Abstract
Aims
We aimed to evaluate bleeding risk in clinical practice in patients with atrial fibrillation (AF) being prescribed dabigatran, rivaroxaban, or apixaban compared with warfarin.
Methods
Using nationwide registries (Norwegian Patient Registry and Norwegian Prescription Database), we identified AF patients with a first prescription of oral anticoagulants between January 2013 and June 2015. Patients were followed until discontinuation or switching of oral anticoagulants, death, or end of follow-up. The primary endpoint was major or clinically relevant non-major (CRNM) bleeding.
Results
In total 32 675 AF patients were identified (58% men, median age 74 years): 11 427 patients used warfarin, 7925 dabigatran, 6817 rivaroxaban, and 6506 apixaban. After a median follow-up of 173 days (25th, 75th percentile 84, 340), 2081 (6.37%) patients experienced a first major or CRNM bleeding. Using a Cox proportional hazard model adjusting for baseline characteristics, use of apixaban [hazard ratio (HR) 0.70, 95% confidence interval (CI) 0.61–0.80, P < 0.001] and dabigatran (HR 0.74, 95% CI 0.66–0.84, P < 0.001) were associated with a lower risk of major or CRNM bleeding compared with warfarin whereas use of rivaroxaban was not (HR: 1.05, 95% CI 0.94–1.17, P = 0.400). Use of dabigatran and rivaroxaban were associated with higher risk of gastrointestinal bleeding, whereas use of apixaban and dabigatran were associated with lower risk of intracranial bleeding, compared with warfarin.
Conclusion
In this nationwide cohort study in AF patients, apixaban and dabigatran were associated with a lower risk of major or CRNM bleeding compared with warfarin. The risk of gastrointestinal bleeding was higher with rivaroxaban and dabigatran compared with warfarin.
Keywords: Atrial fibrillation, Oral anticoagulants, Non-vitamin K antagonist oral anticoagulants, Warfarin, Dabigatran, Rivaroxaban, Apixaban, Bleeding
Introduction
Patients with atrial fibrillation (AF) are at increased risk of stroke.1 Warfarin and other vitamin K antagonists are effective treatments, reducing the risk of stroke by about two-thirds. The limitations with warfarin are a narrow therapeutic range, the need for monitoring, drug and food interactions, and risk of bleeding.2 In recent years, non-vitamin K antagonist oral anticoagulants (NOACs) (apixaban, dabigatran, and rivaroxaban) have been introduced as therapeutic alternatives to warfarin.3–5 NOACs are given at fixed doses and do not require regular monitoring. Information on the characteristics of patients being treated with NOACs in routine clinical practice in the early period after introduction is of interest to clinicians, in order to secure safe use of these drugs. In particular, there is an interest to know whether the outcomes observed in randomized clinical trials, especially the rates of bleeding events, are reflected in routine clinical practice, and whether there are differences between NOACs with regard to the risk of bleeding. Using two nationwide registries, we evaluated the bleeding outcomes in patients with AF being dispensed dabigatran, rivaroxaban, or apixaban compared with patients treated with warfarin.
Methods
Data sources
This study was based on data from two nationwide registries; the Norwegian Patient Registry (NPR) and the Norwegian Prescription Database (NorPD).6,7 The NPR was established in 2008 and contains all hospital visits (emergency visits, hospitalizations, and outpatient consultations), length of stay, and procedures (surgical and medical) from all hospitals in Norway. Diagnoses are coded according to the International Classification of Diseases, 10th revision (ICD10). Medical and surgical procedures are coded based on the Nordic Medico-Statistical Committee (NOMESCO) coding system. Both primary and subsequent codes related to each admission were taken into account in the analyses. The NorPD is a registry covering all prescriptions dispensed at pharmacies nationwide and data are available from 1 January 2004. Each medication is coded according to the anatomical therapeutic chemical system. The NorPD also includes information about date of dispensation, quantity and strength dispensed and the time of all-cause death. Any resident in Norway has a unique personal identifier that allows datasets to be merged on an individual level. The registry holder generated the datasets and released it in a coded and de-identified form, but with a unique identifier common to the two datasets making individual merging of the datasets possible. The two registries are mandatory in Norway and legally exempted from requirement of obtaining patient consent. The study was approved by the Regional Ethics Committee of Mid-Norway (Reference number 2015/162/REK midt).
Study population
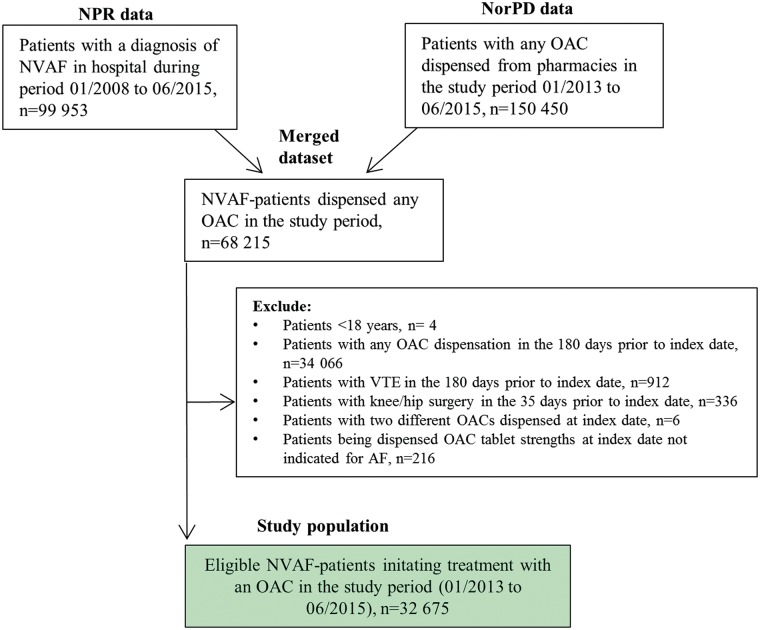
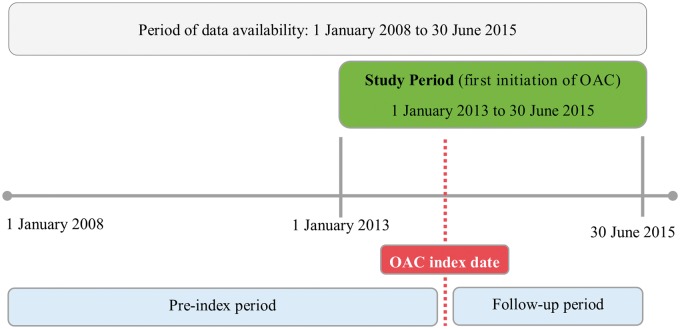
The study included all patients ≥18 years diagnosed with non-valvular AF with at least one warfarin or NOAC dispensation in the study period (1 January 2013–30 June 2015), but being anti-coagulant naïve before start of the study. Non-valvular AF was defined in accordance with the updated American Heart Association/American College of Cardiology 2014 guidelines as AF in the absence of rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.8 OAC naïve was defined as no OAC exposure in the preceding 180 days before index date. The index date was defined as the first dispensation of an OAC (warfarin 2.5 mg, dabigatran 110 or 150 mg, rivaroxaban 15 or 20 mg, and apixaban 2.5 or 5 mg) in the study period. Patients with venous thromboembolism during the last 180 days and those who had knee or hip replacement surgery during the last 35 days before starting OAC were excluded. A cohort creation flowchart is presented in Figure 1, and the study design in Figure 2.
Figure 1.
Cohort creation flow-chart. NorPD, Norwegian Prescription Database; NPR, Norwegian Patient Registry; NVAF, non-valvular atrial fibrillation; OAC, oral anticoagulant; VTE, venous thromboembolism.
Figure 2.
Study design. OAC index date was the date of the first OAC dispensation (warfarin, apixaban, rivaroxaban, dabigatran) in the study period (January 2013–June 2015). Each patient was followed from the index date to the date of discontinuation or switching of OAC therapy, date of death, or end of the study period. OAC, oral anticoagulant.
Co-morbidities and co-medication (listed in Table 1) were retrieved from NPR and NorPD (see Supplementary material online, Table S1 for code definitions). We calculated CHA2DS2VASc (congestive heart failure, hypertension, age ≥65, diabetes, prior stroke/TIA, vascular disease, and female sex category) score9,10 for assessing stroke risk, and a modified HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history/predisposition, labile international normalised ratio (INR), elderly ≥65, and drugs/alcohol abuse) score11 as a measure of bleeding risk and a co-morbidity score (see Supplementary material online, Tables S2–S4 for definitions of scores).
Table 1.
Baseline characteristics of the study population according to OAC treatment
| Warfarin | Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|---|
| Number of patients | 11 427 | 7925 | 6817 | 6506 |
| Men | 6737 (59.0) | 4915 (62.0) | 3711 (54.4) | 3579 (55.0) |
| Age, years | ||||
| Mean (SE) | 74.6 (11.9) | 70.8 (11.3) | 74.7 (10.7) | 74.5 (11.1) |
| Median (25th–75th percentile) | 76 (67–84) | 71 (64–79) | 75 (68–83) | 75 (68-83) |
| ≥75 years | 6248 (54.7) | 2967 (37.4) | 3524 (51.7) | 3295 (50.6) |
| Medical history | ||||
| Chronic kidney disease | 569 (5.0) | 58 (0.73) | 135 (2.0) | 163 (2.5) |
| Chronic heart failure | 3316 (29.0) | 1250 (15.8) | 1388 (20.4) | 1341 (20.6) |
| Diabetes | 1674 (14.7) | 822 (10.4) | 794 (11.7) | 797 (12.3) |
| Stroke, TIA, and thromboembolism | 1329 (11.6) | 745 (9.4) | 1096 (16.1) | 905 (13.9) |
| Ischaemic heart disease | 4102 (35.9) | 1699 (21.4) | 1736 (25.5) | 1795 (27.6) |
| Previous bleeding hospitalization | 1922 (16.8) | 890 (11.2) | 1009 (14.8) | 982 (15.1) |
| Previous OAC (>180 days prior to index) | 2910 (25.5) | 900 (11.4) | 748 (11.0) | 527 (8.1) |
| Active cancer (last year) | 1145 (10.0) | 589 (7.4) | 625 (9.2) | 562 (8.6) |
| COPD | 1064 (9.3) | 518 (6.5) | 580 (8.5) | 567 (8.7) |
| Hypertension | 7654 (67.0) | 4677 (59.0) | 4500 (66.0) | 4254 (65.4) |
| Anaemia (last year) | 553 (4.8) | 155 (2.0) | 203 (3.0) | 201 (3.1) |
| Viral hepatitis | 25 (0.22) | 16 (0.20) | 7 (0.10) | 11 (0.17) |
| Hospital admission last year | 7734 (67.7) | 4422 (55.8) | 4460 (65.4) | 4412 (67.8) |
| Co-medication | ||||
| Low-dose aspirin (last year) | 5420 (47.4) | 3687 (46.5) | 3621 (53.1) | 3304 (50.8) |
| NSAID (last year) | 2264 (19.8) | 1937 (24.4) | 1583 (23.2) | 1498 (23.0) |
| Non-aspirin anti-platelet inhibitors (last year) | 278 (2.4) | 185 (2.3) | 231 (3.4) | 189 (2.9) |
| Risk scores | ||||
| Modified HAS-BLED score ≥ 3 | 4894 (42.8) | 2934 (37.0) | 3206 (47.0) | 3029 (46.6) |
| CHA2DS2-VASc score | ||||
| Mean | 3.09 | 2.46 | 2.94 | 2.93 |
| ≥2 | 9449 (82.7) | 5785 (73.0) | 5709 (83.7) | 5411 (83.2) |
| Co-morbidity score ≥ 1 | 7527 (65.6) | 3851 (48.6) | 4124 (60.5) | 3916 (60.2) |
| Reduced NOAC dose at index date | NA | 2758 (34.8) | 1824 (26.8) | 1901 (29.2) |
Values are numbers (percentages) unless otherwise stated.
COPD, chronic obstructive lung disease; NSAID, non-steroidal anti-inflammatory drug; OAC, oral anticoagulant; NOAC, non-vitamin K oral anticoagulant; SE, standard error; TIA, transitoric ischaemic attack.
Definition of bleeding events and endpoints of the study
Bleeding was defined as all bleeding events recorded in NPR between index date and 30 days after the calculated end of OAC supply. Bleeding events were categorized as major or clinically relevant non-major (CRNM) bleeding based on available information from NPR. Major bleeding was defined as any bleeding event which occurred in a critical area or organ or any bleeding event that was accompanied by blood transfusion ≤10 days after hospital admission date. This is a slight modification of the International Society on Thrombosis and Haemostasis (ISTH) classification of major bleeding12 because no information was available in our data set on haemoglobin levels. A CRNM bleeding was defined in accordance with the ISTH classification12 as any bleeding requiring medical intervention by a health care professional, leading to hospitalization or increased level of care or prompting a face-to-face evaluation, that did not fit the criteria for major bleeding. The bleeding events were also categorized by organ system into gastrointestinal (GI), intracranial (ICH), or bleeding from other sites. Bleeding endpoints took into account all bleeds with the pre-specified ICD10 codes and were not restricted to admissions with bleeding as the primary (first) code. The primary endpoint of the study was a composite of major or CRNM bleeding. Secondary endpoints were major bleeding, CRNM bleeding, GI bleeding, ICH, and bleeds from other organ systems. See Supplementary material online, Table S5 for further details on bleeding codes.
Oral anticoagulant supply
For each dispensation, the OAC days of supply were computed using information on date of dispensation, the number of packages, and the pack-size dispensed. As NOACs are prescribed in a fixed dose, the number of days of supply strictly corresponds to amount dispensed. The NorPD contains information on tablet strength, pack-size and number of packages dispensed, and we assumed, according to the labelling, twice daily dosing for apixaban and dabigatran and once daily dosing for rivaroxaban, e.g. a patient supplied one package of a 60 tablet package of apixaban will have a supply lasting for 30 days whereas a rivaroxaban patient supplied one 100 tablet package will have a supply lasting 100 days. Computing the warfarin supply is not straightforward as we lack information on both dosing instructions and international normalized reference values. We therefore first calculated a median mg/day for all patients using warfarin in the study period (4.688 mg/day) and subsequently used this in the computation of warfarin supply for each dispensation, e.g. a patient dispensed one 100 tablet package of 2.5 mg strength will have a supply lasting for 53 days. We also needed to set the end of OAC supply date during the pre-index period to be able to determine whether a patient was OAC naïve or not (≥180 days without OAC supply prior to index date). We repeated the procedure for all warfarin dispensations during the pre-index period (median mg/day was estimated to 4.388 mg/day) and used this to estimate end of supply for each warfarin dispensation. To account for incomplete adherence, a gap period of 30 days within the calculated end of OAC supply was allowed. A patient continued treatment if next dispensation for the same OAC was within the 30 days after the calculated end of OAC supply. A patient switched treatment if another OAC was dispensed within 30 days after the calculated end of supply and finally the patient discontinued index OAC treatment if next OAC dispensation was more than 30 days after the calculated end of supply. Patients were censored if discontinuing or switching OAC, death, or end of follow-up, whichever occurred first.
Statistical analysis
Cox proportional hazard regression analyses were conducted to determine the risk of bleeding for the different NOACs vs. warfarin, both unadjusted and adjusted for known patient characteristics: age, gender, previous bleeding, previous OAC use, co-morbidities, and concomitant medications at baseline. Hence, the independent exposure of interest was which OAC patients used (with warfarin as the reference drug). Age was the only continuous patient characteristic. The linear assumption was checked by considering a model for the time to bleeding as a function of age, where the function was allowed to be non-linear (using splines). A final model was selected by backwards stepwise selection, using the Akaike information criterion as a measure of model fit. Each bleeding endpoint was compared with the entire cohort and not in contrast to non-bleeders only, e.g. for the major bleeding endpoint the comparison was with all non-major bleedings. The continuous variable (age) was described by the mean, standard deviation, median, and first and third quartiles. Categorical variables were described by the number and percentage of patients in each category. Crude incidence rates (IR) were also calculated as first bleeding episode per 100 person-years. Relative risks were given as hazard ratios (HRs) with 95% confidence intervals (CIs). Post hoc subgroup analyses, for the primary endpoint of major or CRNM bleeding, were performed for elderly patients (≥75 year) as well as for OAC dose levels at index date (standard and reduced dose) in comparison with warfarin. Power calculation was based on reported annual rates of major or CRNM bleeding in the pivotal clinical trials.3–5 These calculations indicated that with a sample size of approximately 2000 apixaban patients (apixaban was chosen because it had the shortest exposure time among the NOACs at the time of planning) and with a minimum of 1 year of follow-up, there would be acceptable levels of power (70–80%) in the comparison with warfarin. All statistical tests were two-tailed and P-values <0.05 were considered significant. Statistical analyses were performed using R (version 3.1.1, R Development Core Team).13
Results
The study population comprised a total of 32 675 patients. The mean age was 73.6 years (median 74 years) and 58% were males. Baseline characteristics in relation to the type of OAC being prescribed are presented in Table 1. Patients treated with dabigatran were younger, more likely to be men, had a lower co-morbidity load and lower baseline risk for stroke (CHA2DS2-VASc score) and bleeding (modified HAS-BLED) than patients treated with the other OACs. Warfarin patients had to a larger extent previously been exposed to OAC (>180 days prior to index) compared with the NOAC-treated patients. Apixaban- and rivaroxaban-treated patients had higher baseline risk of bleeding (modified HAS-BLED) compared with the other OACs.
The median follow-up time was as follows: warfarin 156 (25th, 75th percentile; 84, 309) days, dabigatran 212 (97, 413) days, rivaroxaban 209 (105, 410) days, and apixaban 143 (73, 247) days. A total of 2081 (6.37%) patients experienced a first major or CRNM bleeding episode; 419 patients (1.28%) experienced a major bleeding and 1662 patients (5.09%) a CRNM bleeding. By organ system, 594 patients (1.82%) experienced a GI bleeding, 207 patients (0.63%) an ICH, and 1280 patients (3.92%) experienced bleeding in other sites. Number and percentages of first time bleeding events for the different OACs are presented in Table 2.
Table 2.
Oral anticoagulant follow-up time and number (percentage) of patients experiencing a first time bleeding episode after initiating oral anticoagulant (subsequent bleeding episodes not considered) for the different bleeding endpoints
| Warfarin (n = 11 427) | Dabigatran (n = 7925) | Rivaroxaban (n = 6817) | Apixaban (n = 6506) | Total (n = 32 675) | |
|---|---|---|---|---|---|
| Follow-up time (days), median (25th–75th percentile) | 156 (84–309) | 212 (97–413) | 209 (105–410) | 143 (73–247) | 173 (84–340) |
| Major or CRNM bleeding: | 824 (7.21) | 407 (5.14) | 578 (8.48) | 272 (4.18) | 2081 (6.37) |
| Severity | |||||
| Major bleeding | 181 (1.58) | 80 (1.01) | 109 (1.60) | 49 (0.75) | 419 (1.28) |
| CRNM bleeding | 643 (5.63) | 327 (4.13) | 469 (6.88) | 223 (3.43) | 1662 (5.09) |
| Organ system | |||||
| GI bleeding | 199 (1.74) | 150 (1.89) | 175 (2.57) | 70 (1.08) | 594 (1.82) |
| ICH bleeding | 90 (0.79) | 28 (0.35) | 63 (0.92) | 26 (0.40) | 207 (0.63) |
| Other bleeding | 535 (4.68) | 229 (2.89) | 340 (4.99) | 176 (2.71) | 1280 (3.92) |
CRNM, Clinically relevant non-major; GI, gastrointestinal; ICH, intracranial haemorrhage.
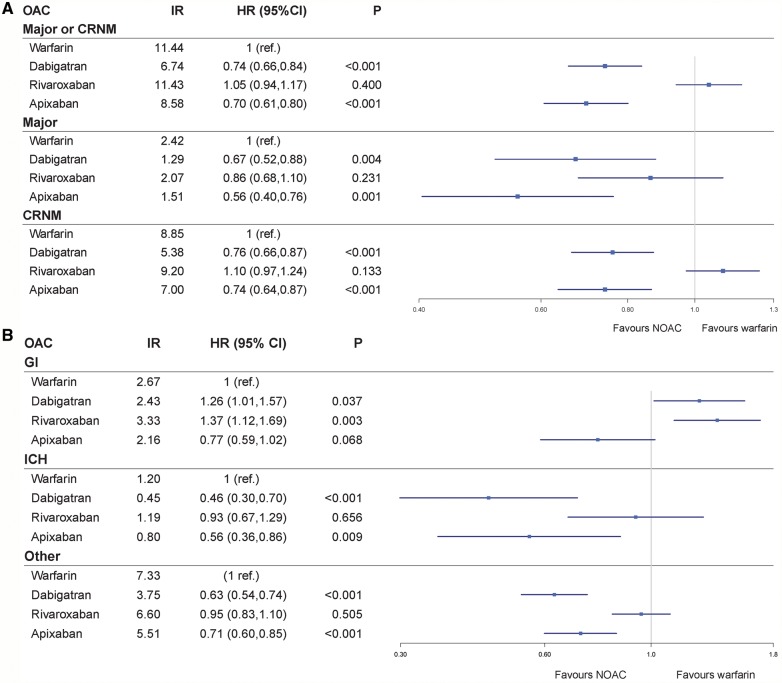
Crude IR for first bleeding events on warfarin and NOACs, and Forest plots showing the adjusted HRs for first bleeding episode for dabigatran, rivaroxaban and apixaban compared with warfarin, are presented in Figure 3. In Supplementary material online, Table S6, using the primary endpoint of major or CRNM bleeding, HRs and P-values for background variables for four different models are provided: (i) one variable at the time model; (ii) an age and OAC adjusted model; (iii) a model based on age, gender, OAC, and risk scores (CHA2DS2VASc, HAS-BLED, and co-morbidity scores); and (iv) the chosen final and optimal fitted Cox regression model including all variables (not risk scores) using the backward stepwise elimination function in R. Supplementary material online, Table S7 gives HRs for the endpoint of major or CRNM bleeding using apixaban as reference instead of warfarin.
Figure 3.
Forest plots showing the adjusted hazard ratios for first bleeding episode for dabigatran, rivaroxaban, and apixaban compared with warfarin. (A) Major or CRNM bleeding. (B) GI bleeding, ICH bleeding, and bleeding from other sites. Crude IR for first bleeding episode are given as events per 100 person-years. CI, confidence interval; CRNM, clinically relevant non-major bleeding; GI, gastrointestinal; HR, adjusted hazard ratio; ICH, intracranial haemorrhage; IR, incidence rate; OAC, oral anticoagulant.
After adjusting for differences in baseline characteristics, both dabigatran (adjusted HR 0.74, 95% CI 0.66–0.84, P < 0.001) and apixaban (adjusted HR 0.70, 95% CI 0.61–0.80, P < 0.001) were associated with a significant lower risk of major or CRNM bleeding compared with warfarin. There was no significant difference in major or CRNM bleeding risk between rivaroxaban and warfarin (adjusted HR 1.05, 95% CI 0.94–1.17, P = 0.400).
A time-restricted major or CRNM bleeding analysis with a cut-off at 180 days showed that dabigatran (adjusted HR 0.79, 95% CI 0.68–0.92, P = 0.002) and apixaban (adjusted HR 0.72, 95% CI 0.62–0.85, P < 0.001) both were associated with a significant lower risk of major or CRNM bleeding compared with warfarin. There was no significant difference in major or CRNM bleeding risk between rivaroxaban and warfarin (adjusted HR 1.06, 95% CI 0.93–1.21, P = 0.400).
Dabigatran (adjusted HR 1.26, 95% CI 1.01–1.57, P = 0.037) and rivaroxaban (adjusted HR 1.37, 95% CI 1.12–1.69, P = 0.003) use were both associated with a higher risk of GI bleeding compared with warfarin. There was no significant difference in the risk of GI bleeding using apixaban compared with warfarin (adjusted HR 0.77, 95% CI 0.59–1.02, P = 0.068).
The risk of ICH was lower in patients using dabigatran (adjusted HR 0.46, 95% CI 0.30–0.70, P < 0.001) and apixaban (adjusted HR 0.56, 95% CI 0.36–0.86, P = 0.009) compared with warfarin, but there was no significant difference between patients using rivaroxaban vs. warfarin (adjusted HR 0.93, 95% CI 0.67–1.29, P = 0.656).
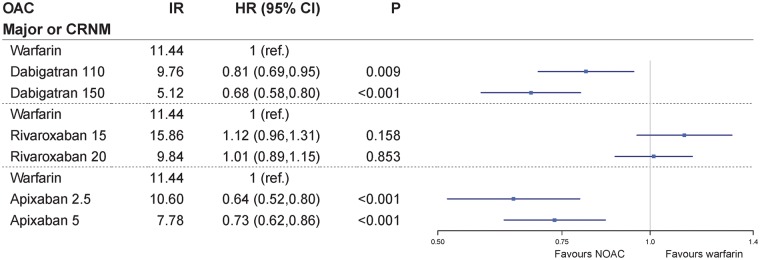
In the total population, 35% of dabigatran (n = 2758), 27% of rivaroxaban (n = 1824), and 29% of apixaban patients (n = 1901) initiated treatment on the reduced dose for stroke prevention (e.g. dabigatran 110 mg twice daily, rivaroxaban 15 mg once daily, or apixaban 2.5 mg twice daily) (Table 1). As many as 82% of patients receiving the reduced dose were ≥75 years, they were more likely to have other comorbidities as chronic kidney disease, hypertension and/or heart failure, and also more likely to have CHA2DS2-VASc score ≥2 and HAS-BLED score ≥3 (Supplementary material online, Table S8). A subgroup analysis of major or CRNM bleeding for the reduced and standard doses of each NOAC compared with warfarin is presented in Figure 4. Both the standard and reduced doses of apixaban and dabigatran were associated with a significant reduction in the primary endpoint of major or CRNM bleeding compared with warfarin. With respect to rivaroxaban, neither the reduced nor the standard dose was significantly different from warfarin.
Figure 4.
Risk of major or CRNM bleeding for the reduced and standard dose of dabigatran, rivaroxaban, and apixaban compared with warfarin. Crude IR for first bleeding episode are given as events per 100 person-years. CI, confidence interval; CRNM, clinically relevant non-major bleeding; HR, adjusted hazard ratio; IR, incidence rate; OAC, oral anticoagulant.
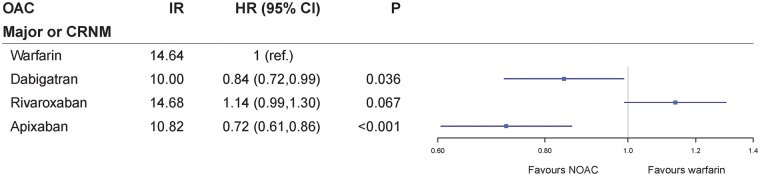
A total of 16 034 patients (49%) were ≥75 years (Table 1). In the subgroup of these elderly patients, dabigatran (adjusted HR 0.84, 95% CI 0.72–0.99, P = 0.036) and apixaban (adjusted HR 0.72, 95% CI 0.61–0.86, P < 0.001) were still associated with a lower risk of major or CRNM bleeding compared with warfarin, whereas rivaroxaban remained insignificant compared with warfarin (adjusted HR 1.14, 95% CI 0.99–1.30, P = 0.067) (Figure 5).
Figure 5.
Risk of major or CRNM bleeding for dabigatran, rivaroxaban, and apixaban compared with warfarin in the subgroup of patients ≥75 years. Crude IR for first bleeding episode are given as events per 100 person-years. CI, confidence interval; CRNM, clinically relevant non-major bleeding; HR, adjusted hazard ratio; IR, incidence rate; OAC, oral anticoagulant.
Discussion
In this large nationwide cohort of 32 675 patients (median age 74 years) with AF initiating OAC, the adjusted risk of major or CRNM bleeding was lower in patients treated with dabigatran and apixaban compared with patients treated with warfarin. In patients treated with rivaroxaban, the risk of major or CRNM bleeding was not significantly different from that of warfarin. For organ system divided analyses, this study demonstrated a similar risk of GI bleeding with apixaban compared with warfarin, whereas both dabigatran and rivaroxaban were associated with a higher risk of GI bleeding. Treatment with dabigatran and apixaban were both associated with a lower risk of ICH compared with warfarin, whereas treatment with rivaroxaban was not.
Our findings correspond with the bleeding outcomes reported in the pivotal outcome trials of the NOACs. A lower risk of bleeding with apixaban compared with warfarin was found in the ARISTOTLE trial.3,14,15 For dabigatran, our results are similar to the findings in the RE-LY trial of a higher risk of GI bleeding and a lower risk of ICH compared with warfarin, but differ with respect to the primary bleeding endpoint where we demonstrated a significantly lower risk of major or CRNM bleeding for both doses of dabigatran compared with warfarin.4 The higher risk of GI bleeding with rivaroxaban compared with warfarin was in line with the results of the ROCKET trial.5
A few observational studies assessing the comparative effectiveness and safety of dabigatran, rivaroxaban, and apixaban in comparison with warfarin in routine clinical practice have recently been reported. One recent study compared dabigatran and warfarin using US Medicare data and demonstrated a reduced risk of ischaemic stroke (dabigatran HR 0.80, 95% CI 0.67–0.96) and ICH (dabigatran HR 0.34, 95% CI 0.26–0.46), and an increased risk of major GI bleeding (dabigatran HR 1.28, 95% CI 1.14–1.44), with dabigatran compared with warfarin.16 Another study using a large US insurance database demonstrated that apixaban was associated with a lower risk of stroke or systemic embolism (apixaban HR 0.67, 95% CI 0.46–0.98, P = 0.04) and that dabigatran and apixaban were associated with a lower risk of major bleeding (dabigatran HR 0.79, 95% CI 0.67–0.94, P < 0.01; apixaban HR: 0.45, 95%CI: 0.34–0.59, P < 0.001).17 Interestingly, the findings on GI bleeding were in line with our results, showing a higher risk of GI bleeding in dabigatran- and rivaroxaban-treated patients compared with warfarin, whereas for apixaban the risk of GI bleeding was similar to warfarin. The study by Larsen et al.18 using nationwide Danish registries showed no significant difference in the rate of ischaemic stroke between any of the NOACs and warfarin. Dabigatran and apixaban were both associated with a statistically significant lower risk of major bleeding compared with warfarin, also in line with our findings. However, the study population was restricted to patients with standard doses of NOAC (dabigatran 150 mg twice daily, rivaroxaban 20 mg once daily, or apixaban 5 mg twice daily) and the study population was also younger (median age 71 years) compared with ours. Comparing the findings from our study with the reported pivotal outcome trials as well as observational studies must be interpreted with caution as there are differences in study populations, bleeding definitions and health care systems as well as other factors that are difficult to account for.
A high proportion of patients (27–35% of patients) in our study initiated NOAC therapy on the reduced dose for stroke prevention (e.g. dabigatran 110 mg twice daily, rivaroxaban 15 mg once daily, or apixaban 2.5 mg twice daily). Due to lack of information on creatinine levels, weight and bleeding diathesis, we do not know how many of these patients that fulfilled the criteria for dose reduction for stroke prevention for the different NOACs. However, 82% of these patients were ≥75 years, and also had a high baseline risk profile with respect to bleeding.
There was a difference in age distribution between the OACs at baseline with the proportion of patients being ≥75 years ranging from 55% for warfarin, 52% for rivaroxaban, 51% for apixaban, and 37% for dabigatran. This distribution is similar to what was seen in the US-based study by Yao et al.17 Notably, these data suggest that a higher proportion of NOAC-treated patients in routine clinical practice are ≥75 years compared with the pivotal outcome trials; e.g. in the ARISTOTLE trial only 31% of patients were reported being ≥75 years.15 Age is an established and strong predictor of increased bleeding risk in OAC-treated patients with higher absolute risks of bleeding reported among the elderly; however, the relative bleeding risk of NOACs in comparison to warfarin in the elderly was not markedly different from the overall population (Figure 5).
Strength and limitations
The strength of our study is that it retrieves data from mandatory and nationwide registries in a public health care system that covers all residents. As a result, the dataset at hand gave us a complete picture of all hospitalizations and prescriptions dispensed nationwide for the entire study period. This complete coverage of data eliminates also selection bias and recall bias that is an apparent problem using other databases being based on selected hospitals, health insurance schemes, or self-reported questionnaires.
An obvious limitation of this study is that we have not considered effectiveness in stroke prevention. As stroke events occur less frequently than overall bleeding complications of OAC treatment, and also considering the still early days of NOAC exposure, this study was not planned for, and deemed sufficiently powered to, evaluating NOAC effectiveness on stroke outcomes. More research is therefore needed to address the bleeding complications of NOACs in the context of stroke prevention in an unselected clinical practice setting.
Although we have adjusted for baseline differences, we are unlikely to have captured the full extent and effect of different prescribing behaviour, especially in this early phase of NOAC introduction, and some unmeasured and residual confounding is undoubtedly still present. With the exception of apixaban being granted general reimbursement 6 months after rivaroxaban and dabigatran, the same conditions for OAC prescribing were valid nationwide and throughout the study period. The time-restricted 180-day bleeding risk analysis gives support to our chosen approach and that results are robust irrespective of differences in OAC follow-up time. We did not have access to information on time in therapeutic range among warfarin users; nor did we have information on laboratory tests and other characteristics such as smoking and weight. One other caveat that influences the external validity of the results is that the AF diagnosis was retrieved from the hospital level only, meaning that AF patients that were solely managed in primary care were not included in the study. Apart from co-medication, co-morbidities from primary care can be underrepresented. There is also a risk of misclassification related to coding errors of hospital admissions; however for serious conditions like bleeding this is not very likely. No formal validation studies of the AF diagnosis in NPR against health records have been conducted. We studied drug exposure at the level of pharmacy dispensation and have no information on patient’s real OAC intake.
Conclusion
In this nationwide cohort study on AF patients being prescribed OAC, use of apixaban and dabigatran were associated with a lower risk of major or CRNM bleeding compared with the use of warfarin. The risk of GI bleeding was higher among users of dabigatran and rivaroxaban compared with warfarin, whereas users of apixaban and dabigatran had a lower risk of ICH compared with users of warfarin. The risk of stroke was not addressed in this study, and hence the optimal benefit to risk balance between stroke prevention and bleeding could not be evaluated.
Disclaimer
Data from the Norwegian Patient Registry (NPR) have been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the NPR is intended nor should be inferred.
Supplementary material
Supplementary material is available at European Heart Journal - Cardiovascular Pharmacotherapy online.
Funding
This study was sponsored by Pfizer Inc., New York, NY, USA.
Conflict of interest: S.H. reports personal fees from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Sanofi, Merck, Novartis. C.J. reports personal fees from Pfizer, during the conduct of the study; personal fees from Pfizer and Bayer, outside the submitted work. I.F.T. reports grant from Pfizer, during the conduct of the study. P.F. is an employee of Pfizer. O.S. is an employee of Pfizer. C.H. is an employee of Bristol-Myers Squibb. W.G. reports grants and personal fees from Bayer, personal fees from Pfizer, Boehringer Ingelheim and Novartis, grants from Roche, outside the submitted work.
References
- 1.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 2.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 5.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 6.Wettermark B, Zoëga H, Furu K, Korhonen M, Hallas J, Nørgaard M, Almarsdottir A, Andersen M, Andersson Sundell K, Bergman U, Helin-Salmivaara A, Hoffmann M, Kieler H, Martikainen J, Mortensen M, Petzold M, Wallach-Kildemoes H, Wallin C, Sørensen H. The Nordic prescription databases as a resource for pharmacoepidemiological research–a literature review. Pharmacoepidemiol Drug Saf 2013;22: 691–699. [DOI] [PubMed] [Google Scholar]
- 7.Bakken IJ, Nyland K, Halsteinli V, Kvam UH, Skjeldestad FE. The Norwegian patient registry. Nor J Epidemiol 2004;14:65–69. [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, ACC/AHA Task Force Members. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071–2104. 2014. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 10.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, ESC Committee for Practice Guidelines (CPG). Focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–2747. [DOI] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 12.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 2015;13:2119–2126. [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing; http://www.R-project.org/ (10 September 2016). [Google Scholar]
- 14.Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, Huber K, Jansky P, Steg PG, Hanna M, Thomas L, Wallentin L, Granger CB. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE trial: predictors, characteristics, and clinical outcomes. J Am Coll Cardiol 2014;63:2141–2147. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, Granger CB, Hanna M, Held C, Husted S, Hylek EM, Jansky P, Lopes RD, Ruzyllo W, Thomas L, Wallentin L. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J 2014;35:1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 17.Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsen TB, Skjoth F, Nielsen PB, Kjaeldgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ 2016;353:i3189.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.