Abstract
Background
Previous studies found conflicting results about whether childhood atopic dermatitis (AD) persists into adulthood.
Objective
We sought to determine persistence rates and clinical factors associated with prolonged AD.
Methods
A systematic review was performed in MEDLINE, EMBASE, Scopus, GREAT, LILACS, Web of Science, Academic Search Complete, and Cochrane Library. Meta-analysis was performed using Kaplan-Meier plots and random-effects proportional hazards regression.
Results
In total, 45 studies including 110,651 subjects spanning 434,992 patient-years from 15 countries were included. In pooled analysis, 80% of childhood AD did not persist by 8 years and less than 5% persisted by 20 years after diagnosis (mean ± SE: 6.1 ± 0.02 years). Children with AD that persisted already for more than 10 years (8.3 ± 0.08 years) had longer persistence than those with 3 (3.2 ± 0.02 years) or 5 (6.8 ± 0.06 years) years of persistence. Children who developed AD by age 2 years had less persistent disease (P < .0001). Persistence was greater in studies using patient-/caregiver-assessed versus physician-assessed outcomes, female versus male patients (P ≤ .0006), but not in those with sensitivity to allergens (P = .90). Three studies found prolonged persistence with more severe AD.
Limitations
Some studies did not capture recurrences later in life.
Conclusions
Most childhood AD remitted by adulthood. However, children with already persistent disease, later onset, and/or more severe disease have increased persistence.
Keywords: atopic dermatitis, eczema, epidemiology, persistence, prognosis
Atopic dermatitis (AD)/eczema is a chronic inflammatory skin disease with a clinical course that varies widely between patients. Some children have disease activity that persists well into adolescence and adulthood, although most children are thought to “grow out of it.” Much of the data to support this notion originate from smaller studies and did not account for a number of clinical factors that might modify disease persistence. Recent studies suggest AD may persist more commonly than previously recognized.1 We sought to systematically analyze the extant literature of birth cohorts and observational studies to determine the rates of disease persistence over time. Moreover, we examined which clinical factors associated with AD persistence may be useful for prognostication in AD.
METHODS
Literature search
We searched the following databases through June 14, 2015: MEDLINE (1946-present), EMBASE (1947-present), Scopus (1966-present), Web of Science (1900-present), Academic Search Complete (1887-present), LILACS (1982-present),GREAT (2000-present), and the Cochrane Library (1992-present). The search strategy was based on a previous Cochrane review2 with inclusion of “persisten*,” “recurren*,” “natural history,” “secular history,” “disease course,” “longitudinal,” “remission,” and “cohort” as additional search terms related to disease persistence (Table I).
Table I.
Literature search scheme for OVID MEDLINE
| 1. explode DERMATITIS, ATOPIC/ |
| 2. atopic dermatitis.mp. |
| 3. dermatitis atopic.mp. |
| 4. explode ECZEMA/or eczema.mp |
| 5. childhood eczema.mp. |
| 6. infantile eczema.mp. |
| 7. neurodermatitis.mp. or exp Neurodermatitis/ |
| 8. Besnier’s prurigo.mp |
| 9. or/1–8 |
| 10. persisten* |
| 11. recurren* |
| 12. natural history |
| 13. secular history |
| 14. disease course |
| 15. longitudinal |
| 16. remission |
| 17. cohort |
| 18. or/10–17 |
| 19. 9 and 18 |
Indicate wildcard characters.
Studies published online, published in print, and in press from all years were considered. All search results with titles and abstracts written in any language were eligible for inclusion. Studies were excluded based on the title, abstract, or both if there was no clear indication they investigated persistence of AD. If data were duplicated in more than 1 study, the most recent and complete study was included in the meta-analysis.
Data extraction
Two reviewers (J. P. K. and L. X. C.) independently performed data extraction from these studies and any differences were resolved by discussion. The following data items were collected: first author; year of publication; study design; how AD was diagnosed; country of study; frequency of initial AD diagnosis; age and frequency of AD at follow-up; history of hypersensitivity to 1 or more allergens; severity of AD; mean age of patients and percent of males in the study. The persistence of AD was stratified by gender, allergen hypersensitivity, and baseline AD severity where available.
Statistical analysis
Statistical analyses were performed using software (SAS, Version 9.4, SAS Institute Inc, Cary, NC).
Survival analyses using the Kaplan-Meier and Cox proportional hazards regression models were used to determine the overall persistence of AD. The time-to-event variable in all models was the time from AD diagnosis to either persistence of AD or censoring. The models accounted for both left and right censoring, ie, AD diagnosis and follow-up could happen at any age or period of observation. For studies that presented age of AD diagnosis or persistence across a range of ages, the median of the age range was selected. Models included a random effect for the study to address potential issues of heterogeneity. In addition, models were stratified by age of AD onset, number of years of already persistent disease, self-report versus physician assessment of AD, sex, and hypersensitivity to 1 or more allergens where available. Median, mean, and SE of mean AD persistence time were estimated. Hazard ratios (HR) (95% confidence intervals [CI]) were calculated for each strata level. A 2-sided P value of .05 was taken as statistically significant.
RESULTS
Literature search
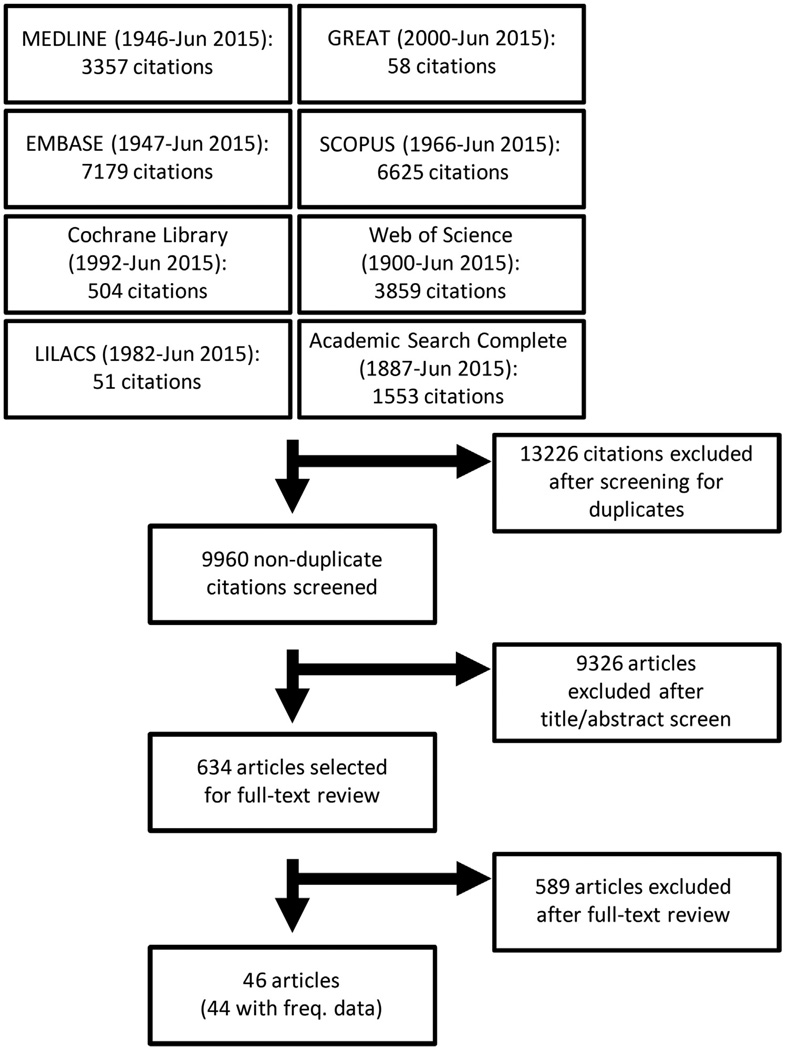
The literature search yielded 9960 nonduplicate articles. After review of the titles and abstracts, 9326 were excluded; an additional 634 articles were excluded after full-text review. In total, 46 studies3–48 were included in the review, of which 44 had valid frequency data that could be pooled, 7 had valid data to assess the effects of gender,6,23,28,31,44,45,48 6 for allergen sensitization,6,17,23,36,40,45 and 3 for baseline AD severity17,28,40 on disease persistence. The Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) flow diagram is presented in Fig 1.
Fig 1.
PRISMA flow diagram of literature search and study selection for meta-analysis of atopic dermatitis persistence.
Study characteristics
The studies were all longitudinal with respect to persistence of AD, either retrospective (n = 3) or prospective (n = 42) data collection, included both males and females, and encompassed subjects of all ages. Published years of studies ranged from 1955 to 2015. The 45 studies included 110,651 subjects spanning 434,992 patient-years from 15 countries (Supplemental Tables I to IV; available at http://www.jaad.org). The age of entry into the cohort, ie, the age of confirmed AD, ranged from 0.04 to 17.5 years, with a mean (SD) of 1.6 ± 1.3 years. AD was diagnosed in 87.7% of subjects by age 5 years. Duration of follow-up ranged from 0.25 to 23.0 years, with a mean (SD) of 3.9 (2.8) years. Duration of follow-up was 5 years or longer in 55.7% of studies and 10 years or longer in 20.8% of studies. All subjects had AD at baseline, of which 80,477 (72.7%) had persistent AD upon follow-up at various time points.
Overall persistence of AD
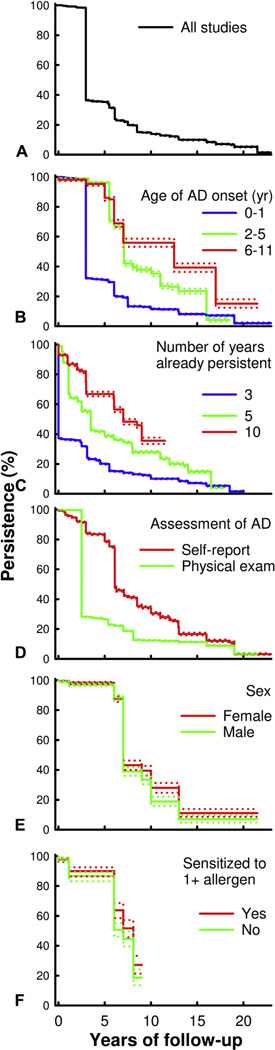
In the pooled analysis, there was a precipitous decline in the overall rates of AD persistence after 3 years of follow-up (Fig 2, A). The median duration of AD persistence was 3.0 years (mean ± SE: 6.1 ± 0.02 years). In addition, persistence rates slowly continued to decrease, such that 80% of AD did not persist by 8 years and less than 5% of AD persisted by 20 years of follow-up.
Fig 2.
Predictors of persistence of atopic dermatitis (AD). Kaplan-Meier survival plots and 95% log-log confidence intervals of AD persistence are presented overall (A) and stratified by age of AD onset (0–1, 2–5, 6–11 years) (B), already persistent AD (3, 5, 10 years) (C), assessment of AD (self-report, physician assessment) (D), sex (male, female) (E), and sensitization to 1 or more allergens (no, yes) (F).
Persistence of AD significantly varied by whether patients already had persistent disease. After 3 years of persistent AD, the mean (SE) additional duration of AD persistence was only 3.2 (0.02) years (median not estimable) (Fig 2, B). In patients with 5 or 10 years of persistent AD, the additional duration of persistence increased (median: 4.0 vs 8.5 years, respectively; mean ± SE: 6.8 ± 0.06 vs 8.3 ± 0.08 years, respectively).
AD persistence by age of disease onset
Persistence of AD significantly varied by age of entry into the cohort, ie, age of first confirmed AD. The median duration of AD persistence was 3.0 years (mean ± SE: 5.8 ± 0.03 years) for subjects who were given a diagnosis of AD at age 0 to 1 year (Fig 2, C). However, the duration of persistent AD increased for subjects with AD onset by ages 2 to 5 years (median: 8.0 years; mean±SE: 10.5±0.09 years), 6 to 11 years (median: 12.5 years; mean ± SE: 14.8 ± 0.2 years), and 12 to 17 years (median not estimable; mean ± SE: 7.5 ± 0.03 years). In proportional hazards regression models, the risk for overall disease persistence was significantly higher in children with AD onset at age 2 to 5 years (HR 2.65; 95% CI 2.54–2.75) and 12 to 17 years (HR 2.04; 95% CI 1.66–2.49) compared with age of onset at 0 to 1 years, and peaked in those with onset at age 6 to 11 years (HR 4.22; 95% CI 3.86–4.61; P < .0001 for all).
AD persistence by self-report
There were significant differences between studies that used self-reported versus physician-assessed measures of AD at follow-up. The duration of AD persistence was significantly higher in studies that used self-reported AD (median: 6.2 years; mean±SE: 9.6 ± 0.06 years) compared with physician-assessed AD (median: 3.0 years; mean ± SE: 5.8 ± 0.03 years) (HR 1.65; 95% CI 1.61–1.69) (Fig 2, D).
AD persistence by gender
There were 7 studies that stratified AD persistence by subjects’ gender.6,23,28,31,44,45,48 The duration of AD persistence was significantly higher in females (median: 9.0 years; mean ± SE: 12.7 ± 0.2 years) compared with males (median: 7.0 years; mean±SE: 11.7 ± 0.2 years) (HR 1.15; 95% CI 1.04–1.27; P = .006) (Fig 2, E).
AD persistence by sensitivity to allergens
There were 6 studies that stratified AD persistence by subjects’ reactivity to skin-prick testing and/or antigen-specific serum IgE.6,17,23,36,40,45 There was no significant difference of AD persistence between those with hypersensitivity to 1 or more allergens (median: 8.1 years; mean ± SE: 8.0 ± 0.1 years) compared with none (median: 7.0 years; mean ± SE: 7.7 ± 0.1 years) (HR 1.11; 95% CI 0.92–1.33; P = .90) (Fig 2, F).
Similar results were found in sensitivity analyses for age of onset, self-reported disease activity, gender, and allergen sensitivity that excluded any retrospective studies.
AD persistence by baseline AD severity
There were 3 studies that stratified AD persistence by subjects’ baseline AD severity,17,28,40 although the inconsistent measures preclude pooling of results for meta-analysis. One study found that children with AD onset at 0 to 2 years of age who had frequent parental-reported scratching were more likely to have persistent AD by age 7 years (81.3% vs 44.5%).17 One study found that children with AD at age 9 to 16 months who had moderate to severe compared with mild disease as judged by Scoring Atopic Dermatitis (SCORAD) were more likely to have persistent AD 6 to 12 years later (SCORAD 25–50: 51.8% and SCORAD[50: 53.8% vs SCORAD < 25: 32.5%).40 Finally, a study found that children with AD onset within the first 3 years of life who had less than or equal to 1 or more than 1 night compared with 0 nights per week of being kept awake by their rash had higher rates of persistent AD 5 years later (52.9% and 66.7% vs 42.0%).28
DISCUSSION
The results of this meta-analysis suggest that, overall, 80% of childhood AD did not persist by 8 years and less than 5% persisted by 20 years after diagnosis. Individuals with more severe disease at the time of diagnosis had increased risk for persistent disease in 3 studies. The longer the AD already persisted, the more likely it was to continue to persist. Moreover, children who developed AD in the first 2 years of life had significantly lower risk of persistent disease than those who developed AD later in childhood or adolescence. Later onset disease might be related to exposure to irritants and contact allergens in personal care products; environmental allergens; or both. However, there were no differences of AD persistence by baseline sensitivity to 1 or more allergens. It is interesting that studies using patient-reported assessment of AD found higher risk of persistent AD compared with physician assessment. This is likely a result of the waxing and waning nature of AD, where disease activity might not be present at clinical or research visits but is present during interval periods. There was a small but significantly increased risk of persistence in females compared with males. There appeared to be more persistent disease in children with more severe AD at baseline; however, the few studies found used different severity scales that did not allow for pooled meta-analysis. Overall, the results suggest that early onset AD does tend to “burn out” and improve by adulthood. However, children with later onset, more persistent AD, and severe AD are much more likely to have prolonged persistence into adolescence and adulthood.
It is important to distinguish between AD persistence and severity. For example, even mild disease can be persistent and severe disease can spontaneously remit. The current study demonstrates that there are distinct predictors of AD persistence. We identified disease severity, older age of onset, and female gender as risk factors for persistence. Moreover, we found that cases of already persistent disease continued to persist. This suggests that the duration of AD persistence may be in a sense programmed from the disease onset. Alternatively, some environmental or behavioral risk factors for persistence may be ongoing and facilitate AD persistence. These factors may be useful for clinicians to predict which patients will have persistent AD. Long-term persistence of AD is an important consideration for therapeutic management of AD. Patients with persistent disease often require a treatment approach that is both effective and well-tolerated over an extended period of time. Future studies are needed to develop validated tools for clinical prediction of AD persistence.
Recent studies have challenged the long-standing dogma that most childhood AD resolved by adulthood. Margolis et al1 assessed 7157 children from the Pediatric Eczema Elective Registry, a phase IV registry of pimecrolimus users, and found that more than 80% of children had persistent disease at all ages and that only by age 20 years did 50% of patients achieve a 6-month disease-free period. The considerably higher rates of AD persistence observed in that study compared with most cohort studies were likely because of inclusion of children with more severe disease at baseline and those who already had persistent disease at enrollment. However, the results of our meta-analysis suggest that overall AD does not persist in most, when including unselected patients from birth cohorts and observational studies.
There are multiple strengths to this meta-analysis, including synthesis of a large number of international studies, including more than 100,000 patients and more than 400,000 patient-years. Kaplan-Meier and proportional hazards regression were used to account for the left and right censoring of study data. Random-effect proportional hazards models were used to address potential issues of heterogeneity. Finally, multiple sensitivity analyses were performed, which revealed important predictors of AD persistence, including age of AD onset, previous persistence of AD, baseline AD severity, and sex. We suggest that future studies stratify AD persistence by these variables to achieve more reliable estimates of AD persistence. However, this study has limitations that should be considered, including inconsistent measures of disease activity among studies. Studies were predominantly prospective with a minority being retrospective. However, there were no major differences in sensitivity analyses that excluded retrospective studies. Longer, more consistent duration of follow-up for all studies would have been preferable. There were only a few studies that stratified AD persistence by gender, sensitization to allergens, and baseline AD severity. Studies that relied on physician assessment of AD might have missed skin disease that had a more sporadic course or was clear on the day of the assessment. This is supported by our finding that AD persistence was higher in studies using self-/patient-reported assessments. Finally, we were not able to assess whether patients had AD recurrence or flares after the observed period of remittance in the study. Thus, it is possible that some of the patients reported to have AD remittance had unobserved recurrences later in life.
In conclusion, only 1 in 5 children with AD had disease persistence beyond 8 years. However, children with already persistent disease, later onset, and more severe disease were more likely to have disease persistence into adolescence and adulthood. Future investigation into risk factors for disease persistence is warranted.
Supplementary Material
CAPSULE SUMMARY.
Previous studies have reported conflicting results regarding the persistence of childhood atopic dermatitis into adulthood.
Only 1 in 5 children with atopic dermatitis had disease persistence beyond 8 years. Children with already persistent disease, later onset, and more severe disease were more likely to have disease persist into adolescence and adulthood.
These risk factors may be useful to predict which children will have persistent atopic dermatitis.
Acknowledgments
This publication was made possible with support from the Agency for Healthcare Research and Quality, grant number K12HS023011, and the Dermatology Foundation. No honorarium, grant, or other form of payment was given to anyone to produce the article.
Abbreviations used
- AD
atopic dermatitis
- CI
confidence interval
- HR
hazard ratio
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Margolis JS, Abuabara K, Bilker W, Hoffstad O, Margolis DJ. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593–600. doi: 10.1001/jamadermatol.2013.10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nankervis H, Pynn EV, Boyle RJ, et al. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev. 2015;1:CD008426. doi: 10.1002/14651858.CD008426.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuzumi T, Kobayashi E, Aoki T, Sasai Y, Kanamori S, Yamada I. The influence of ageing and seasons on infantile eczema - a population-based cohort study of babies aged 4 months and 10 months [in Japanese] Arerugi. 2005;54:455–463. [PubMed] [Google Scholar]
- 4.Sangsupawanich P, Chongsuvivatwong V, Mo-Suwan L, Choprapawon C. Relationship between atopic dermatitis and wheeze in the first year of life: analysis of a prospective cohort of Thai children. J Investig Allergol Clin Immunol. 2007;17:292–296. [PubMed] [Google Scholar]
- 5.Morales E, Garcia-Esteban R, Guxens M, et al. Effects of prolonged breastfeeding and colostrum fatty acids on allergic manifestations and infections in infancy. Clin Exp Allergy. 2012;42:918–928. doi: 10.1111/j.1365-2222.2012.03969.x. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto N, Fukao T, Kaneko H, et al. Total IgE at 6 months predicts remittance or persistence of atopic dermatitis at 14 months. Allergy Asthma Proc. 2013;34:362–369. doi: 10.2500/aap.2013.34.3678. [DOI] [PubMed] [Google Scholar]
- 7.Perkin MR, Strachan DP, Williams HC, Kennedy CT, Golding J ALSPAC Study Team. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221–229. doi: 10.1111/j.1399-3038.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- 8.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Development of atopic dermatitis in the DARC birth cohort. Pediatr Allergy Immunol. 2010;21:307–314. doi: 10.1111/j.1399-3038.2009.00914.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K, Shimanouchi Y, Kawakubo K, et al. Infantile eczema at one month of age is associated with cord blood eosinophilia and subsequent development of atopic dermatitis and wheezing illness until two years of age. Int Arch Allergy Immunol. 2005;137(Suppl 1):69–76. doi: 10.1159/000085435. [DOI] [PubMed] [Google Scholar]
- 10.Ballardini N, Kull I, Lind T, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy. 2012;67:537–544. doi: 10.1111/j.1398-9995.2012.02786.x. [DOI] [PubMed] [Google Scholar]
- 11.Amberbir A, Medhin G, Alem A, Britton J, Davey G, Venn A. The role of acetaminophen and geohelminth infection on the incidence of wheeze and eczema: a longitudinal birth-cohort study. Am J Respir Crit Care Med. 2011;183:165–170. doi: 10.1164/rccm.201006-0989OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purvis DJ, Thompson JM, Clark PM, et al. Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. Br J Dermatol. 2005;152:742–749. doi: 10.1111/j.1365-2133.2005.06540.x. [DOI] [PubMed] [Google Scholar]
- 13.Bleiker TO, Shahidullah H, Dutton E, Graham-Brown RA. The prevalence and incidence of atopic dermatitis in a birth cohort: the importance of a family history of atopy. Arch Dermatol. 2000;136:274. doi: 10.1001/archderm.136.2.274. [DOI] [PubMed] [Google Scholar]
- 14.Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101:587–593. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 15.Kull I, Bergstrom A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. 2006;61:1009–1015. doi: 10.1111/j.1398-9995.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 16.Ekback M, Tedner M, Devenney I, et al. Severe eczema in infancy can predict asthma development. A prospective study to the age of 10 years. PLoS One. 2014;9:e99609. doi: 10.1371/journal.pone.0099609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Illi S, von Mutius E, Lau S, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113:925–931. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 18.Singh AGR, Evans M, et al. Risk factors for the persistent expression of atopic dermatitis in a high-risk birth cohort. J Allergy Clin Immunol. 2006;117:S178. [Google Scholar]
- 19.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitisea prospective follow-up to 7 years of age. Allergy. 2000;55:240–245. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 20.van Asperen PP, Kemp AS. The natural history of IgE sensitization and atopic disease in early childhood. Acta Paediatr Scand. 1989;78:239–245. doi: 10.1111/j.1651-2227.1989.tb11063.x. [DOI] [PubMed] [Google Scholar]
- 21.Kissling S, Wuthrich B. Follow-up of atopic dermatitis after early childhood [in German] Hautarzt. 1993;44:569–573. [PubMed] [Google Scholar]
- 22.Shen CY, Lin MC, Lin HK, Lin CH, Fu LS, Fu YC. The natural course of eczema from birth to age 7 years and the association with asthma and allergic rhinitis: a population-based birth cohort study. Allergy Asthma Proc. 2013;34:78–83. doi: 10.2500/aap.2013.34.3625. [DOI] [PubMed] [Google Scholar]
- 23.Carlsten C, Dimich-Ward H, Ferguson A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. 2013;110:24–28. doi: 10.1016/j.anai.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Harpsoe MC, Basit S, Bager P, et al. Maternal obesity, gestational weight gain, and risk of asthma and atopic disease in offspring: a study within the Danish National Birth Cohort. J Allergy Clin Immunol. 2013;131:1033–1040. doi: 10.1016/j.jaci.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Burr ML, Dunstan FD, Hand S, Ingram JR, Jones KP. The natural history of eczema from birth to adult life: a cohort study. Br J Dermatol. 2013;168:1339–1342. doi: 10.1111/bjd.12216. [DOI] [PubMed] [Google Scholar]
- 26.Bohme M, Soderhall C, Kull I, Bergstrom A, van Hage M, Wahlgren CF. Filaggrin mutations increase the risk for persistent dry skin and eczema independent of sensitization. J Allergy Clin Immunol. 2012;129:1153–1155. doi: 10.1016/j.jaci.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 27.Semic-Jusufagic A, Gevaert P, Bachert C, Murray C, Simpson A, Custovic A. Increased serum-soluble interleukin-5 receptor alpha level precedes the development of eczema in children. Pediatr Allergy Immunol. 2010;21:1052–1058. doi: 10.1111/j.1399-3038.2010.01077.x. [DOI] [PubMed] [Google Scholar]
- 28.von Kobyletzki LB, Bornehag CG, Breeze E, Larsson M, Lindstrom CB, Svensson A. Factors associated with remission of eczema in children: a population-based follow-up study. Acta Derm Venereol. 2014;94:179–184. doi: 10.2340/00015555-1681. [DOI] [PubMed] [Google Scholar]
- 29.Sumikawa YMA, Yamashita T. A survey of the prevalence of atopic dermatitis in school students in Hokkaido. J Dermatol. 2014;41:72. [Google Scholar]
- 30.Schmitt J, Apfelbacher C, Chen CM, et al. Infant-onset eczema in relation to mental health problems at age 10 years: results from a prospective birth cohort study (German Infant Nutrition Intervention plus) J Allergy Clin Immunol. 2010;125:404–410. doi: 10.1016/j.jaci.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 31.Hua TC, Hwang CY, Chen YJ, et al. The natural course of early-onset atopic dermatitis in Taiwan: a population-based cohort study. Br J Dermatol. 2014;170:130–135. doi: 10.1111/bjd.12603. [DOI] [PubMed] [Google Scholar]
- 32.Kurukulaaratchy R, Fenn M, Matthews S, Hasan Arshad S. The prevalence, characteristics of and early life risk factors for eczema in 10-year-old children. Pediatr Allergy Immunol. 2003;14:178–183. doi: 10.1034/j.1399-3038.2003.00036.x. [DOI] [PubMed] [Google Scholar]
- 33.Kekki OM, Scheynius A, Poikonen S, Koskinen A, Kautiainen H, Turjanmaa K. Sensitization to Malassezia in children with atopic dermatitis combined with food allergy. Pediatr Allergy Immunol. 2013;24:244–249. doi: 10.1111/pai.12057. [DOI] [PubMed] [Google Scholar]
- 34.Filipiak-Pittroff B, Schnopp C, Berdel D, et al. Predictive value of food sensitization and filaggrin mutations in children with eczema. J Allergy Clin Immunol. 2011;128:1235.e5–1241.e5. doi: 10.1016/j.jaci.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Williams HC, Strachan DP. The natural history of childhood eczema: observations from the British 1958 birth cohort study. Br J Dermatol. 1998;139:834–839. doi: 10.1046/j.1365-2133.1998.02509.x. [DOI] [PubMed] [Google Scholar]
- 36.Novembre E, Cianferoni A, Lombardi E, Bernardini R, Pucci N, Vierucci A. Natural history of “intrinsic” atopic dermatitis. Allergy. 2001;56:452–453. doi: 10.1034/j.1398-9995.2001.056005452.x. [DOI] [PubMed] [Google Scholar]
- 37.Wuthrich B, Schmid-Grendelmeier P. Natural course of AEDS. Allergy. 2002;57:267–268. doi: 10.1034/j.1398-9995.2002.1n3572.x. [DOI] [PubMed] [Google Scholar]
- 38.Wakamori T, Katoh N, Hirano S, Kishimoto S, Ozasa K. Atopic dermatitis, dry skin and serum IgE in children in a community in japan. Int Arch Allergy Immunol. 2009;149:103–110. doi: 10.1159/000189192. [DOI] [PubMed] [Google Scholar]
- 39.Vowles M, Warin RP, Apley J. Infantile eczema: observations on natural history and prognosis. Br J Dermatol. 1955;67:53–59. doi: 10.1111/j.1365-2133.1955.tb12687.x. [DOI] [PubMed] [Google Scholar]
- 40.Ricci G, Patrizi A, Giannetti A, Dondi A, Bendandi B, Masi M. Does improvement management of atopic dermatitis influence the appearance of respiratory allergic diseases? A follow-up study. Clin Mol Allergy. 2010;8:8. doi: 10.1186/1476-7961-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garmhausen D, Hagemann T, Bieber T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy. 2013;68:498–506. doi: 10.1111/all.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartwig IR, Sly PD, Schmidt LA, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. 2014;134:160–169. doi: 10.1016/j.jaci.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 43.Aberg N, Engstrom I. Natural history of allergic diseases in children. Acta Paediatr Scand. 1990;79:206–211. doi: 10.1111/j.1651-2227.1990.tb11440.x. [DOI] [PubMed] [Google Scholar]
- 44.Ziyab AH, Raza A, Karmaus W, et al. Trends in eczema in the first 18 years of life: results from the Isle of Wight 1989 birth cohort study. Clin Exp Allergy. 2010;40:1776–1784. doi: 10.1111/j.1365-2222.2010.03633.x. [DOI] [PubMed] [Google Scholar]
- 45.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. J Allergy Clin Immunol. 2010;126:590–595. e1–e3. doi: 10.1016/j.jaci.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Musgrove K, Morgan JK. Infantile eczema: a long-term follow-up study. Br J Dermatol. 1976;95:365–372. doi: 10.1111/j.1365-2133.1976.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 47.Rystedt I. Long term follow-up in atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1985;114:117–120. doi: 10.2340/00015555114117120. [DOI] [PubMed] [Google Scholar]
- 48.Mortz CG, Andersen KE, Dellgren C, Barington T, Bindslev-Jensen C. Atopic dermatitis from adolescence to adulthood in the TOACS cohort: prevalence, persistence and comorbidities. Allergy. 2015;70:836–845. doi: 10.1111/all.12619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.