Abstract
Objective Recently, tenofovir disoproxil fumatate (TDF)-related side effects, such as renal nephrotoxicity and reduction of bone mineral density, have been reported. Consequently, increased switching from fixed-dose tablet TDF and emtricitabine (TDF/FTC) to abacavir and lamivudine (ABC/3TC) has occurred. Interestingly, while TDF has a lipid-lowering property, one of the ABC-related side effects is hyperlipidemia. Therefore, such switching could cause lipid elevation. To evaluate the change in lipid levels associated with switching from TDF/FTC to ABC/3TC in virologically-suppressed human immunodeficiency virus (HIV)-infected patients.
Methods This is a retrospective, single-center study. We included the HIV-infected patients whose therapy included a drug switch from TDF/FTC to ABC/3TC between September 2009 and December 2012 at Ryukyu University Hospital. The exclusion criteria were HIV-RNA >40 copies/mL on the switching day, and a documented therapy change to a lipid-lowering agent or any other antiretroviral agents within 3 months before or after switching. We compared the low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), and triglyceride (TG) levels before switching to three months after.
Results A total of 18 patients met the inclusion criteria. The LDL, HDL, and TC levels significantly increased three months following the switch (p<0.05), with median (interquartile range) values of 17 (7, 32), 6 (2, 13), and 27 (10, 45) mg/dL, respectively. The TG values did not markedly change.
Conclusion Switching from TDF/FTC to ABC/3TC resulted in significantly increased LDL, HDL, and TC levels.
Keywords: tenofovir disoproxil fumatate, abacavir, antiretrovirus therapy, dyslipidemia, human immunodeficiency virus
Introduction
Since the introduction of combination antiretroviral therapy (cART), the mortality and prognosis of human immunodeficiency virus (HIV)-infected patients have improved (1-3). At the same time, the rates of acute myocardial infarction (AMI) and cardiovascular risk factors have also increased compared with the rates in non-HIV patients (4). Dyslipidemia remains an independent risk factor for cardiovascular disease in HIV-infected patients (4). Therefore, lipid management is an important consideration in the cardiac risk modification strategies for the long-term care of HIV patients.
The recommended initial therapy for HIV infection consists of two nucleoside reverse transcriptase inhibitors (NRTIs) combined with a third antiretroviral agent. Among NRTI combinations, the international treatment guidelines list tenofovir disoproxil fumatate/emtricitabine (TDF/FTC) as the preferred regimen and abacavir sulfate/lamivudine (ABC/3TC) as an alternative, except when combined with doltegravir (5,6). TDF/FTC has superior virologic response to ABC/3TC in patients with a high viral load, and ABC may cause hypersensitivity reactions (7).
However, side effects associated with the long-term use of TDF have been appeared. A meta-analysis concluded that TDF use was associated with a modest loss of renal function, as evidenced by an average reduction in creatinine clearance of 3.9 mL/min (8). The risk was even greater in Japanese patients in particular, in whom the reduction was about four times as high, with an average glomerular filtration rate of 17 mL/min/1.73 m2 (8,9). Given that a low body weight has also been identified as an independent risk factor for tenofovir-associated renal dysfunction (10), the difference between the Japanese and European populations in rates of tenofovir-related renal dysfunction was largely attributed to differences in body weight. Additionally, TDF is associated with a reduction in bone mineral density and increased risk of osteoporotic fracture (11,12). However, the prevalence of HLA-B*5701, which has a high correlation with ABC-related severe hypersensitivity, is 0.1% in Japanese, which is lower than that in other races (13,14). This high risk of renal failure and osteoporotic fracture and low risk of hypersensitivity has therefore prompted switching from TDF/FTC to ABC/3TC in Japan.
Interestingly, TDF has a lipid-lowering property (15-18). Withdrawing TDF from cART regimens has been shown to significantly increase total cholesterol (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels by mean degrees of 19, 4, and 11 mg/dL, respectively, over 12 weeks (17). Conversely, ABC might increase the lipid levels. Indeed, according to a Japanese post-marketing survey of ABC/3TC, the incidence of major lipid-related adverse events are 9.5% for hyperlipidemia, 3.4% for hypertriglyceridemia, and 2.2% for an increase in blood triglyceride levels (19). However, to our knowledge, no study has yet examined the incremental fluctuations in lipid levels as a result of switching therapies.
It is therefore regarded that switching TDF/FTC to ABC/3TC would increase the lipid level by abolishing the lipid lowering effect of TDF and by inducing a lipid increasing effect of ABC. However, to the best of our knowledge, no prior study has measured the incremental changes which occur as a result of a change in therapy.
Objective
We herein evaluated the change in lipid levels associated with switching from TDF/FTC to ABC/3TC in virologically-suppressed HIV-infected patients.
Materials and Methods
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and national and institutional standards and was approved by the Ethics Committee of Ryukyu University (No. 721).
Study design
This is a retrospective, single-center study. We compared LDL, HDL, TC, and triglyceride (TG) levels before switching and three months after. The baseline data were obtained from the records compiled on the blood sampling day closest to the ABC/3TC initiation. The later data were recorded as “post-three months” and were obtained on the blood sampling day closest to 90 days after switching. To investigate the existence of possible external factors, we also compared LDL, HDL, TC, and TG changes from baseline to post-three months in a control group whose medication was consistently maintained.
Study patients
We evaluated HIV-infected patients switched from a TDF/FTC to an ABC/3TC regimen between September 2009 and December 2012 at Ryukyu University Hospital. The exclusion criteria were HIV-RNA >40 copies/mL on the day of switching and a documented therapy change to a lipid-lowering agent or any other antiretroviral agents within 3 months before or after switching. We also included patients who remained on TDF/FTC therapy as controls, applying the same exclusion criteria described above. These groups were referred to as the “switching” and “non-switching” groups, respectively.
Statistical analyses
The baseline characteristics between the switching and non-switching groups were compared using Fisher's exact test or Mann-Whitney's U test. The p value for intervals from baseline between the two groups were determined using Mann-Whitney's U test. The p value for comparison between the blood concentrations of lipid parameters at baseline and those at post-three months in the switching group were determined using Wilcoxon signed-rank test. The p value for comparison of the changes in lipid levels between the two groups were determined using Mann-Whitney's U test. The proportions of subjects outside the Japan Atherosclerosis Society (JAS) (2012)-recommended lipid levels at baseline were compared with the proportions at post-three months using Fisher's exact test. All of the analyses used a two-sided alpha of 0.05. The statistical analyses were performed using the SPSS Statistics software program, ver. 21 (IBM).
Results
A total of 27 patients switched from TDF/FTC to ABC/3TC between September 2009 and December 2012 at Ryukyu University Hospital, and 18 of these were included in this analysis, with 9 excluded (3 for changing to a third antiretroviral agent in the period of three months before switching and three month after, 2 for HIV-RNA >40 copies/mL at baseline, 2 who discontinued ABC/3TC due to nausea, 1 who reduced the dose of mevalostatin at baseline, and 1 who added another antiretroviral agent after switching).
The patient baseline characteristics are shown in Table 1. In the switching group, all of the patients were Japanese except for one Caucasian, the median duration of TDF/FTC use was 48 months, and the median CD4+ cell count was 551 /μL. No significant differences were noted between the groups for any baseline characteristic. Table 2 shows the actual interval of blood sampling between the baseline and the post-three months measurement. The interval in the switching group had a median (interquartile range) of 88 (84-98) days. There were no significant differences in this interval between the groups.
Table 1.
Baseline Characteristics.
| Switch group (n=18) | non-switch group (n=19) | p value | |||
|---|---|---|---|---|---|
| Male | 18 | 19 | 1.000 | ||
| Median age, years | 44 | ( 38 - 68) | 39 | ( 24 - 54) | 0.423 |
| Japanese, (%) | 17 | ( 94 ) | 19 | 1.000 | |
| Median weight, kg | 67 | ( 59 - 76) | 67 | ( 50 - 89) | 0.423 |
| Median TDF/FTC use, months (IQR) | 48 | ( 24 - 64) | 33 | ( 14 , 63) | 0.463 |
| Median CD4+cells, /µL (IQR) | 551 | ( 347 , 690) | 568 | ( 507 , 754) | 0.525 |
| CD4+ cells bellow 200 /µL(%) | 2 | ( 11 ) | 2 | ( 11 ) | 1.000 |
| Comorbidity | |||||
| Dyslipidemia, (%) | 12 | ( 67 ) | 10 | ( 53 ) | 0.508 |
| Type II diabetes mellitus, (%) | 0 | ( 0 ) | 2 | ( 11 ) | 0.486 |
| HCV infection, (%) | 2 | ( 11 ) | 1 | ( 5 ) | 0.604 |
| Hemophilia B, (%) | 2 | ( 11 ) | 0 | ( 0 ) | 0.230 |
| Smoker, (%) | 8 | ( 44 ) | 8 | ( 42 ) | 1.000 |
| Third antiretroviral agent, (%) | |||||
| RAL | 8 | ( 44 ) | 9 | ( 47 ) | 1.000 |
| EFV | 6 | ( 33 ) | 6 | ( 32 ) | 1.000 |
| ATV/r | 2 | ( 11 ) | 2 | ( 11 ) | 1.000 |
| LPV/r | 1 | ( 6 ) | 1 | ( 5 ) | 1.000 |
| DRV/r | 1 | ( 6 ) | 1 | ( 5 ) | 1.000 |
| Stable lipid-lowering treatments at baseline, (%) | |||||
| Statins | 1 | ( 6 ) | 1 | ( 5 ) | 1.000 |
| Fibrates | 2 | ( 11 ) | 1 | ( 5 ) | 0.604 |
| Median lipid parameters, mg/dL (IQR) | |||||
| LDL | 92 | ( 81 , 105) | 97 | ( 83 , 107) | 0.456 |
| HDL | 43 | ( 36 , 49) | 41 | ( 37 , 50) | 0.860 |
| TG | 166 | ( 100 , 212) | 126 | ( 109 , 159) | 0.284 |
| TC | 159 | ( 143 , 178) | 157 | ( 146 , 172) | 0.203 |
p values for comparison between the switch group and the non-switch group are determined by Fisher’s exact tests or by Mann-Whitney’s U tests. Abbreviations: IQR: interquartile range, HCV: hepatitis C virus, TDF/FTC: tenofovir/emtricitabine, RAL: raltegravir, EFV: efavirenz, ATV: atazanavir, /r: ritonavir, LPV: lopinavir, DRV: darunavir, LDL: low-density lipoprotein, HDL: high-density lipoprotein, TG: triglyceride, TC: total cholesterol
Table 2.
Intervals from Baseline.
| Median Days (interquartile range) | p value | ||
|---|---|---|---|
| switch group | non-switch group | ||
| Post three months | 88 ( 84, 98) | 84 ( 84, 91) | 0.822 |
Post three months is defined as the closest day to post 90 days from the baseline measurement. p value for comparison between the switch group and the non- switch group is determined by Mann-Whitney's U test.
The lipid parameters at the baseline and post-three months in the switching group are shown in Table 3. A significant increase was noted in the LDL, HDL, and TC levels three months after switching. Table 4 shows the lipid changes from baseline to post-three months in both groups. The changes in the LDL, HDL, and TC levels were significantly greater in the switching group than in the non-switching group, although no marked differences were noted in the TG levels between the groups.
Table 3.
Lipid Parameters at Baseline and Post Three Months in Patients who Switched TDF/FTC to ABC/3TC.
| Median (IQR) n=18 | ||||||
|---|---|---|---|---|---|---|
| Baseline | Post three months | p values | ||||
| LDL, mg/dL | 92 | ( 82 , 105) | 114 | ( 97 , 137) | < 0.001 | |
| HDL, mg/dL | 43 | ( 36 , 49) | 48 | ( 41 , 54) | 0.001 | |
| TG, mg/dL | 166 | ( 100 , 212) | 142 | ( 111 , 177) | 0.948 | |
| TC, mg/dLα | 159 | ( 143 , 178) | 184 | ( 174 , 218) | 0.009 | |
p values for comparison between blood concentrations of lipid parameters at baseline and those of at post three months are determined by Wilcoxon signed-rank tests. Baseline is defined as the first day when ABC/3TC.was prescribed. Abbreviations: IQR: interquartile range, TDF: tenofovir, FTC: emtricitabine, ABC: abacavir, 3TC: lamivdine, α TC n=12
Table 4.
Lipid Change from Baseline at Post Three Months.
| Median (IQR) | ||||||
|---|---|---|---|---|---|---|
| switch group (n=18) | non-switch group (n=19) | p | ||||
| LDL, mg/dL | 17 | ( 6 , 31) | -6 | ( -11 , -1) | < 0.001 | |
| HDL, mg/dL | 6 | ( 2 , 13) | 1 | ( -3 , 5) | 0.034 | |
| TG, mg/dL† | -20 | ( -61 , 31) | -3 | ( -20 , 20) | 0.563 | |
| TC, mg/dL‡ | 27 | ( 10 , 45) | -1 | ( -10 , 9) | 0.002 | |
p values for comparison between the switch group and the non-switch group in lipid changes are detemined by Mann-Whitney’s U tests. Abbreviations: IQR: interquartile range, LDL: low-density lipoprotein, HDL: high-density lipoprotein. TG: triglyceride, TC: total cholesterol.
† TG n=18 in the non-switch group.
‡ TC n=12 in the switch group, whereas n=15 in the non-swtich group.
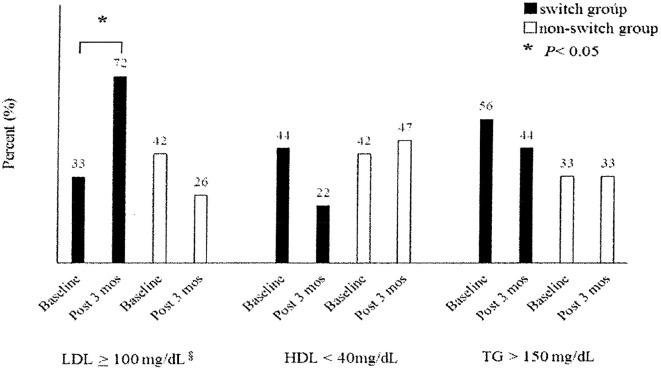
In the switching group, the LDL, HDL, and TC levels increased by a median (interquartile range) of 17 (7, 32), 6 (2, 13), and 27 (10, 45) mg/dL, respectively. The Figure shows the proportion of subjects outside the JAS 2012 recommended lipid level. In the switching group, the proportion of patients with an LDL level ≥100 mg/dL at post-three months was significantly higher than at baseline. The proportion of patients with an HDL level <40 mg/dL at post-three months was lower than at baseline, but not significantly.
Figure.
Proportion of subjects outside JAS (2012) recommended lipid level. Fisher’s exact tests were applied to compare proportions at baselines with those of post three months. JAS: Japan Atherosclerosis Society, LDL: low-density lipoprotein, HDL: high-density lipoprotein, TG: triglyceride. § recommended as secondary prevention of coronary artery disease.
Discussion
To the best of our knowledge, this is the first study evaluating the lipid changes associated with switching from TDF/FTC to ABC/3TC in virologically-suppressed HIV-infected patients. In our results, the LDL, HDL, and TC levels significantly increased three months after switching by median (interquartile range) values of 17 (7, 32), 6 (2, 13), 27 (10, 45) mg/dL, respectively. However, the TG levels did not significantly change.
To investigate the potential influence of external factors, such as recovery from low immunity or malnutrition and diet education in outpatient care, we compared the changes in the lipid levels of patients who switched to the changes in those who continuously used TDF/FTC. No significant differences in any baseline characteristic were noted between these groups. The changes in the LDL, HDL, and TC levels-but not the TG levels - were significantly greater in the switching group than in the non-switching group. We therefore concluded that no external factors had affected our results.
Our findings regarding the changes (or lack thereof) in TC, LDL, HDL, and TG levels agreed with the previous study, which withdrew TDF from cART (17). While the absolute values for the changes in lipids in our study were greater than those in the study that only withdrew TDF from cART, we were unable to compare the fluctuation range between the studies because the preceding study lacked any data on basic statistics, such as the population variance (17).
Observational and clinical studies conducted in Japan have shown that the likelihood of recurrence of cardiovascular disease decreases in association with a decrease in the LDL level to 100 mg/dL (20,21). Many studies have shown that an HDL level <40 mg/dL is associated with an increased risk of cardiovascular disease compare to a level ≥40 mg/dL (21-26). In the present study, not only TC and LDL levels but also HDL levels were increased when TDF/FTC was switched to ABC/3TC. In addition, the proportion of patients with LDL levels ≥100 mg/dL significantly increased after switching. In contrast, the proportion of patients with HDL levels <40 mg/dL decreased, but not significantly. This indicates that the increase in the HDL level was not enough to exceed the threshold of 40 mg/dL. Therefore, the increase in lipid levels with drug switching increased the risk of recurrence of cardiovascular disease in our study population.
Several limitations associated with the present study warrant mention. First, our study was retrospective. To reduce the risk of any biases, we initially included all of the patients who switched from TDF/FTC to ABC/3TC and then excluded the inappropriate patients. Second, the number of analyzed patients was small. Our results may therefore not apply to the general population. Third, some patients may have eaten a meal before the blood sampling. Although we attempted to only examine fasting blood samples from HIV-infected outpatients, since we did not record the meal intake status before the sampling, fasting blood sampling might not have been strictly observed in all of the patients. The results from non-fasting blood sampling might have affected our findings, mainly via an observed increase in TG levels. Fourth, we could not clarify the degree to which the discontinuation of TDF or addition of ABC contributed to the lipid change. Resolving this issue will require investigating the change in the lipid levels by adding ABC to virologically-suppressed patients whose regimen does not contain ABC. However, administering unnecessary drugs is ethically inappropriate and could cause adverse events or worsen adherence. In clinical practice, we most often switch TDF/FTC to ABC/3TC since both are fixed-dose tablets. As such, investigating the degree to which each substance contributes to the lipid change is likely not actually meaningful.
A previous study showed the lipid-lowering effects of TDF (15-18) and the incidence of ABC-associated lipid elevation (19). However, our study specifically demonstrated the lipid elevation associated with switching TDF/FTC to ABC/3TC, as well as the fluctuation range. Since HIV-infected patients have increased cardiovascular risk factors and switching from TDF/FTC to ABC/3TC is increasingly common in Japan, thus demonstrating the lipid elevation and the fluctuation range associated with switching therapies is clinically meaningful.
Conclusion
Switching from TDF/FTC to ABC/3TC resulted in an increase in the LDL, HDL, and TC levels.
The authors state that they have no Conflict of Interest (COI).
References
- 1. May MT, Sterne JA, Costagliola D, et al. . HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analyses. Lancet 368: 451-458, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Obel N, Omland LH, Kronborg G, et al. . Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: a population-based nationwide cohort study. PLoS One 6: e22698, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewden C, Chene G, Morlat P, et al. . HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 46: 72-77, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 92: 2506-2512, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Department of Health and Human Services (DHHS). Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, 2015 at: http://AIDSinfo.nih.gov [PubMed] [Google Scholar]
- 6. European AIDS Clinical Society (EACS). Guidelines for treatment of HIV-infected adults in Europe, version 8.0, 2015 at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html [Google Scholar]
- 7. Sax PE, Tierney C, Collier AC, et al. . Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis 204: 1191-1201, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin infect Dis 51: 496-505, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses 25: 387-394, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Nishijima T, Komatsu H, Gatanaga H, et al. . Impact of small body weight on tenofovir-associated renal dysfunction in HIV-infected patients: a retrospective cohort study of Japanese patients. PLoS One 6: e22661, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stellbrink HJ, Orkin C, Arribas JR, et al. . Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 51: 963-972, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Bedimo R, Maalouf NM, Zhang S, Drechsler H, Tebas P. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS 26: 825-831, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Hughes AR, Mosteller M, Bansal AT, et al. . Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics 5: 203-211, 2004. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka H, Akaza T, Juji T. Report of the Japanese Central Bone Marrow Data Center. Clin Transpl 139-144, 1996. [PubMed] [Google Scholar]
- 15. Randell PA, Jackson AG, Zhong L, Yale K, Moyle GJ. The effect of tenofovir disoproxil fumarate on whole-body insulin sensitivity, lipids and adipokines in healthy volunteers. Antivir Ther 15: 227-233, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Tungsiripat M, Kitch D, Glesby MJ, et al. . A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG5206. AIDS 24: 1781-1784, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fabbiani M, Bracciale L, Doino M, et al. . Lipid-lowering effect of tenofovir in HIV-infected patients. J Antimicrob Chemother 66: 682-683, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Di Giambenedetto S, Fabbiani M, Colafigli M, et al. . Safety and feasibility of treatment simplification to atazanavir/ritonavir+lamivudine in HIV-infected patients onstable treatment with two nucleos(t)ide reverse transcriptase inhibitors+atazanavir/ritonavir with virologicalsuppression (Atazanavir and Lamivudine for treatment Simplification, AtLaS pilot study). J Antimicrob Chemother 68: 1364-1372, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of EpzicomⓇ (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan. Pharmacoepidemiol Drug Saf 23: 372-381, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sakamoto T, Kojima S, Ogawa H, et al. . Effects of early statin treatment on symptomatic heart failure and ischemic events after acute myocardial infarction in Japanese. Am J Cardiol 97: 1165-1171, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Mabuchi H, Kita T, Matsuzaki M, et al. . Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simbastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J 66: 1096-1100, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Mabuchi H, Kita T, Matsuzaki M, et al. . Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low-dose simbastatin therapy in Japanese patients with hypercholesterolemia: primary prevention cohort study of the Japan Lipid Intervention Trial (J-LIT). Circ J 66: 1087-1095, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Kitamura A, Iso H, Naito Y, et al. . High-density lipoprotein cholesterol and premature coronary heart disease in urban Japanese men. Circulation 89: 2533-2539, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Satoh H, Nishino T, Tomita K, et al. . Risk factors and the incidence of coronary artery disease in young middle-aged Japanese men: results from a 10-year cohort study. Intern Med 45: 235-239, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Maruyama K, Hirobe K, Noda H, et al. . Associations between blood lipid profiles and risk of myocardial infarction among Japanese male workers: 3M study. J Atheroscler Thromb 16: 714-721, 2009. [DOI] [PubMed] [Google Scholar]
- 26. Noda H, Iso H, Saito I, et al. . The impact of the metabolic syndrome and its components on the incidence of ischemic heart disease and stroke: the Japan public health center-based study. Hypertens Res 32: 289-290, 2009. [DOI] [PubMed] [Google Scholar]