Abstract
Introduction
Invasive aspergillosis (IA) is associated with a significant clinical and economic burden. The phase III SECURE trial demonstrated non-inferiority in clinical efficacy between isavuconazole and voriconazole. No studies have evaluated the cost-effectiveness of isavuconazole compared to voriconazole. The objective of this study was to evaluate the costs and cost-effectiveness of isavuconazole vs. voriconazole for the first-line treatment of IA from the US hospital perspective.
Methods
An economic model was developed to assess the costs and cost-effectiveness of isavuconazole vs. voriconazole in hospitalized patients with IA. The time horizon was the duration of hospitalization. Length of stay for the initial admission, incidence of readmission, clinical response, overall survival rates, and experience of adverse events (AEs) came from the SECURE trial. Unit costs were from the literature. Total costs per patient were estimated, composed of drug costs, costs of AEs, and costs of hospitalizations. Incremental costs per death avoided and per additional clinical responders were reported. Deterministic and probabilistic sensitivity analyses (DSA and PSA) were conducted.
Results
Base case analysis showed that isavuconazole was associated with a $7418 lower total cost per patient than voriconazole. In both incremental costs per death avoided and incremental costs per additional clinical responder, isavuconazole dominated voriconazole. Results were robust in sensitivity analysis. Isavuconazole was cost saving and dominant vs. voriconazole in most DSA. In PSA, isavuconazole was cost saving in 80.2% of the simulations and cost-effective in 82.0% of the simulations at the $50,000 willingness to pay threshold per additional outcome.
Conclusion
Isavuconazole is a cost-effective option for the treatment of IA among hospitalized patients.
Funding
Astellas Pharma Global Development, Inc.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-016-0443-1) contains supplementary material, which is available to authorized users.
Keywords: Cost-effectiveness, Invasive aspergillosis, Infectious diseases, Isavuconazole, Phase III trial, Sensitivity analyses, Voriconazole
Introduction
Invasive aspergillosis (IA) is a fungal infection that primarily affects immunocompromised individuals. In 2013, a total of 2990 hospitalizations with a primary diagnosis of IA, and 14,470 hospitalizations with either a primary or secondary diagnosis of IA, occurred in the USA [1]. Risk factors for IA include the presence of hematological malignancies, hematopoietic stem cell or solid organ transplant, severe and/or prolonged neutropenia, prolonged and/or high-dose immunosuppressive therapy, and chemotherapy [2]. The majority of cases of IA are encountered in patients with hematological malignancies and bone marrow transplant patients (43.0–68.0%), with the remainder of cases occurring in solid organ transplant recipients (13.0–17.0%) and other immunocompromised hosts (10.0–15.0%) [3, 4].
IA is a serious, life-threatening condition. The reported 1-year overall survival rate following a diagnosis of IA among bone marrow transplant patients ranges from 10.0 to 40.0% in the literature [5, 6]. Among critically ill patients admitted to an intensive care unit with proven IA, overall survival rates at 84 days after diagnosis of IA have been reported to be 21.0% [7]. A 2010 analysis of a national hospital administrative database found that the median length of hospitalization among patients diagnosed with IA was 18–26 days (depending on whether IA was a primary or secondary diagnosis) [8]. During this time, hospitals incurred a median cost between $32,465 and $68,008 (2006 USD), with antifungal medications accounting for 6.0–10.2% of those costs [8]. A 2009 analysis of data from the 2003 Nationwide Inpatient Sample concluded that the length of hospitalization for immunocompromised patients with IA was 1.8–12.4 times higher than the length of hospitalization of similarly high-risk immunocompromised patients without IA and the median hospital charges were 2.3–12.7 times higher (depending on diagnosis-related group) [9]. The annual total cost of IA in the USA, including hospitalizations, subsequent home healthcare, and outpatient medications, was estimated to be $674 million in 1998 [10], the equivalent of $1.3 billion today.
Current treatment guidelines, published before FDA approval of isavuconazonium sulfate, recommend several antifungal agents for the treatment of IA, including voriconazole, amphotericin B and its lipid formulations, itraconazole, posaconazole, and caspofungin [11, 12]. Voriconazole, an antifungal triazole that inhibits fungal respiration and cell membrane function, is currently the preferred agent for first-line treatment of IA, both from a clinical [11] and an economic perspective [13, 14]. Amphotericin B has a less favorable safety profile [15] and lower efficacy [15] compared to voriconazole, and it is recommended for patients who cannot be treated with voriconazole [11]. Other available agents offer limited efficacy benefits compared to voriconazole and therefore are recommended as salvage therapy agents after voriconazole or amphotericin B treatment [11]. A literature review conducted by Krueger and Nelson identified 10 pharmacoeconomic analyses in six different countries comparing voriconazole to other options for the treatment of IA, and all these analyses concluded that voriconazole was the most cost-effective therapy compared to other treatment options discussed above [13]. Another advantage of voriconazole is that it can be administered both orally and intravenously (IV). Voriconazole does present several possible disadvantages: possible toxicity of the IV formulation in patients with renal failure (due to required co-administration with solubility additive sulfobutylether-β-cyclodextrin) [11, 14], a twice daily dosing schedule of the oral medication because of relatively rapid metabolization [14], and a known risk of drug–drug interactions (due to its effect on the CYP2C19, CYP2C9, and CYP3A4 metabolic pathways) [11, 14].
Isavuconazonium sulfate, a novel triazole and prodrug of isavuconazole, received FDA approval for the treatment of IA and invasive mucormycosis in March 2015 [16]. The approval was granted on the basis of data from the SECURE clinical trial (NCT00412893), which showed that isavuconazole has comparable efficacy with voriconazole [17]. All-cause mortality at day 42 (the SECURE trial primary endpoint) was similar in both treatment groups (18.6% in the isavuconazole-treated group and 20.2% in the voriconazole-treated group). Overall adverse event (AE) rates were also similar (96.1% of isavuconazole-treated patients and 98.5% of voriconazole-treated patients experienced at least one treatment-emergent AE) [17]. However, statistically significant differences were observed in eye disorders, hepatobiliary disorders, and skin disorders, with isavuconazole-treated patients having significantly lower rates of these events [17]. Similar to voriconazole, isavuconazole is also available in both oral and IV formulations. Its IV formulation, however, does not require solubility additive sulfobutylether-β-cyclodextrin, which has been associated with potential nephrotoxicity in the voriconazole IV formulation [18]. Oral isavuconazole has a longer half-life than oral voriconazole, allowing for once daily dosing. In addition, the metabolism of isavuconazole is limited to the CYP3A4 and CYP3A5 pathway [18].
In order to determine the optimal treatment option, it is important to thoroughly evaluate new therapies against existing standards by comparing efficacy, safety, costs, and cost-effectiveness. No existing study compares the costs and cost-effectiveness of isavuconazole vs. voriconazole. To fill this gap, the current study has been conducted to estimate the total cost per treated patient and evaluate the cost-effectiveness of isavuconazole vs. voriconazole from a US hospital perspective.
Methods
Model Overview, Perspective, and Population
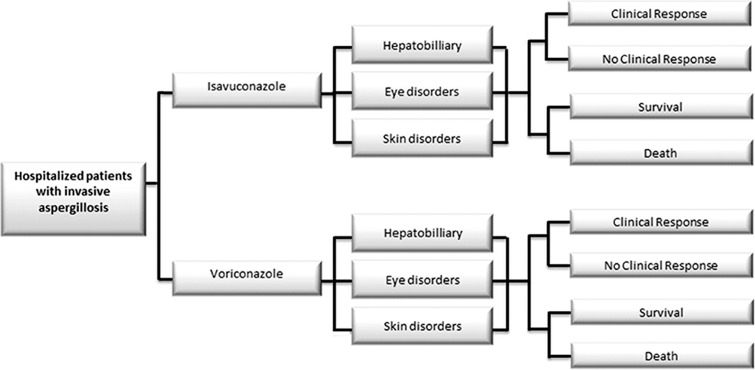
A cost-effectiveness decision tree model was developed in Microsoft Excel 2010 (Fig. 1). The target population included patients with proven, probable, or possible invasive fungal disease caused by the Aspergillus species or other filamentous fungi (reflecting the SECURE trial population) [17]. Two treatments, isavuconazole and voriconazole, were compared. The model estimated the total cost per IA patient treated with each product, with total cost defined as the sum of drug, AE, and hospital stay costs. Incremental cost per death avoided and the incremental cost per additional responder comparing isavuconazole to voriconazole were also estimated. The model was developed from a US hospital perspective, in which only direct costs incurred during the hospitalization were considered, with a time horizon of one hospital stay (including readmissions occurring within 30 days of the original discharge). Readmission within 30 days was considered given that it was a prespecified endpoint in the clinical trial. Because this time horizon is less than 1 year, discounting of costs and effectiveness measures was not necessary. This article does not contain any new studies with human or animal subjects performed by any of the authors. The model relied only on the summary statistics from the SECURE trial (ClinicalTrials.gov identifier NCT00412893) and patient level data was not used. Institutional review board (IRB) review was not needed.
Fig. 1.
Clinical progression of patients through the model. IA invasive aspergillosis. Hospitalized patients with IA would enter the model and they could receive either isavuconazole or voriconazole. While receiving treatments, patients could experience adverse events related to hepatobiliary, eye, and skin disorders. Patients were evaluated for survival and clinical response at the end of model cycle. Length of stay and likelihood of readmission (not depicted here) are also considered in the calculation of healthcare costs
Efficacy and Safety Inputs
Efficacy inputs, mortality and clinical response, for both treatment arms were extracted from the SECURE trial (Table 1) [17]. Mortality was the primary efficacy endpoint and clinical response was the secondary endpoint in the SECURE trial. As was previously described, all-cause mortality at day 42 was 18.6% for isavuconazole-treated patients and 20.2% for voriconazole-treated patients [adjusted difference = −1.0%, 95% CI (−7.8, 5.7%)] [17]. Clinical response rates were 62.0% and 60.3%, respectively [adjusted difference = 0.4%, 95% CI (−10.64, 11.53%)] [17]. The differences in these two outcomes were not statistically significant. The median length of stay of the initial hospitalization was 13 days for isavuconazole-treated patients and 15 days for voriconazole-treated patients [19]. Following discharge from the initial hospitalization, 18.3% of isavuconazole-treated patients and 24.4% of voriconazole-treated patients had a readmission within 30 days [adjusted difference = −6.0%, 95% CI (−13.3, 1.3%)]. This difference was not statistically significant [19]. The median length of stay for readmissions (6 days) was obtained from an analysis of the Premier database of inpatients with a diagnosis of IA and who had a readmission [20]; in the absence of other information, it was assumed to be equal across treatment arms.
Table 1.
Model inputs: efficacy, dosing schedule, and costs
| Definition | Base case value | Source/notes | |
|---|---|---|---|
| Efficacy | ISAV | VORI | |
| Mortality rate | 18.6% | 20.2% | SECURE trial [17]; measured at day 42 on the basis of the intent to treat (ITT) population |
| Clinical response rate | 62.0% | 60.3% | SECURE trial [17]; measured at end of treatment on the basis of the modified ITT population |
| Initial hospital length of stay, days | 13.0 days | 15.0 days | SECURE trial [19] |
| 30-day readmission rate | 18.3% | 24.4% | SECURE trial [19] |
| Readmission length of stay, days | 6.0 days | Estimated from an administrative database study of hospitalized patients with IA [20] | |
| Dosing schedule | Dose | Frequency (per day) | |
|---|---|---|---|
| ISAV | Product label [16, 22] | ||
| Day 1: IV infusion | 372 mg | 3 | |
| Day 2: IV infusion | 372 mg | 3 | |
| Day 3 to end of treatment | |||
| IV infusion | 372 mg | 1 | |
| Oral (tablet) | 372 mg | 1 | |
| VORI | |||
| Day 1: IV infusion | 6 mg/kg | 2 | |
| Day 2: IV infusion | 4 mg/kg | 2 | |
| Day 3 to end of treatment | |||
| IV infusion | 4 mg/kg | 2 | |
| Oral (tablet) | 200 mg | 2 | |
| Treatment duration within hospital, days | IV infusion | Oral | |
|---|---|---|---|
| ISAV | 8.1 | 4.9 | SECURE clinical trial data [17] |
| VORI | 8.9 | 6.1 |
| Drug cost (WAC) | Cost per unit | ||
|---|---|---|---|
| ISAV | ReadyPrice® (Thomson) [23] | ||
| IV infusion (372 mg) | $238.50 | ||
| Oral (tablet) (186 mg) | $70.00 | ||
| VORI (generic) | |||
| IV infusion (200 mg) | $122.07 | ||
| Oral (tablet) (200 mg) | $35.05 | ||
| Hospitalization costa | |||
|---|---|---|---|
| Per diem cost of hospitalization related to IA | $2898.31 | HCUP NIS [1] | |
ISAV isavuconazole, VORI voriconazole, IV intravenous, HCUP NIS Healthcare Cost and Utilization Project National Inpatient Sample
aInflated to 2015 USD
The model considered grade III and IV AEs whose overall incidence significantly differed between isavuconazole- and voriconazole-treated patients in the SECURE trial, i.e., eye disorders, hepatobiliary disorders, and skin disorders (individually described in ESM Appendix 1) [21].
Dosing Frequency and Duration Inputs
The dosing schedules for both voriconazole and isavuconazole were based on the doses administered in the SECURE trial [17], and they are consistent with their respective product labels [16, 22]. The current model assumed there was no vial wastage. Both isavuconazole and voriconazole were initiated as an IV infusion and could subsequently be converted to an oral formulation as early as day 3, on the basis of physician evaluation. IV voriconazole was dosed on the basis of patients’ weight, while administration of oral voriconazole, IV and oral isavuconazole was based on fixed dosing. The model assumed an average patient weight of 75 kg to estimate costs of IV voriconazole, which was the midpoint of the average weight of 82 kg of adults older than 20 years of age in the USA and the mean weight of 69 kg in the SECURE trial [21]. Details regarding the dosing of each treatment arm can be found in Table 1. The total duration of isavuconazole treatment (including IV and oral formulations) reported in the SECURE trial was 47.0 days on average, 8.1 days of which were IV treatment [17]. Only drugs administered within the hospital were considered; thus, the model included the costs of 8.1 days of IV and 4.9 days (13.0–8.1 days) of oral isavuconazole treatment. The total duration of voriconazole treatment was 46.4 days in the SECURE trial, 8.9 days of which were IV treatment [17]. The model considered voriconazole treatment as 8.9 days of IV and 6.1 days (15.0–8.9 days) of oral dosing.
Cost Inputs
Prices of isavuconazole and voriconazole were estimated on the basis of the wholesale acquisition costs (WACs), obtained from ReadyPrice® (Thomson) (Table 1) [23]. Given that generic voriconazole is already available and with a lower price, the price of generic voriconazole was used. The price of 200 mg of voriconazole was $122.07 and $35.05 for the IV and oral formulations, respectively. The price of 372 mg of isavuconazonium sulfate (equivalent to 200 mg of isavuconazole) was $238.50 and $70.00 for the IV and oral formulations, respectively.
The per diem cost of hospitalization due to IA was estimated at $2898.31, using the 2013 HCUP Nationwide Inpatient Sample (NIS), a publicly available database created and maintained by the Agency for Healthcare Research and Quality (AHRQ) [1]. The same per diem cost of hospitalization was assumed for initial hospitalizations and readmissions (Table 1). All costs were inflated to 2015 USD [24].
The costs of managing AEs were estimated using published literature [25–37]. For acute AEs, the cost of managing the entire AE episode was included and this cost was assumed to be equal among the treatment arms. For AEs which could last beyond the initial hospitalization, the cost per AE reported in literature (typically reported as an annual cost) was prorated on the basis of the length of initial hospital stay (i.e., 13 days for isavuconazole and 15 days for voriconazole) for each treatment arm. Prorating AEs this way makes the assumption that those AEs begin on the first day of IA therapy. This assumption is used given the lack of detailed information on time of AE onset and the fact that costs of AE do not have a significant impact on the model’s results. When no literature was available, the cost of each AE was assumed to be the cost of an inpatient consultation of moderate complexity. In the case of hepatobiliary disorders, the cost of a liver function panel was also included.
Deterministic and Probabilistic Sensitivity Analyses (DSA and PSA)
Deterministic sensitivity analysis (DSA) was carried out by increasing and decreasing one parameter at a time while maintaining the rest of the inputs at the base case value. Treatment duration, length of hospitalization (length of stay), readmission rate, per diem cost of hospitalization, drug costs, costs of AE treatment, clinical response rates, and mortality rates were all varied in the DSA to evaluate their impact. In addition, a sensitivity analysis assuming that a US payer perspective was included. This payer perspective included drug and AE costs throughout the total duration of isavuconazole and voriconazole treatment. The total duration was reported by Horn et al. (including inpatient and outpatient) [19]. Another sensitivity analysis assumed equal hospital length of stay, mortality, and clinical response rate among isavuconazole- and voriconazole-treated patients. For this analysis, the average of each input for isavuconazole and voriconazole was used for both treatments. Sensitivity analyses were also conducted by changing only the inputs of isavuconazole to make it less favorable than voriconazole. The parameter inputs were varied on the basis of either the confidence interval, if available, or a predefined percentage of the base case value (ESM Appendix 2).
Probabilistic sensitivity analysis (PSA) was carried out by assuming parametric distributions for each input parameter and resampling each parameter simultaneously from their respective distributions to re-estimate model outputs (ESM Appendix 2). Treatment duration was assumed to follow normal distributions, and mortality and clinical response rates were assumed to follow beta distributions. The standard errors of these parameters were estimated from the SECURE trial. The initial hospitalization length of stay follows a uniform distribution, with the min and max specified as ∓25.0% of the base case value. The per diem cost of hospitalization and the costs of managing AEs were assumed to follow gamma distributions, with standard errors assumed to be equal to one quarter of their respective means. Five-thousand random draws were conducted. PSA was conducted for all three outcomes: incremental cost per patient, incremental cost per death avoided, and incremental cost per clinical responder. For the incremental cost per patient, the results of the PSA were summarized in a cost-minimization acceptability curve based on incremental cost per patient. For the incremental cost per death avoided and the incremental cost per clinical responder, cost-effectiveness acceptability curves were plotted on the basis of net monetary benefit (NMB) values, which were estimated on the basis of willingness to pay threshold × incremental benefit − incremental costs.
Results
The model estimated the total costs per treated patient receiving isavuconazole and voriconazole, the incremental cost per patient treated with isavuconazole vs. voriconazole, the incremental cost per death avoided (isavuconazole vs. voriconazole), and the incremental cost per clinical responder (isavuconazole vs. voriconazole).
Isavuconazole was associated with a lower total cost per patient compared to voriconazole ($44,748.38 vs. $52,166.16). This was a result of lower drug costs ($3571.85 vs. $3869.99), lower costs of AEs ($317.42 vs. $576.74), lower costs of initial hospitalization ($37,678.04 vs. $43,474.66), and lower costs of hospital readmissions ($3181.07 vs. $4244.77) among isavuconazole-treated patients than voriconazole-treated patients. Isavuconazole was dominant (lower costs and greater health benefits) against voriconazole with respect to incremental cost per death avoided and incremental cost per clinical responder (Table 2).
Table 2.
Base case results: costs and cost-effectiveness of ISAV vs. VORI
| Parameter | ISAV | VORI |
|---|---|---|
| Total cost per patient | $44,748.38 | $52,166.16 |
| Total drug cost | $3571.85 | $3869.99 |
| AE cost | $317.42 | $576.74 |
| Initial hospitalization cost | $37,678.04 | $43,474.66 |
| Hospital readmission cost | $3181.07 | $4244.77 |
| Incremental cost per death avoided | ISAV is dominant over VORIa | |
| Incremental cost per additional clinical responder | ISAV is dominant over VORIa | |
ISAV isavuconazole, VORI voriconazole, AE adverse event
a“Dominant” indicates greater health benefits (survival, clinical response) and lower cost
Isavuconazole was predicted to be associated with lower total cost per patient than voriconazole in each DSA except for when the initial hospitalization length was increased by 25.0% for isavuconazole-treated patients and left unchanged for voriconazole-treated patients (Table 3). All other DSAs estimated a lower cost for isavuconazole than voriconazole, ranging from $1411.05 to $17,292.29 savings per patient. The DSA using the US payer perspective (including both inpatient and outpatient drug and AE costs) estimated a cost saving of $4849.26 for isavuconazole-treated patients, compared to voriconazole-treated patients. The DSA assuming equal LOS, mortality, and clinical response for isavuconazole and voriconazole estimated a cost saving of $1411.05 for isavuconazole.
Table 3.
Deterministic sensitivity analysis results, ISAV vs. VORI
| Parameter | Total cost per patient | Incremental cost per death avoided | Incremental cost per clinical responder | |||
|---|---|---|---|---|---|---|
| Low SA input | High SA input | Low SA input | High SA input | Low SA input | High SA input | |
| Changing the inputs for both ISAV and VORI | ||||||
| Treatment duration (IV) | −$7173.91 | −$7661.64 | Dominant | Dominant | Dominant | Dominant |
| Treatment duration (oral) | −$7703.00 | −$7132.55 | Dominant | Dominant | Dominant | Dominant |
| Total treatment duration | −$7522.57 | −$7312.98 | Dominant | Dominant | Dominant | Dominant |
| Initial hospital length of stay (±25.0%) | −$6161.21 | −$8674.34 | Dominant | Dominant | Dominant | Dominant |
| Initial hospital length of stay (mean values) | −$2200.00 | Dominant | Dominant | |||
| Readmission length of stay | −$7151.85 | −$7683.70 | Dominant | Dominant | Dominant | Dominant |
| Readmission rate | −$7327.91 | −$7484.42 | Dominant | Dominant | Dominant | Dominant |
| Per diem cost of hospitalization | −$5702.70 | −$9132.86 | Dominant | Dominant | Dominant | Dominant |
| Total cost of AEs | −$7352.95 | −$7482.61 | Dominant | Dominant | Dominant | Dominant |
| Drug prices (IV and oral) | −$7343.24 | −$7492.31 | Dominant | Dominant | Dominant | Dominant |
| Payer perspective | −$4833.26 | Dominant | Dominant | |||
| Equal initial length of stay, mortality rate, and clinical response rate | −$1411.05 | – | – | |||
| Mortality rate | – | – | Dominant | Dominant | Dominant | Dominant |
| Clinical response rate | – | – | Dominant | Dominant | Dominant | Dominant |
| Changing the inputs for ISAV only | ||||||
| Initial hospital length of stay | −$17,295.94 | $2460.39 | Dominant | $158,459.35 | Dominant | $143,398.88 |
| Readmission rate | −$8216.44 | −$6477.45 | Dominant | Dominant | Dominant | Dominant |
| Mortality rate | – | – | Dominant | $198,126.42 | – | – |
| Clinical response rate | – | – | – | – | $106,054.92 | Dominant |
ISAV isavuconazole, VORI voriconazole, IV intravenous, AE adverse event
DSAs for incremental cost per death avoided and incremental cost per clinical responder showed isavuconazole was dominating against voriconazole in all but two cases: when the initial hospitalization length increased by 25.0% for isavuconazole-treated patients but remained unchanged for voriconazole-treated patients, and when the relevant efficacy outcome, i.e., 42-day mortality rate or clinical response rate, changed unfavorably for isavuconazole but remained unchanged for voriconazole (making isavuconazole worse than voriconazole).
In all PSA simulations of the total cost per patient, the incremental cost per patient (comparing isavuconazole vs. voriconazole) was less than $50,000 (ESM Appendix 3). In 80.2% of those simulations, isavuconazole was associated with a lower total cost per patient than voriconazole. At a willingness to pay (WTP) of $50,000 per death avoided, 82.4% of simulations found isavuconazole to be cost-effective compared to voriconazole on the basis of NMB values (ESM Appendix 3). Similar results were seen when the cost per clinical responder was considered. At a WTP of $50,000 per clinical responder, 81.8% of simulations indicated isavuconazole to be cost-effective compared to voriconazole on the basis of NMB values (ESM Appendix 3).
Discussion
The current standard of care for the treatment of IA is voriconazole. However, isavuconazole, a novel triazole, was recently introduced in the USA for the treatment of IA. An appealing alternative treatment option, isavuconazole has comparable efficacy as voriconazole and isavuconazole is well tolerated compared with voriconazole, with fewer drug-related adverse events. Findings from this economic evaluation show that isavuconazole is associated with lower costs per treated patient and is a cost-effective option compared to voriconazole for the treatment of IA. Isavuconazole dominates voriconazole in terms of incremental cost per additional death avoided and per additional clinical responder.
In our analyses, the difference in the cost of hospitalization was the main driver of the difference in total costs between isavuconazole and voriconazole, contributing to 92.0% of the difference in total cost. Isavuconazole-treated patients were associated with a shorter duration of initial stay and lower rate of readmission compared to voriconazole-treated patients, leading to a lower hospitalization cost. Isavuconazole’s shorter duration of treatment and fewer doses per day from day 3 onward led to lower isavuconazole drug cost, compared to voriconazole, despite a higher drug cost per day. Isavuconazole’s lower incidence rates of AEs also translated into additional cost saving. As a result, the use of isavuconazole may lead to a cost saving of $7417.78 per patient compared to voriconazole.
The study results were robust. In most sensitivity analyses, isavuconazole was associated with lower cost and greater health benefits (dominating scenario) compared to voriconazole. Only in situations where the initial length of hospital stay, mortality rate, and clinical response rate were made wholly unfavorable for isavuconazole (while maintaining voriconazole inputs) was isavuconazole no longer associated with cost savings or dominance, compared to voriconazole. Given the fact that isavuconazole is associated with numerically shorter length of stay, lower mortality, and higher response rates compared to voriconazole in the SECURE trial, these worst-case scenarios seem highly unlikely [17]. Even when LOS, mortality rate, and clinical response rate were assumed to be equal, isavuconazole was still associated with a lower cost per patient owing to the lower rate of readmission and associated readmission costs. In about 80.0% of the PSA simulations, isavuconazole is less costly than voriconazole; in the majority of the simulations (approx. 82% of cases), isavuconazole is also more cost-effective than voriconazole at a WTP of $50,000 per additional outcome.
No prior studies have compared the cost-effectiveness of isavuconazole vs. voriconazole in the treatment of IA; however, there are multiple studies comparing voriconazole to other IA treatments [13, 38]. Those studies have concluded that voriconazole is the preferred therapy for IA in terms of cost-effectiveness. The current study utilized a similar methodology as these prior studies and found that isavuconazole is associated with a lower cost per patient and dominates voriconazole in cost-effectiveness analysis for the treatment of IA in the hospital setting.
The results of this study demonstrate that isavuconazole is a valuable addition to the IA armamentarium. The efficacy of isavuconazole is similar to that of voriconazole. Isavuconazole, however, is associated with a lower total cost per patient and is cost-effective compared to voriconazole. In the USA, where the cost of healthcare is steadily increasing, cost-effectiveness analyses are increasingly important in helping healthcare decision-makers to evaluate different reimbursement decisions and in helping physicians and patients make the optimal selection of therapy for a given condition. Given that IA imposes a substantial economic and clinical burden on society [8, 9], optimizing the treatment of IA by evaluating the cost-effectiveness of alternative treatment options could help improve the care and reduce the overall healthcare spending. Evidence from the current study could support payers and physicians in the process of therapy selection for the treatment of IA by providing valuable information for effective decision-making.
Limitations
This economic evaluation is subject to several limitations. First, the model did not consider costs of treating any underlying conditions. The costs associated with treating underlying diseases can be substantial and may vary greatly by disease condition. There is, however, no evidence that treatment with isavuconazole or voriconazole could impact the costs of underlying disease conditions. Second, in order to make an informed comparison of first-line treatment with isavuconazole vs. voriconazole, no subsequent therapy was considered. The results of the SECURE trial indicated that the clinical response rate was numerically higher (not statistically significant) with isavuconazole than with voriconazole treatment; therefore, not including subsequent treatment is expected to be conservative against the isavuconazole arm, where a lower proportion of patients might receive subsequent therapy and incur associated costs. Additionally, although clinical trials are the gold standard for determining the comparative efficacy of alternative treatments, they often have restrictive inclusion and exclusion criteria and thus patients in the trial may lack the heterogeneity presented in real-world clinical practice. Finally, as the perspective of this study was that of a US hospital (and US payer, in sensitivity analysis), our results may not be generalizable to other countries with different healthcare systems, practices, and prices.
Conclusions
This economic evaluation shows that isavuconazole may be a cost-effective option compared to voriconazole for the treatment of IA in the hospital setting. Isavuconazole was associated with a lower total cost per patient and dominated voriconazole in terms of incremental costs per additional death avoided and cost per additional clinical responder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Sponsorship, article processing charges, and the open access charge for this study were funded by Astellas Pharma Global Development, Inc. The study sponsor was involved in all stages of the study research and manuscript preparation, but all authors participated in the design of the study and contributed to manuscript development. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. The model was developed by Analysis Group, Inc. and analyzed and interpreted in collaboration with all authors. Manuscript drafts were prepared by the authors with editorial assistance provided by Ana Bozas, PhD, a professional medical writer employed by Analysis Group, Inc. and funded by Astellas Pharma Global Development Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All the authors made the decision to submit the manuscript for publication.
Disclosures
Rachel Harrington is an employee of Astellas Pharma Global Development, Inc. Edward Lee is an employee of Astellas Pharma Global Development, Inc. James R Spalding is an employee of Astellas Pharma Global Development, Inc. Nkechi Azie was an employee of Astellas Pharma Global Development, Inc. at the time of the study. Hongbo Yang is an employee of Analysis Group Inc. Jin Wei is an employee of Analysis Group Inc. Andrew Messali is an employee of Analysis Group Inc. Erice Wu is an employee of Analysis Group Inc. Analysis Group Inc. received research funding from Astellas Pharma Global Development Inc.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. The model relied only on the summary statistics from the SECURE trial (ClinicalTrials.gov identifier NCT00412893) and patient level data was not used. Institutional review board (IRB) review was not needed.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
A synopsis of the current research was presented in poster format at the AMCP Nexus 2015 meeting, which took place in Orlando, Florida, during October 26–29, 2015.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/0717F06062D4B7B0.
References
- 1.HCUPnet: National and regional estimates on hospital use for all patients from the HCUP National Inpatient Sample (NIS). 2013 [cited 2015 November 30, 2015]. Available from: http://hcupnet.ahrq.gov/.
- 2.Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32(3):358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 4.Perkhofer S, Lass-Florl C, Hell M, et al. The Nationwide Austrian Aspergillus Registry: a prospective data collection on epidemiology, therapy and outcome of invasive mould infections in immunocompromised and/or immunosuppressed patients. Int J Antimicrob Agents. 2010;36(6):531–536. doi: 10.1016/j.ijantimicag.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(7):909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 6.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44(4):531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 7.Taccone FS, Van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care. 2015;19:7. [DOI] [PMC free article] [PubMed]
- 8.Kim A, Nicolau DP, Kuti JL. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses. 2011;54(5):e301–e312. doi: 10.1111/j.1439-0507.2010.01903.x. [DOI] [PubMed] [Google Scholar]
- 9.Tong KB, Lau CJ, Murtagh K, Layton AJ, Seifeldin R. The economic impact of aspergillosis: analysis of hospital expenditures across patient subgroups. Int J Infect Dis. 2009;13(1):24–36. doi: 10.1016/j.ijid.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Wilson LS, Reyes CM, Stolpman M, Speckman J, Allen K, Beney J. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5(1):26–34. [DOI] [PubMed]
- 11.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 12.Denning DW. Invasive Aspergillosis. Clin Infect Dis. 1998;26(4):781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 13.Krueger KP, Nelson AC. Economic considerations in the treatment of invasive aspergillosis: a review of voriconazole pharmacoeconomic studies. Clinicoecon Outcomes Res. 2009;1:35–43. doi: 10.2147/CEOR.S4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikulska M, Novelli A, Aversa F, et al. Voriconazole in clinical practice. J Chemother. 2012;24(6):311–327. doi: 10.1179/1973947812Y.0000000051. [DOI] [PubMed] [Google Scholar]
- 15.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–415. doi: 10.1056/NEJMoa020191. [DOI] [PubMed] [Google Scholar]
- 16.Astellas Pharma U.S. Inc. CRESEMBA® (isavuconazonium sulfate). 2015; 1–28]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500s001,207501s001lbl.pdf.
- 17.Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387:760–9. [DOI] [PubMed]
- 18.Thompson GR, Wiederhold NP. Isavuconazole: a comprehensive review of spectrum of activity of a new triazole. Mycopathologia. 2010;170(5):291–313. doi: 10.1007/s11046-010-9324-3. [DOI] [PubMed] [Google Scholar]
- 19.Horn D, Goff D, Khandelwal N, et al. Hospital resource use of patients receiving isavuconazole vs voriconazole for invasive mold infections in the phase III SECURE trial. J Med Econ. 2016;19(7):728–734. doi: 10.3111/13696998.2016.1164175. [DOI] [PubMed] [Google Scholar]
- 20.Data on file. Analysis using Premier data to estimate the length of stay for 30-day readmission among patients who were initially hospitalized with invasive aspergillus infection. Astellas Pharma U.S. Inc.
- 21.Data on file. SECURE trial Clinical Data. Astellas Pharma U.S. Inc.
- 22.Pfizer I. VFEND (voriconazole) Prescribing Information. 2015 [cited 2015 November 30]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=618.
- 23.ReadyPrice. 2009 [cited 2016 April 11]. Available from: http://www.rubali.com/thomsonreuters/drug_information/index.php?frame=ready_price.html.
- 24.Consumer Price Index—All Urban Consumers. US Medical Care Services [cited 2016 April 11]. Available from: http://data.bls.gov/cgi-bin/surveymost?cu.
- 25.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55(3):490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45(2):e17–e24. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 27.Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic urticaria: a cost analysis of 50 nonimmunosuppressed patients. Arch Dermatol. 2008;144(1):35–39. doi: 10.1001/archdermatol.2007.5. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Stephens JM, Carter JA, Haider S, Rustgi VK. Impact of adverse events on costs and quality of life in protease inhibitor-based combination therapy for hepatitis C. Expert Rev Pharmacoecon Outcomes Res. 2012;12(3):335–343. doi: 10.1586/erp.12.10. [DOI] [PubMed] [Google Scholar]
- 29.Johner A, Raymakers A, Wiseman SM. Cost utility of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Surg Endosc. 2013;27(1):256–262. doi: 10.1007/s00464-012-2430-1. [DOI] [PubMed] [Google Scholar]
- 30.Koberlein J, Beifus K, Schaffert C, Finger RP. The economic burden of visual impairment and blindness: a systematic review. BMJ Open. 2013;3(11):e003471. doi: 10.1136/bmjopen-2013-003471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122(5):1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 32.Schackman BR, Haas DW, Becker JE, et al. Cost-effectiveness analysis of UGT1A1 genetic testing to inform antiretroviral prescribing in HIV disease. Antivir Ther. 2013;18(3):399–408. doi: 10.3851/IMP2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udeh BL, Schneider JE, Ohsfeldt RL. Cost effectiveness of a point-of-care test for adenoviral conjunctivitis. Am J Med Sci. 2008;336(3):254–264. doi: 10.1097/MAJ.0b013e3181637417. [DOI] [PubMed] [Google Scholar]
- 34.Xakellis GC, Frantz R. The cost of healing pressure ulcers across multiple health care settings. Adv Wound Care. 1996;9(6):18–22. [PubMed]
- 35.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379–387. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 36.Yun HR, Bae SC. Cost-effectiveness analysis of NSAIDs, NSAIDs with concomitant therapy to prevent gastrointestinal toxicity, and COX-2 specific inhibitors in the treatment of rheumatoid arthritis. Rheumatol Int. 2005;25(1):9–14. doi: 10.1007/s00296-003-0392-2. [DOI] [PubMed] [Google Scholar]
- 37.Shadick NA, Liang MH, Phillips CB, Fossel K, Kuntz KM. The cost-effectiveness of vaccination against Lyme disease. Arch Intern Med. 2001;161(4):554–561. doi: 10.1001/archinte.161.4.554. [DOI] [PubMed] [Google Scholar]
- 38.Al-Badriyeh D, Heng SC, Neoh CF, Slavin M, Stewart K, Kong DC. Pharmacoeconomics of voriconazole in the management of invasive fungal infections. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):623–636. doi: 10.1586/erp.10.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
A synopsis of the current research was presented in poster format at the AMCP Nexus 2015 meeting, which took place in Orlando, Florida, during October 26–29, 2015.