni Valenti, Alberto Bucceri, Alma Kasa, Paola Budel, Anna Caramellino, Paola Ballarino, Emanuela Donelli, Stefania Silvestro, Rossella Tonetti, Samuela Bozzoni, Angela Cocquio, Carmela Romano, Anna Nappi, Lucrezia Silvestro, Chiara De Divitiis, Silvia Ghidoni, Rosalba Cortinovis, Roberta Marchesi, Michele Fortis, Loredana Palermo, Giovanni Maria Pisanu, Maura Carboni, Francesca Meloni, Daniele Barillari, Luca Imperatori, Gianni Grilli, Gianluca Laici, Sara Diacciati, Paolo Petreni, Boaz Samolsky Dekel, Federica Marsigli, Miriam Manfreda, Silvia Ghedini, Emanuela Bruno, Emanuele Salvini, Davide Gerboni, Serena Saulle, Roberta Carella, Gaetano Pascoletti, Silvia Bolzonello, Marta Bonotto, Silvia Ellero, Gianpiero Fasola, Elena Ongaro, Cristina Borghesi, Lucia Germani, Edoardo De Ruvo, Federica Marchetti, Milena Pasquale, Lucia Masu, Dario Tammaro, Elisabetta Saracco, Maria Teresa Di Dato, Maurizio Ferrara, Andrea Pironti, Pietro Buonavolontà, Laura Ginocchi, Gianna Musettini, Andrea Antonuzzo, Chiara Caparello, Maurizio Lucchesi, Azzurra Farnesi, Mari Zampieri, Manuela Pisciotta, Iacopo Fioroni, Raffaele Ratta, Maria Concetta Cursano, Daniele Santini, Cinzia Potestà, Gloria Gallo, and Benhaz Saber
References
- 1.Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- 2.Mercadante S. The use of opioids for treatment of cancer pain. Expert Opin Pharmacother. 2015;16:389–394. doi: 10.1517/14656566.2015.989213. [DOI] [PubMed] [Google Scholar]
- 3.Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 4.Portenoy RK, Payne D, Jacobson P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81:129–134. doi: 10.1016/S0304-3959(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 5.Mercadante S, Marchetti P, Cuomo A, Mammucari M, Caraceni A, IOPS MS Study Group Breakthrough pain and its treatment: critical review and recommendations of IOPS (Italian Oncologic Pain Survey) expert group. Support Care Cancer. 2016;24:961–968. doi: 10.1007/s00520-015-2951-y. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante S, Valle A, Porzio G, et al. Relationship between background cancer pain, breakthrough pain, and analgesic treatment: a preliminary study for a better interpretation of epidemiological and clinical studies. Curr Med Res Opin. 2013;29:667–671. doi: 10.1185/03007995.2013.792247. [DOI] [PubMed] [Google Scholar]
- 7.Haugen DF, Hjermstad MJ, Hagen N, Caraceni A, Kaasa S, European Palliative Care Research Collaborative (EPCRC). Assessment and classification of cancer breakthrough pain: a systematic literature review. Pain. 2010;149:476–82. [DOI] [PubMed]
- 8.Løhre ET, Klepstad P, Bennett MI, et al. European Association for Palliative Care Research Network. From “breakthrough” to “episodic” cancer pain? A European Association for Palliative Care Research Network expert Delphi survey toward a common terminology and classification of transient cancer pain exacerbations. J Pain Symptom Manage. 2016;51:1013–1019. doi: 10.1016/j.jpainsymman.2015.12.329. [DOI] [PubMed] [Google Scholar]
- 9.Mercadante S, Lazzari M, Reale C, IOPS Study Group et al. Italian Oncological Pain Survey (IOPS): a multicentre Italian study of breakthrough pain performed in different settings. Clin J Pain. 2015;31:214–221. doi: 10.1097/AJP.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 10.Davies A, Zeppetella G, Andersen S, et al. Multicentre European study of breakthrough cancer pain: characteristics and patient perceptions of current and potential management strategies. Eur J Pain. 2011;15:756–763. doi: 10.1016/j.ejpain.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Mercadante S. Breakthrough pain in cancer patients: prevalence, mechanisms and treatment options. Curr Opin Anaesthesiol. 2015;28:559–564. doi: 10.1097/ACO.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 12.Mercadante S, Adile C, Torta R, et al. Meaningful cut-off pain intensity for breakthrough pain changes in advanced cancer patients. Curr Med Res Opin. 2013;29:93–97. doi: 10.1185/03007995.2012.755120. [DOI] [PubMed] [Google Scholar]
- 13.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(Suppl. 1):1995–2025. [DOI] [PubMed]
- 14.Mercadante S, Costanzo BV, Fusco F, et al. Breakthrough pain in advanced cancer patients followed at home: a longitudinal study. J Pain Symptom Manage. 2009;38:554–560. doi: 10.1016/j.jpainsymman.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Mercadante S, Zagonel V, Breda E, et al. Breakthrough pain in oncology: a longitudinal study. J Pain Symptom Manage. 2010;40:183–190. doi: 10.1016/j.jpainsymman.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Deandrea S, Corli O, Consonni D, et al. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage. 2014;47:57–76. doi: 10.1016/j.jpainsymman.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Mercadante S, Maddaloni S, Roccella S, Salvaggio L. Predictive factors in advanced cancer pain treated only by analgesics. Pain. 1992;50:151–155. doi: 10.1016/0304-3959(92)90155-5. [DOI] [PubMed] [Google Scholar]
- 18.Hui D, Bruera E. Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol. 2016;13:159–171. doi: 10.1038/nrclinonc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mercadante S, Roila F, Berretto O, Labianca R, Casilini S, DOMAIN-AIOM study group Prevalence and treatment of cancer pain in Italian oncological wards centres: a cross-sectional survey. Support Care Cancer. 2008;16:1203–1211. doi: 10.1007/s00520-008-0456-7. [DOI] [PubMed] [Google Scholar]
- 20.Breuer B, Chang VT, Von Roenn JH, et al. How well do medical oncologists manage chronic cancer pain? A national survey. Oncologist. 2015;20:202–209. doi: 10.1634/theoncologist.2014-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portenoy RK, Bruns D, Shoemaker B, Shoemaker SA. Breakthrough pain in community-dwelling patients with cancer pain and noncancer pain: part 1-prevalence and characteristics. J Opioid Manage. 2010;6:97–108. doi: 10.5055/jom.2010.0009. [DOI] [PubMed] [Google Scholar]
- 22.Nekolaichuk CL, Fainsinger RL, Aass N, et al. The Edmonton Classification System for Cancer Pain: comparison of pain classification features and pain intensity across diverse palliative care settings in eight countries. J Palliat Med. 2013;16:516–523. doi: 10.1089/jpm.2012.0390. [DOI] [PubMed] [Google Scholar]
- 23.Brunelli C, Kaasa S, Knudsen AK, Hjermstad MJ, Pigni A, Caraceni A. Comparisons of patient and physician assessment of pain-related domains in cancer pain classification: results from a large international multicenter study. J Pain. 2014;15:59–67. doi: 10.1016/j.jpain.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Mercadante S, Aielli F, Adile C, et al. Prevalence of oral mucositis, dry mouth, and dysphagia in advanced cancer patients. Support Care Cancer. 2015;23:3249–3255. doi: 10.1007/s00520-015-2720-y. [DOI] [PubMed] [Google Scholar]
- 25.Mercadante S. The use of rapid onset opioids for breakthrough cancer pain: the challenge of its dosing. Crit Rev Oncol Hematol. 2011;80:460–465. doi: 10.1016/j.critrevonc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Mercadante S. Rapid onset opioids for breakthrough pain: titrating or not titrating, this is the question. Eur J Pain. 2011;5:443–8.
- 27.Mercadante S, Gatti A, Porzio G, et al. Dosing fentanyl buccal tablet for breakthrough cancer pain: dose titration versus proportional doses. Curr Med Res Opin. 2012;28:963–968. doi: 10.1185/03007995.2012.683112. [DOI] [PubMed] [Google Scholar]
- 28.Mercadante S, Villari P, Ferrera P, Mangione S, Casuccio A. The use of opioids for breakthrough pain in acute palliative care unit by using doses proportional to opioid basal regimen. Clin J Pain. 2010;26:306–309. doi: 10.1097/AJP.0b013e3181c4458a. [DOI] [PubMed] [Google Scholar]
- 29.Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur J Pain. 2009;13:331–338. doi: 10.1016/j.ejpain.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Gatti A, Mediati RD, Reale C, et al. Breakthrough pain in patients referred to pain clinics: the Italian pain network retrospective study. Adv Ther. 2012;29:464–472. doi: 10.1007/s12325-012-0022-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.