Abstract
Immunoglobulin G4-related disease (IgG4-RD) is a new disease category involving many organ systems, including the endocrine system in general and the thyroid in particular. Since an initial association was made between hypothyroidism and autoimmune (IgG4-related) pancreatitis, more forms of IgG4-related thyroid disease (IgG4-RTD) have been recognized. Four subcategories of IgG4-RTD have so far been identified: Riedel thyroiditis (RT), fibrosing variant of Hashimoto thyroiditis (FVHT), IgG4-related Hashimoto thyroiditis, and Graves disease with elevated IgG4 levels. Although a male predominance is seen for IgG4-RD in general, RT and FVHT have a female preponderance. The pathogenesis of IgG4-RD is not completely understood; however, genetic factors, antigen-antibody reactions, and an allergic phenomenon have been described. Diagnosis of IgG4-RD requires a combination of clinical features, serological evidence, and histological features. Histology is the mainstay of diagnosis, with IgG4 immunostaining. Although serum IgG4 levels are usually elevated in IgG4-RD, raised serum IgG4 is neither necessary nor adequate for diagnosis. Imaging supports the diagnosis and is a useful tool in disease monitoring. Management of IgG4-RTD is both medical and surgical. Steroids are the first-line treatment and may produce a swift response. Tamoxifen and rituximab are second-line agents used in steroid-resistant patients. Surgical debulking is carried out in RT solely as a procedure to relieve obstruction. Other endocrine associations described with IgG4-RD are hypophysitis and Hashimoto encephalopathy. IgG4-RTD is an uncommon disease entity, and prompt diagnosis and treatment can improve outcomes.
Key Words: Immunoglobulin G4, Hashimoto thyroiditis, Riedel thyroiditis, Graves disease, Tamoxifen, Rituximab
Introduction
Immunoglobulin G4-related disease (IgG4-RD) is a new clinical disease entity which is characterized by a dense lymphoplasmacytic infiltrate rich in IgG4-positive plasma cells in the affected tissues, with a tendency to form tumefactive lesions, and storiform fibrosis with frequent but not invariable elevations of serum IgG4 levels, and a swift initial response to glucocorticoids [1]. IgG4-RD was first identified in 2001, when sclerosing pancreatitis was found to be associated with high serum IgG4 levels and to respond to glucocorticoid therapy [2]. This disease entity seems to have unified several diseases described decades ago, which were previously thought to occur in isolation, such as Mikulicz syndrome [3], retroperitoneal fibrosis [4], Küttner tumour [5], and Riedel thyroiditis (RT) [6].
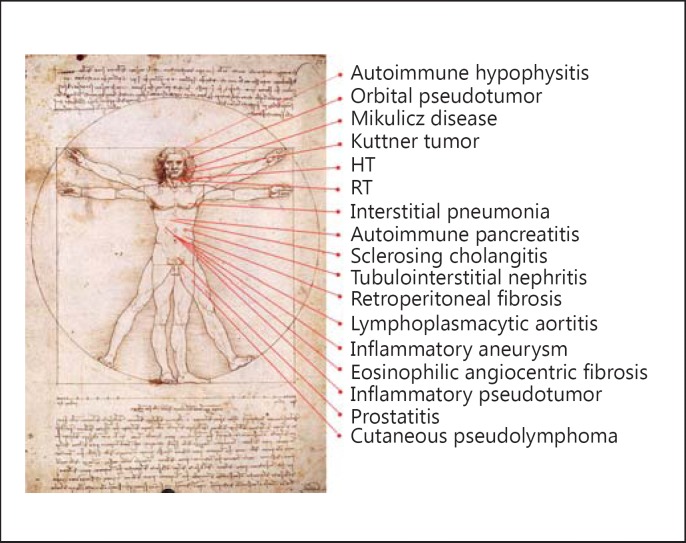
In 2012 a consensus guideline on the nomenclature of IgG4-RD was released, which brought together the disparate organ diseases into 1 disease spectrum [1]. Figure 1 demonstrates the spectrum of IgG4-RD known so far [7]. In 2005 an association of hypothyroidism with positive thyroglobulin antibody was noted in autoimmune pancreatitis patients [8], prompting examination of the spectrum of IgG4-related thyroid disease (IgG4-RTD). IgG4-RTD is thus a new disease classification linked to IgG4-RD, but the precise relationship is not yet fully defined. In this review, we will describe the current understanding of IgG4-RTD.
Fig. 1.
IgG4-related conditions. Many diseases have been reported to be IgG4-related. Modified from Umehara et al. [7] with permission.
Sparse data exist on the epidemiology of IgG4-RD. Asian data predominates, with 1 Japanese study estimating the annual incidence of IgG4-RD to be 0.28-1.08/ 100,000, with a total incidence of 8,000 patients predicted in Japan in 2009, which accounted for a prevalence of approximately 62 patients per million inhabitants [9]. There is a male predominance with a 4:1 ratio and a peak incidence between 50 and 70 years of age [10].
Classification of IgG4-RTD
We have categorized IgG4-RTD as:
a RT
b Fibrosing variant of Hashimoto thyroiditis (FVHT)
c IgG4-related Hashimoto thyroiditis (IgG4-RHT)
d Graves disease with elevated IgG4 level
Although the above disease entities are described as separate diseases, overlap among these has been observed. However, there are insufficient data at present to completely link each of them together into a single disease entity.
Pathogenesis
Most studies of the pathogenesis of IgG4-RD are based on autoimmune pancreatitis alone. Genetic factors, antigen-antibody reactions, and allergic phenomenon seem to play a pivotal role. The pathogenesis of IgG4-RTD, in relation to IgG4-RD, is poorly understood.
Genetic Factors
A few genetic factors have been reported to be associated with the susceptibility to autoimmune pancreatitis, such as HLA-DRB1*0405 and HLA-DQB1*0401 haplotypes in Japanese patients, which may play a functional role in antigen presentation and in the induction of an autoimmune response [11]. Although familial cases of IgG4-RD appear to be rare, 2 siblings with multifocal fibrosclerosis and RT have been reported [12], and it is quite possible that some familial forms might have been missed in the past because the entity had not been recognized.
Antigen-Antibody Reactions and Allergic Phenomena
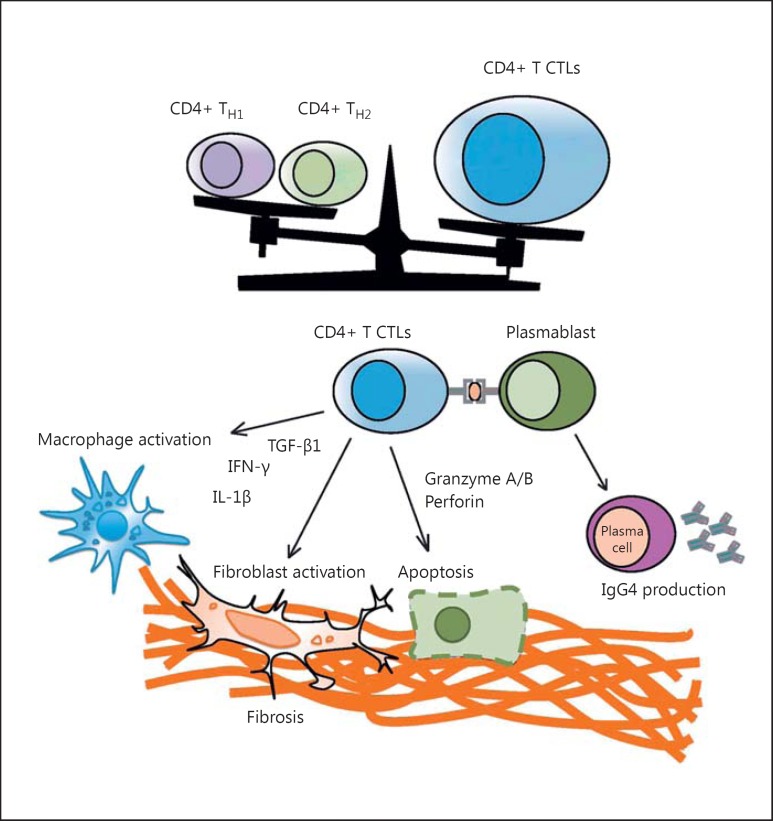
A model for the pathogenesis of IgG4-RD was developed by Maehara et al. [13]. According to the model, the pathogenic processes of IgG4-RD could be initiated by immune responses to infections, commensal microbes, food, and environmental allergens, with tissue damage leading to activation of CD4+ cytotoxic T lymphocytes, which infiltrate affected tissues and mediate inflammation and fibrosis. Activated T cells produce inflammatory cytokines, including multiple interleukins (IL) such as IL-4, IL-5, IL-10, and IL-13, interferon gamma (INF-γ), and transcription growth factor beta (TGF-β) [14]. The major function of IL-4 and IL-10 is to initiate class switching of autoreactive B lymphocytes to IgE and IgG4 production and to promote the differentiation of IgG4-positive plasma cells. IL-5, IL-13, and TGF-β play a major role in the recruitment of eosinophils as well as fibroblast activation, causing dense fibrosis and obliterative phlebitis [13] with tissue damage. IgG4 and IgE antibodies cross-react to self-antigens. B cells that recognize self-antigens may then present those self-antigens to autoreactive T cells, leading to a vicious circle between T cells and B cells [15]. It is not yet clear whether IgG4 plays a central role in the pathogenesis of IgG4-RD or is the result of the fibroinflammatory process, as IgG4 antibodies are unable to cross-link antigens to form immune complexes and activate the complement system [16]. Figure 2 illustrates the pathogenesis of IgG4-RD [13].
Fig. 2.
Schematic model of CD4+ cytotoxic T lymphocytes (CTLs) in IgG4-RD pathogenesis. Clonal expansion of CD4+ CTLs and their infiltration into tissue sites may be the cause of this disease state. Reactivation of these CD4+ CTL cells may require antigen presentation by plasmablasts or other activated B cells in tissue sites. Activated CD4+ CTLs presumably mediate fibrosis and inflammation as a result of cytokine secretion or possibly the induction of cell death. The mechanisms by which CD4+ CTLs may cause disease remain speculative. Reproduced from Maehara et al. [13] with permission.
Histological Features
A characteristic histological appearance of IgG4-RD is essential for a definitive diagnosis. There are 3 major features: (1) a lymphoplasmacytic infiltrate rich in IgG4 plasma cells; (2) fibrosis arranged at least focally in a storiform (irregularly whorled) pattern; (3) obliterative phlebitis; and 2 minor features that can be associated but are nonspecific: (4) phlebitis without obliteration of the lumen, and (5) an increased number of eosinophils [17]. For a confident pathological diagnosis, at least 2 of the major features are necessary. The histological features of IgG4-RHT are a predominant interfollicular pattern of fibrosis, small thyroid follicles, marked follicular cell degeneration, and increased giant cell/histiocyte infiltration [18], whereas predominant interlobular fibrosis, and mild or no other histological features are seen in Hashimoto thyroiditis (HT) unrelated to IgG4.
IgG4 immunostaining is important for the diagnosis, especially when serum IgG4 is not elevated. Although IgG4 plasma cells per high-power field has an acceptable specificity, an IgG4/IgG plasma cell ratio of greater than 40% is considered more valuable, as some inflammatory lesions have high IgG4 plasma cells. Caveats also exist for the IgG4/IgG plasma cell ratio as inflammatory conditions, such as rheumatoid arthritis and Castleman syndrome, can also have elevated ratios without IgG4-RD [17]. Different cut-offs have been suggested for disease in different organs, including for the thyroid gland. More than 20 IgG4 plasma cells per high-power field and an IgG4/IgG ratio greater than 30% have been adopted by Li et al. [18] in diagnosing IgG4-RT, with claimed higher sensitivity and specificity.
Serum IgG4 Levels
Serum IgG4 levels are usually elevated to greater than 135 mg/dL in IgG4-RD, but this is neither necessary nor adequate for diagnosis. Carruthers et al. [19] described conditions that can cause elevated IgG4, such as infections, connective tissue diseases, and immunodeficiency states, and demonstrated a sensitivity and specificity of 90 and 60%, respectively, for diagnosing IgG4-RD when the cut-off for serum IgG4 was set at greater than 135 mg/dL. The measurement of serum IgG4 is also useful to assess treatment response and recurrence [20].
Diagnosis
Diagnostic criteria for IgG4-RD were proposed in 2012 utilizing clinical features, serological evidence, and histological features (Table 1) [21]. Diagnosis in each category of IgG4-RT is mentioned separately.
Table 1.
Comprehensive diagnostic criteria for IgG4-RD
| 1 | Clinical examination: clinical history, physical examination, imaging |
| 2 | Immunological examination: IgG4 in serum >135 mg/dL or elevated IgG4/IgG ratio; possibly accompanied by other laboratory tests, such as IgE, γ-globulin, or a complement |
| 3 | Histopathologic examination: lymphoplasmocytic infiltration with storiform fibrosis and obliterative phlebitis, infiltration by IgG4-positive plasma cells (IgG4+/IgG+ >40%) |
1 + 2 = possible IgG4-RD; 1 + 3 = probable IgG4-RD; 1 + 2 + 3 = definite IgG4-RD. Adapted from Umehara et al. [21].
Imaging
Imaging in IgG4-RTD may support the diagnosis, but findings are not specific for the disease. Ultrasound of the thyroid has been the most commonly used modality and it usually shows diffuse low echogenicity of the thyroid gland in IgG4-related HT, whereas non-IgG4 thyroiditis is associated with diffuse, coarse echogenicity [22]. This may be due to the higher degree of stromal fibrosis and follicular cell degeneration in IgG4-HT. In RT cases, while ultrasound shows hypoechogenicity, computed tomography (CT) shows a hypodense, infiltrative mass, which slightly enhances after contrast. MRI shows hypointensity in T1 and T2 images [23]. CT/MRI with PET scans have been utilized for the diagnosis, especially in the setting of ruling out malignancy in other IgG4-RD and also for disease monitoring after treatment [24]. When extrathyroidal disease is suspected, CT and FDG-PET scanning may assist in defining the disease extent and activity.
Riedel Thyroiditis
RT is a rare form of chronic thyroiditis that is characterized by extensive fibrosis involving the thyroid gland and its surrounding tissues [25]. In 1896, Riedel initially recognized hard and infiltrative lesions in the thyroid gland in 2 cases which were thought to be malignant but were histologically proven to be otherwise [26]. RT appears to be the thyroid disease with the strongest association to IgG4-RD. At the Mayo Clinic, over a period of 64 years until 1985, only 37 patients with RT were encountered (of 56,700 patients who underwent thyroidectomy [27]), highlighting the rarity of the disease, with an estimated incidence of 1.06 cases per 100,000 outpatients. In contrast to IgG4-RD, RT is more common in women, with a recent case series showing 81% of confirmed Riedel's diagnoses from 1976 to 2008 in women [28].
RT was linked with other fibrosclerotic diseases and thought to be a part of IgG4-RD due to the extensive thyroidal fibrosis and due to the discovery of associated organ involvement, such as retroperitoneal fibrosis [25, 29], pancreatic fibrosis, mediastinal fibrosis, orbital pseudotumour [28], and sclerosing cholangitis [12]. RT usually presents as a firm mass in the neck with the compressive symptoms of tracheal narrowing, dysphagia, and vocal cord paralysis [25]. However, in about a third of patients with RT, 1 or more of the other fibrosing disorders develops over the next decade [28]. Although most patients are clinically euthyroid at presentation, 30-40% eventually develop hypothyroidism as the gland is progressively infiltrated [30]. Antibody titres against thyroglobulin (Tg-Ab) and thyroperoxidase (TPO-Ab) are elevated in 90% [25] in RT, but most likely due to concurrent HT rather than an autoimmune etiology of RT [31]. Hypocalcaemia is common due to the involvement of parathyroid glands in the fibroinflammatory process [32]. The differential diagnosis of RT includes anaplastic thyroid cancer [33], lymphoma [34], and FVHT [35].
The histological criteria of RT are: (1) a fibroinflammatory process involving all or a portion of the thyroid gland; (2) evidence of extension into the surrounding tissues, including strap muscles; (3) infiltrates of inflammatory cells without giant cells, lymphoid follicles, oncocytes, or granulomas; (4) evidence of occlusive phlebitis, and (5) absence of neoplasm [36]. Immunohistochemistry staining is not part of the histological diagnostic criteria yet, although a link between IgG4-RD and RT was shown for the first time by Dahlgren et al. [37], who demonstrated excessive numbers of IgG4-positive plasma cells in 3 RT histological samples with histopathology otherwise consistent with a diagnosis of IgG4-RD. In a recent study performed at the Mayo Clinic, in 6 cases of RT from 1958 to 2008, 5 cases had IgG4 immunostaining confirming the link [38]. However, until now elevated serum IgG4 levels have not been documented in RT.
Fibrous Variant of HT
FVHT is an uncommon form of HT, seen in about 10% of patients with HT [39]. Katz and Vickery [39] first defined this lesion in 1974 and proposed the histopathological diagnostic criteria of: (1) a marked fibrous replacement of more than one-third of the thyroid parenchyma, and (2) changes typical of HT in the remaining tissue. Distinctive clinical features of FVHT include severe pressure symptoms in the neck and a very firm thyroid gland, with rapid enlargement [40].
The incidence of FVHT is unknown. Katz and Vickery [39] reported that, of 56 cases of FVHT, females predominate, with a female to male ratio of 9:1, mostly consisting of middle-aged women, between 40 and 60 years of age.
Deshpande et al. [40] studied a group of patients with FVHT and compared them with typical HT patients, finding that FVHT patients have more hypothyroidism, a higher mean IgG4-positive cell count in affected thyroid tissue, and a higher IgG4/IgG ratio than typical HT patients. They described the histology as an exaggerated lobular pattern, with the lobules separated by cellular storiform-type fibrosis, resembling the fibrosis seen in other forms of IgG-RD. Based on this evidence, they proposed that FVHT belonged to the IgG4 spectrum of diseases. However, differentiating RT from FVHT can be difficult, and Hennessey [35] described the main distinctions in these 2 entities (Table 2). Other organ involvement does not seem to be a feature of FVHT.
Table 2.
Differentiation of FVHT and RT
| Finding | RT | FVHT |
|---|---|---|
| Thyroid antibodies | Moderate titre | High titre |
| Normal thyroid tissue | Sharply demarcated | Diffuse involvement |
| Venulitis | Yes | No |
| Extrathyroidal invasion | Yes | No |
| Hürthle cells | No | Yes |
| Lymphocyte light chains | λ-dominant | κ-dominant |
| Plasma cell production | Increased IgA (47%) | IgG-dominant, IgA (<15%) |
| Associated autoimmune disease | Yes | Yes |
| Associated de Quervain syndrome | Yes | No |
| Ultrasound appearance | Hypoechoic | Hypoechoic |
| Doppler flow | Diminished | Enhanced |
Adapted from Hennessey [35].
IgG4-Related HT
This new entity was proposed by Li et al. [18] in 2009, with HT divided into 2 groups: IgG4 thyroiditis (IgG4-positive plasma cell-rich group), and non-IgG4 thyroiditis (IgG4-positive plasma cell-poor group). It is an organ-specific disease of the thyroid gland, and, unlike RT, has not been associated with other systemic manifestations of IgG4-RD.
The incidence is unknown. Li et al. [22] reported that IgG4-RHT has a male preponderance with an age range of 47-68 years (mean 52.7 years).
IgG4-RHT is associated with more rapid progress, subclinical hypothyroidism, diffuse low echogenicity on ultrasonography, and a higher level of circulating thyroid autoantibodies than non-IgG4 thyroiditis [22]. It was also found that, after total thyroidectomy, the serum IgG4 concentration in IgG4-RHT showed a significant reduction and often returned to the normal range. IgG4-RHT differs from RT as marked fibrosis, extrathyroidal extension, and other systemic manifestations are absent [6]. FVHT could well be a part of IgG4-RHT, although no distinct histological criteria to differentiate them are available at present.
Graves Disease in the Context of IgG4-RTD
A small subset of patients with Graves disease was found to have elevated serum IgG4 levels. These patients were older and had more hypoechoic areas on ultrasonography, but any histological differences have not been evaluated [41]. An association of Graves ophthalmopathy with elevated serum IgG4 levels has also been found [42], but more data are required to link this to IgG4-RTD.
Management
Therapeutic options in IgG4-RTD are both medical and surgical, and which comes first depends on the stage of the disease. Due to the rarity of these conditions, no randomized control studies have been carried out for any treatment modality.
Medical Management
In RT, L-thyroxine replacement is used for associated primary hypothyroidism, and calcium and calcitriol therapy for hypoparathyroidism, as clinically indicated. Glucocorticoids remain the mainstay of treatment for RT, which can give symptomatic relief for upper airway symptoms, dysphonia, and recurrent laryngeal nerve involvement, and reduce the size and hardness of the pathological tissue mass [43]. Glucocorticoid usage early in the course of the disease has shown to reduce the progression of the disease [44]. Glucocorticoid starting doses range widely from 15 to 100 mg per day, and are generally reduced slowly over weeks to months. Remission rates after glucocorticoid usage are unclear, but relapse can occur during dose reduction. Nonsmokers respond better than smokers [28].
Tamoxifen is the second-line agent which has been used successfully in patients who relapse on glucocorticoids. Tamoxifen inhibits fibroblastic function, perhaps through its influence on the production of TGFß, which is a potent growth inhibitor [45]. Doses of 10-20 mg of tamoxifen either in combination with continued glucocorticoid therapy or as a monotherapy have been reported to be successful, especially in symptom control and reduction in pathological tissue mass [46, 47]. Case reports have suggested that the combination of mycophenolate mofetil, which directly inhibits T and B lymphocytic proliferation and antibody production, with prednisolone can be used in glucocorticoid and tamoxifen- resistant patients [48].
Recent results from a prospective, single-arm pilot trial of rituximab alone in IgG4-RD provided strong evidence that B cell depletion is an effective treatment, although none of the 30 participants had RT [49]. Soh et al. [50] reported that treatment with rituximab was effective in a patient with RT refractory to steroids and tamoxifen in reducing the extrathyroidal inflammatory mass and associated symptoms. Figure 3 illustrates the FDG-PET scan uptake before and after treatment with tamoxifen in this patient.
Fig. 3.
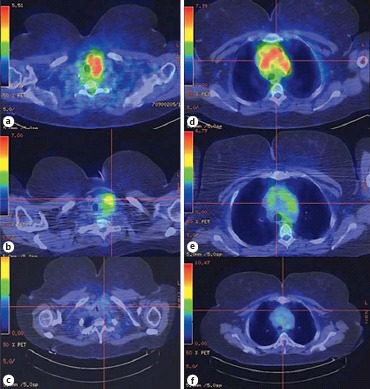
a Active inflammation at the left neck before rituximab (SUVmax, 6.5). b Decrease in inflammation at the left neck 6 weeks after rituximab (SUVmax, 5.2). c Persistent decrease in inflammation at the left neck 10 months after rituximab (SUVmax, 3.7). d Active inflammation at the mediastinum before rituximab (SUVmax, 8.8). e Decrease in inflammation at the mediastinum 6 weeks after rituximab (SUVmax, 4.9). f Persistent decrease in inflammation at the mediastinum 10 months after rituximab (SUVmax, 4.2). Reproduced from Soh et al. [50] with permission.
Surgical Management
Although debulking surgery has been performed for RT, especially isthmectomy to relieve obstruction, more extensive surgical procedures are generally considered inappropriate in the management of RT due to the risk of permanent vocal cord palsy and surgical hypoparathyroidism [51].
Association with Malignancy
Although pancreatic adenocarcinoma [52], non-Hodgkin lymphoma [53], and salivary duct cancer [54] have been linked to IgG4-RD, the actual risk of malignancy is unknown. Studies on the effect of IgG4-positive cells on cancer tissue suggest that IgG4 antibodies can increase tumour-inducing Th2-associated inflammatory conditions and cause loss of antitumour immunity by inhibiting immune effector cell activation [55, 56]. Case reports have shown that RT also has associations with follicular and papillary thyroid cancer [57, 58]. However, further studies are needed to confirm this association.
Other Endocrine Associations
Other endocrine diseases are emerging in the IgG4-RD spectrum. Hashimoto encephalopathy was found to be associated with high serum and intrathecal IgG4 levels in a case report [59]. IgG4-related hypophysitis has been the subject of case reports and a case series [60].
Conclusion
IgG4-RTD is an emerging new disease entity that consists of previously known diseases such as RT and FVHT, as well as new disease categories, such as IgG4-RHT and IgG4-related Graves disease. The pathogenesis of each of these disease categories is still unclear, and precise diagnostic criteria are not yet defined. It also remains unclear whether treatment should be disease specific. Any association with thyroid cancer remains to be confirmed. Although IgG4-RTD is an uncommon form of thyroiditis, its identification in the correct clinical setting is important to further our understanding of the pathogenesis and disease course, and may enable better treatment.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64:3061–3067. doi: 10.1002/art.34593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz's disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335–340. doi: 10.1007/s10165-006-0518-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimori N, Ito T, Igarashi H, Oono T, Nakamura T, Niina Y, Hijioka M, Lee L, Uchida M, Takayanagi R. Retroperitoneal fibrosis associated with immunoglobulin G4-related disease. World J Gastroenterol. 2013;19:35–41. doi: 10.3748/wjg.v19.i1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geyer JT, Ferry JA, Harris NL, Stone JH, Zukerberg LR, Lauwers GY, Pilch BZ, Deshpande V. Chronic sclerosing sialadenitis (Küttner tumor) is an IgG4-associated disease. Am J Surg Pathol. 2010;34:202–210. doi: 10.1097/PAS.0b013e3181c811ad. [DOI] [PubMed] [Google Scholar]
- 6.Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken) 2010;62:1312–1318. doi: 10.1002/acr.20215. [DOI] [PubMed] [Google Scholar]
- 7.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, Tsubota K, Yoshino T, Kawa S, Suzuki R, Takegami T, Tomosugi N, Kurose N, Ishigaki Y, Azumi A, Kojima M, Nakamura S, Inoue D. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22:1–14. doi: 10.1007/s10165-011-0508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komatsu K, Hamano H, Takayama M, Muraki T, Sakurai A, Ota M, Kawa S. High prevalence of hypothyroidism in patients with autoimmune pancreatitis. Dig Dis Sci. 2005;50:1052–1057. doi: 10.1007/s10620-005-2703-9. [DOI] [PubMed] [Google Scholar]
- 9.Uchida K, Masamune A, Shimosegawa T, Okazaki K. Prevalence of IgG4-related disease in Japan based on Nationwide Survey in 2009. Int J Rheumatol. 2012 doi: 10.1155/2012/358371. 358371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, Okumura K, Toshima F, Toyama J, Minami T, Matsui O, Gabata T, Zen Y. IgG4-related disease: dataset of 235 consecutive patients. Medicine. 2015;94:e680. doi: 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawa S, Ota M, Yoshizawa K, Horiuchi A, Hamano H, Ochi Y, Nakayama K, Tokutake Y, Katsuyama Y, Saito S, Hasebe O, Kiyosawa K. HLA DRB10405-DQB10401 haplotype is associated with autoimmune pancreatitis in the Japanese population. Gastroenterology. 2002;122:1264–1269. doi: 10.1053/gast.2002.33022. [DOI] [PubMed] [Google Scholar]
- 12.Comings DE, Skubi KB, van Eyes J, Motulsky AG. Familial multifocal fibrosclerosis: findings suggesting that retroperitoneal fibrosis, mediastinal fibrosis, sclerosing cholangitis, Riedel's thyroiditis and pseudotumor of the orbit may be different manifestations of a single disease. Ann Intern Med. 1967;66:884–892. doi: 10.7326/0003-4819-66-5-884. [DOI] [PubMed] [Google Scholar]
- 13.Maehara T, Mattoo H, Ohta M, et al. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadentis and sialoadenitis. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209139. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lighaam LC, Aalberse RC, Rispens T. IgG4-related fibrotic diseases from an immunological perspective: regulators out of control? Int J Rheumatol. 2012 doi: 10.1155/2012/789164. 789164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 16.Nirula A, Glaser SM, Kalled SL, Taylor FR. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr Opin Rheumatol. 2001;23:119–124. doi: 10.1097/BOR.0b013e3283412fd4. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25:1181–1192. doi: 10.1038/modpathol.2012.72. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Bai Y, Ozaki T, Taniguchi E, Mori I, Nagayama K, Nakamura H, Kakudo K. Immunohistochemistry of IgG4 can help subclassify Hashimoto's autoimmune thyroiditis. Pathol Int. 2009;59:636–641. doi: 10.1111/j.1440-1827.2009.02419.x. [DOI] [PubMed] [Google Scholar]
- 19.Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74:14–18. doi: 10.1136/annrheumdis-2013-204907. [DOI] [PubMed] [Google Scholar]
- 20.Tabata T, Kamisawa T, Takuma K, Egawa N, Setoguchi K, Tsuruta K, Obayashi T, Sasaki T. Serial changes of elevated serum IgG4 levels in IgG4-related systemic disease. Intern Med. 2011;50:69–75. doi: 10.2169/internalmedicine.50.4321. [DOI] [PubMed] [Google Scholar]
- 21.Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Nakamura S, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21–30. doi: 10.1007/s10165-011-0571-z. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Nishihara E, Hirokawa M, Taniguchi E, Miyauchi A, Kakudo K. Distinct clinical, serological, and sonographic characteristics of Hashimoto's thyroiditis based with and without IgG4-positive plasma cells. J Clin Endocrinol Metab. 2010;95:1309–1317. doi: 10.1210/jc.2009-1794. [DOI] [PubMed] [Google Scholar]
- 23.Ozgen A, Cila A. Riedel's thyroiditis in multifocal fibrosclerosis: CT and MR imaging findings. AJNR Am J Neuroradiol. 2000;21:320–321. [PMC free article] [PubMed] [Google Scholar]
- 24.Lang D, Zwerina J, Pieringer H. IgG4-related disease: current challenges and future prospects. Ther Clin Risk Manag. 2016;12:189–199. doi: 10.2147/TCRM.S99985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham A, Gilliland IC. Riedel's thyroiditis. BMJ. 1959;2:225–226. doi: 10.1136/bmj.2.5146.225-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedel BM. Die chronische, zur Bildung eisenharter Tumoren führende Entzündung der Schilddrüse. Verh Dtsch Ges Chir. 1896;25:101–105. [Google Scholar]
- 27.Hay ID. Thyroiditis: a clinical update. Mayo Clin Proc. 1985;60:836–843. doi: 10.1016/s0025-6196(12)64789-2. [DOI] [PubMed] [Google Scholar]
- 28.Fatourechi MM, Hay ID, McIver B, Sebo TJ, Fatourechi V. Invasive fibrous thyroiditis (Riedel's thyroiditis): the Mayo Clinic Experience 1976-2008. Thyroid. 2011;21:765–772. doi: 10.1089/thy.2010.0453. [DOI] [PubMed] [Google Scholar]
- 29.Turner-Warwick R, Nabarro JD, Doniach D. Riedel's thyroiditis and retroperitoneal fibrosis. Proc R Soc Med. 1966;59:596–598. doi: 10.1177/003591576605900704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price DC. Radioisotopic evaluation of the thyroid and the parathyroids. Radiol Clin North Am. 1993;31:991–1015. [PubMed] [Google Scholar]
- 31.Zelmanovitz F, Zelmanovitz T, Beck M, Cerski CT, Schmid H, Czepielewski MA. Riedel's thyroiditis associated with high titers of antimicrosomal and antithyroglobulin antibodies and hypothyroidism. J Endocrinol Invest. 1994;17:733–737. doi: 10.1007/BF03347770. [DOI] [PubMed] [Google Scholar]
- 32.Marín F, Araujo R, Pa´ramo C, Lucas T, Salto L. Riedel's thyroiditis associated with hypothyroidism and hypoparathyroidism. Postgrad Med J. 1989;65:381–383. doi: 10.1136/pgmj.65.764.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan SK, Chan JK, Tang SK. Paucicellular variant of anaplastic thyroid carcinoma: a mimic of Reidel's thyroiditis. Am J Clin Pathol. 1996;105:388–393. doi: 10.1093/ajcp/105.4.388. [DOI] [PubMed] [Google Scholar]
- 34.Vigouroux C, Escourolle H, Mosnier-Pudar H, Thomopoulos P, Louvel A, Chapuis Y, Varet B, Luton JP. Riedel's thyroiditis and lymphoma: diagnostic difficulties (in French) Presse Med. 1996;25:28–30. [PubMed] [Google Scholar]
- 35.Hennessey JV. Riedel's thyroiditis: a clinical review. J Clin Endocrinol Metab. 2011;96:3031–3041. doi: 10.1210/jc.2011-0617. [DOI] [PubMed] [Google Scholar]
- 36.Papi G, LiVolsi VA. Current concepts on Riedel thyroiditis. Am J Clin Pathol. 2004;121:50–63. doi: 10.1309/NUU88VAFR9YEHKNA. [DOI] [PubMed] [Google Scholar]
- 37.Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken) 2010;62:1312–1318. doi: 10.1002/acr.20215. [DOI] [PubMed] [Google Scholar]
- 38.Stan MN, Sonawane V, Sebo TJ, Bahn RS. Riedel's thyroiditis association with IgG4-related disease. Clin Endocrinol. doi: 10.1111/cen.13238. DOI: 10.1111/cen.13238. [DOI] [PubMed] [Google Scholar]
- 39.Katz SM, Vickery AL., Jr The fibrous variant of Hashimoto's thyroiditis. Hum Pathol. 1974;5:161–170. doi: 10.1016/s0046-8177(74)80063-8. [DOI] [PubMed] [Google Scholar]
- 40.Deshpande V, Huck A, Ooi E, Stone JH, Faquin WC, Nielsen GP. Fibrosing variant of Hashimoto thyroiditis is an IgG4 related disease. J Clin Pathol. 2012;65:725–728. doi: 10.1136/jclinpath-2011-200485. [DOI] [PubMed] [Google Scholar]
- 41.Takeshima K, Inaba H, Furukawa Y, Nishi M, Yamaoka H, Miyamoto W, Ota T, Doi A, Kawashima H, Ariyasu H, Wakasaki H, Furuta H, Nakao T, Sasaki H, Akamizu T. Elevated serum immunoglobulin G4 levels in patients with Graves' disease and their clinical implications. Thyroid. 2014;24:736–743. doi: 10.1089/thy.2013.0448. [DOI] [PubMed] [Google Scholar]
- 42.Bozkirli E, Bakiner OS, Bozkirli E, Eksi Haydardedeoglu F, Sizmaz S, Torun AI, Ertorer ME. Serum Immunoglobulin G4 levels are elevated in patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2015;83:962–967. doi: 10.1111/cen.12671. [DOI] [PubMed] [Google Scholar]
- 43.Bagnasco M, Passalacqua G, Pronzato C, Albano M, Torre G, Scordamaglia A. Fibrous invasive (Riedel's) thyroiditis with critical response to steroid treatment. J Endocrinol Invest. 1995;18:305–307. doi: 10.1007/BF03347818. [DOI] [PubMed] [Google Scholar]
- 44.Vaidya B, Harris PE, Barrett P, Kendall-Taylor P. Corticosteroid therapy in Riedel's thyroiditis. Postgrad Med J. 1997;73:817–819. doi: 10.1136/pgmj.73.866.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butta A, MacLennan K, Flanders KC, Sacks NP, Smith I, McKinna A, Dowsett M, Wakefield LM, Sporn MB, Baum M, Colletta AA. Induction of transforming growth factor 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992;52:4261–4264. [PubMed] [Google Scholar]
- 46.Few J, Thompson NW, Angelos P, Simeone D, Giordano T, Reeve T. Riedel's thyroiditis: treatment with tamoxifen. Surgery. 1996;120:993–998. doi: 10.1016/s0039-6060(96)80045-6. [DOI] [PubMed] [Google Scholar]
- 47.Jung YJ, Schaub CR, Rhodes R, Rich FA, Muehlenbein SJ. A case of Riedel's thyroiditis treated with tamoxifen: another successful outcome. Endocr Pract. 2004;10:483–486. doi: 10.4158/EP.10.6.483. [DOI] [PubMed] [Google Scholar]
- 48.Levy JM, Hasney CP, Friedlander PL, Kandil E, Occhipinti EA, Kahn MJ. Combined mycophenolate mofetil and prednisone therapy in tamoxifen- and prednisone-resistant Riedel's thyroiditis. Thyroid. 2010;20:105–107. doi: 10.1089/thy.2009.0324. [DOI] [PubMed] [Google Scholar]
- 49.Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, Deshpande V, Smyrk TC, Chari S, Stone JH. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74:1171–1177. doi: 10.1136/annrheumdis-2014-206605. [DOI] [PubMed] [Google Scholar]
- 50.Soh SB, Pham A, O'Hehir RE, Cherk M, Topliss DJ. Novel use of rituximab in a case of Riedel's thyroiditis refractory to glucocorticoids and tamoxifen. J Clin Endocrinol Metab. 2013;98:3543–3549. doi: 10.1210/jc.2012-4050. [DOI] [PubMed] [Google Scholar]
- 51.Marín F, Araujo R, Páramo C, Lucas T, Salto L. Riedel's thyroiditis associated with hypothyroidism and hypoparathyroidism. Postgrad Med J. 1989;65:381–383. doi: 10.1136/pgmj.65.764.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamisawa T, Chen PY, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A, Hishima T. Pancreatic cancer with a high serum IgG4 concentration. World J Gastroenterol. 2006;12:6225–6228. doi: 10.3748/wjg.v12.i38.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi N, Ghazale AH, Smyrk TC, Mandrekar JN, Chari ST. Possible association between IgG4-associated systemic disease with or without autoimmune pancreatitis and non-Hodgkin lymphoma. Pancreas. 2009;38:523–526. doi: 10.1097/MPA.0b013e31819d73ca. [DOI] [PubMed] [Google Scholar]
- 54.Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in IgG4-related autoimmune disease of the parotid gland. Hum Pathol. 2009;40:881–886. doi: 10.1016/j.humpath.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Karagiannis P, Gilbert AE, Nestle FO, Karagiannis SN. IgG4 antibodies and cancer-associated inflammation: insights into a novel mechanism of immune escape. Oncoimmunology. 2013;2:e24889. doi: 10.4161/onci.24889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harada K, Shimoda S, Kimura Y, Sato Y, Ikeda H, Igarashi S, Ren XS, Sato H, Nakanuma Y. Significance of immunoglobulin G4 (IgG4)-positive cells in extrahepatic cholangiocarcinoma: molecular mechanism of IgG4 reaction in cancer tissue. Hepatology. 2012;56:157–164. doi: 10.1002/hep.25627. [DOI] [PubMed] [Google Scholar]
- 57.Hao SP, Chen JF, Yen KC. Riedel's thyroiditis associated with follicular carcinoma. Eur Arch Otorhinolaryngol. 1999;256:470–472. doi: 10.1007/s004050050192. [DOI] [PubMed] [Google Scholar]
- 58.Baloch ZW, Feldman MD, LiVolsi VA. Combined Riedel's disease and fibrosing Hashimoto's thyroiditis: a report of three cases with two showing coexisting papillary carcinoma. Endocr Pathol. 2000;11:157–167. doi: 10.1385/ep:11:2:157. [DOI] [PubMed] [Google Scholar]
- 59.Hosoi Y, Kono S, Terada T, Konishi T, Miyajima H. Hashimoto's encephalopathy associated with an elevated intrathecal IgG4 level. J Neurol. 2013;260:1174–1176. doi: 10.1007/s00415-013-6878-2. [DOI] [PubMed] [Google Scholar]
- 60.Bando H, Iguchi G, Fukuoka H, Taniguchi M, Yamamoto M, Matsumoto R, Suda K, Nishizawa H, Takahashi M, Kohmura E, Takahashi Y. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. Eur J Endocrinol. 2014;170:161–172. doi: 10.1530/EJE-13-0642. [DOI] [PubMed] [Google Scholar]