Abstract
Aims
Aortic valve stenosis (AS) is the most common valvulopathy and is characterized by inflammation, extracellular matrix (ECM) remodelling and calcification, causing a narrowing of the valve and the consequential obstruction of the cardiac outflow. Although intraleaflet haemorrhage is associated with AS progression, the mechanisms involved are not known. The aims of this study were to identify valvular iron in relation to pathological changes associated with AS and the effects on valvular interstitial cells (VIC) in terms of iron uptake and iron-induced responses.
Methods and results
Valvular iron accumulation was detected by Perls' staining on aortic valve sections and shown to increase with the extent of calcification. Furthermore, qRT–PCR analysis revealed that iron-containing valve regions exhibited increased expression of genes involved in ECM remodelling and calcification. In addition, we demonstrate that iron transporters are regulated by pathways with major impact on AS and that VIC can take up and accumulate iron, which resulted in increased proliferation and decreased elastin production.
Conclusion
Iron, which may accumulate in the aortic valve by means of intraleaflet haemorrhages, can be taken up by VIC in a pro-inflammatory environment and actively contribute to VIC proliferation, ECM remodelling and calcification. These findings suggest a possible mechanism through which iron uptake by VIC may favour AS progression.
Keywords: Aortic valve stenosis, Calcification, Iron, Elastin
Translational perspective.
Biomechanical factors on aortic valves may induce haemorrhages leading to an accumulation of valvular iron. Here, we demonstrate an association of valvular iron with the degree of aortic valve calcification. In addition, we show that iron uptake by valvular interstitial cells stimulated proliferation and extracellular matrix remodelling. Taken together, these results suggest possible mechanisms through which valvular iron favours aortic valve stenosis progression. These findings emphasize the pathophysiological role of valvular haemorrhages and suggest iron transporters as a novel potential therapeutic target to retard hemodynamic progression of aortic stenosis.
Introduction
Aortic valve stenosis (AS), the most common valvulopathy among adults,1 is characterized by inflammatory processes, extracellular matrix (ECM) remodelling and calcification, causing a narrowing of the valve and the consequential obstruction in the cardiac outflow.2 Valvular interstitial cells (VIC) control the structure and function of the aortic valve. During AS progression, VIC differentiate into the pathological myofibroblast and osteoblast-like type of cell that promote inappropriate ECM remodelling and subsequent valve calcification.2
In atherosclerosis, the extravasation of blood inside the lesion, intraplaque haemorrhage, triggers an inflammatory response, increases cell proliferation, and enhances proteolytic activities that lead to ECM remodelling and plaque rupture.3 Two recent observational studies have described internal bleedings in the cusps of stenotic aortic valves.4,5 Although those studies associated intraleaflet haemorrhage with AS progression,4 the possible pathophysiology of iron accumulation in the valve and the role of VIC have remained hitherto unexplored.
The aims of this study are to identify valvular iron in relation to pathological changes associated with AS and the effects on VIC in terms of iron uptake and iron-induced responses. By a combination of observations in human aortic valves in relation to clinical characteristics as well as evaluation of iron-induced responses in VIC, the present study provides a first mechanistic insight into the role of iron accumulation in ECM remodelling and calcification and how VIC participate in the process.
Materials and methods
Clinical data are presented as jittered dot plots and experimental data as mean ± SD. All statistical analyses were two-sided. Adjusted P-values are shown for multiple comparisons. Supplementary material online, Tables S1 and S2 display patient characteristics. Expanded methods are provided in the Supplementary materials online.
Results
Iron positivity according to tissue characteristics and diagnosis
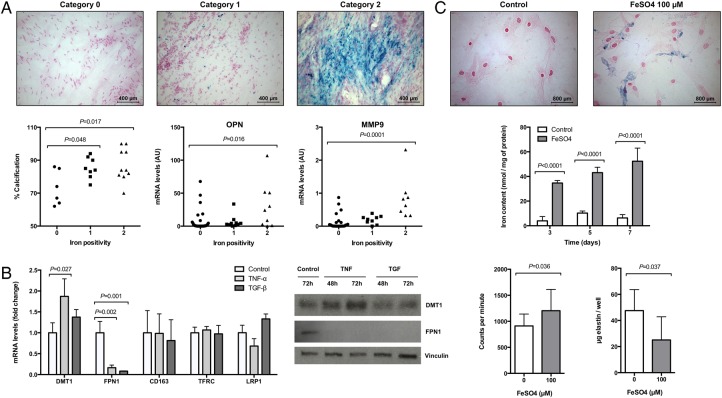
The characteristics of aortic valve regions by degree of iron positivity, as determined by Perls' staining, are shown in Figure 1A. Iron positivity (Categories 1 and 2) detected in non-calcified tissue was 27.6%, which was significantly lower compared with calcified tissue, 60.5% (P = 0.007). Furthermore, tissues derived from bicuspid stenotic valves exhibited a higher proportion of iron positivity, 59.4%, compared with tricuspid stenotic valves, 34.3% (P = 0.039), with the most pronounced iron accumulation in calcified tissue, exhibiting an iron positivity of 82.4 and 42.9% for bicuspid and tricuspid stenotic valves, respectively (P = 0.013). The number of observations is given in Supplementary material online, Table S3.
Figure 1.
Iron accumulation in stenotic valves is associated with calcification and affects valvular interstitial cells function. (A) Histological classification of iron positivity in human aortic valves using Perls' staining and its relation with calcification and gene expression. (B) Changes in mRNA and protein expression in valvular interstitial cells after stimulation with tumour necrosis factor-α and transforming growth factor-β for 24 or 48–72 h, respectively (n = 3). (C) Intracellular iron detection and quantification in valvular interstitial cells incubated with FeSO4 (100 µmol/L) for 72 h (n = 3) and its effect in 3H-thymidine incorporation (expressed in counts per minute; n = 3) and elastin production (n = 6). Multiple comparisons show adjusted P-values.
Iron positivity associations with clinical characteristics and gene expression
Stratifying AS patients according to iron positivity revealed a positive association with the degree of valve calcification (Figure 1A). In addition, gene expression according to the histological classification of directly adjacent tissue was performed in valves derived from 26 patients and revealed an increase in the expression of osteopontin (OPN) and matrix metalloproteinase 9 (MMP9; Figure 1A).
Tumour necrosis factor-α and transforming growth factor-β regulate iron transporters in valvular interstitial cells
In terms of mRNA and protein levels, tumour necrosis factor-α (TNF-α) increased divalent metal transporter 1 (DMT1) and decreased ferroportin 1 (FPN1). Transforming growth factor-β (TGF-β) decreased FPN1 but did not significantly alter DMT1. Neither TNF-α nor TGF-β significantly altered the expression of CD163, transferrin receptor (TFRC), and low-density lipoprotein receptor-related protein 1 (LRP1) (Figure 1B).
Ferroportin 1 expression is reduced in valvular interstitial cells obtained from stenotic valves
Valvular interstitial cells derived from stenotic valves exhibited significantly (n = 3) lower mRNA levels of FPN1 compared with those derived from control valves (fold change: 0.48 ± 0.14; P = 0.022), whereas DMT1 mRNA levels remained similar in the two VIC populations (fold change: 0.93 ± 0.17; P = 0.67).
Valvular interstitial cells and iron
Perls' staining of VIC incubated for 72 h with Ferrous sulfate (FeSO4; 100 µmol/L) revealed an intracellular blue staining (Figure 1C). Quantitative iron measurements revealed a significant and time-dependent increase in intracellular iron concentrations in the presence of FeSO4 (Figure 1C). FeSO4 (0–200 µmol/L) did not affect cell viability (see Supplementary material online, Figure S1).
Changes in VIC mRNA levels (n = 3) encoding components of iron storage, transport, and metabolism were evaluated in the presence of 100 µmol/L FeSO4. The expression of ferritin light (FTL) and heavy (FTH1) chains was significantly up-regulated 1.46 ± 0.25 fold (P = 0.019) and 1.62 ± 0.10 fold (P = 0.025) at 48 h; 1.50 ± 0.27 fold (P = 0.011) and 1.69 ± 0.33 fold (P = 0.013) at 72 h, respectively. In addition, FeSO4 significantly down-regulated TFRC 0.61 ± 0.06 fold (P = 0.011) and up-regulated CD163 1.80 ± 0.76 fold (P = 0.044), and haem oxygenase 1 (HO-1) 1.90 ± 0.64 fold (P = 0.03); whereas LRP1, DMT1, and FPN1 remained unaltered after 24 h incubation (see Supplementary material online, Figure S1).
Valvular interstitial cells incubated for 72 h with 100 µmol/L FeSO4 exhibited significantly increased proliferation and reduced elastin production (Figure 1C).
Discussion
Three novel and important notions emerge from the present study. First, non-haem iron accumulates in AS in relation to the degree of calcification and is associated with an increased expression of genes that promote ECM remodelling and calcification. Second, VIC can take up and accumulate iron, and iron transporters are regulated by pathways with a major impact on AS pathophysiology. Third, iron induced VIC proliferation and decreased elastin production. Taken together, these results provide mechanistic insights and suggest potential pathways linking, for example, intraleaflet haemorrhage to a maladaptive response to injury and valvular calcification.
Using Perls' staining, the present study is the first report of non-haem-bound iron in human aortic valves. Previous studies have used either glycophorin A staining to identify haemorrhages within aortic valve leaflets4,5 or biochemical methods to determine total valvular iron content.6 In the present study, iron positivity prevailed in calcified regions of stenotic aortic valves, albeit also being detected in non-calcified areas. These results were supported by an increased valvular calcification in iron-positive human stenotic aortic valves and by the finding that iron-containing valve regions exhibited increased expression of OPN and MMP9; genes involved in valve remodelling and calcification, suggesting that iron may induce AS progression.
The source of valvular iron may originate from intraleaftlet haemorrhages and haem metabolism by macrophages, which co-localize with extravasated erythrocytes in human aortic valves. Although incompletely explored in valvular pathologies, the clearance of erythrocytes by macrophages in human atherosclerotic lesions results in the release of free iron from phagocytes to the extracellular space through FPN1.7 In further support of a connection between the observed iron positivity and intraleaflet haemorrhages, both are more common in leaflets derived from bicuspid than from tricuspid aortic valves as demonstrated in the present and previous5 studies.
Certain limitations of the histological approach should be acknowledged. Using Perls' staining in sections rather than whole valves may underestimate iron positivity; however, the large number of analysed sections generated a comprehensive map while allowing gene expression measurements. The observational design of the histological part of the study precludes any conclusions as to the causality of the associations between calcification and iron. Therefore, we subsequently sought for potential mechanisms by which iron may be linked to the pathophysiology of AS.
The two cytokines TNF-α and TGF-β that promote the acquisition of VIC phenotypes associated with AS2 induced differential effects on the expression levels of iron transporters. Both mRNA and protein levels of the iron-importer DMT1 were up-regulated by TNF-α. Interestingly, DMT1 transports free iron from the extracellular to the intracellular space. Other iron transporters, which mediate the uptake of iron in its haem-bound form, as haemoglobin–haptoglobin complexes or transferrin, were unaltered by cytokine treatment. These results indicate that the free-iron uptake may be preferentially inducible. Furthermore, both TNF-α and TGF-β almost abolished mRNA and protein levels of the iron-exporter FPN1, suggesting that a decreased iron export can also be induced. In support of these results, VIC isolated from stenotic valves expressed significantly lower levels of FPN1 than those derived from non-calcified valves. Taken together, these results indicate that in the context of AS, VIC may acquire a phenotype that predisposes for both the uptake and accumulation of iron.
One of the major findings of the present study is the discovery of a time-dependent free-iron uptake in VIC. In the presence of FeSO4, also ferritin subunits were up-regulated, indicating a self-regulated increased iron storage capacity. Consistent with these results, an increased expression of both ferritins occurs in α-actin positive regions of advanced atherosclerotic lesions; regions that also exhibited iron positivity.8 These findings suggest that VIC and smooth muscle cells share the characteristics of an inducible iron storage under pathological conditions.
Furthermore, we provide the first piece of evidence linking iron accumulation with increased VIC proliferation. In AS, the Wnt/β-catenin pathway is a main driver of VIC proliferation,9 and increased intracellular iron concentrations promotes Wnt/β-catenin signalling in other cell types.10 In addition, VIC decreased elastin production in response to iron. This finding is in agreement with a previous publication in skin fibroblasts.11 This decreased elastin production demonstrated in vitro and the increased levels of MMP9 demonstrated in human aortic valves, together with the well-established role of decreased elastin production and increased degradation in AS development,12,13 argue in favour of the potential effect of iron as a modifying element in the structure of the aortic valve that can promote AS progression.
In summary, our study provides both observational support from human tissue analysis and mechanistic evidence that non-haem-bound valvular iron is associated with ECM remodelling and calcification in AS. In addition, we demonstrate that VIC can take up and accumulate iron, which affects the response to injury in terms of VIC proliferation and elastin production.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
A.L.-F., G.J., and M.B. performed statistical analysis. P.E., A.F.-C., and M.B. handled funding and supervision. A.L.-F., M.C., G.J., E.N., and M.B. acquired the data. A.L.-F. and M.B. conceived and designed the research. A.L.-F. and M.B. drafted the manuscript. A.L.-F., M.C., G.J., E.N., P.E., G.C., A.F.-C., and M.B. made critical revision of the manuscript for key intellectual content.
Funding
This work was supported by the Swedish Research Council (Grant number 2014-2312); the Swedish Heart and Lung Foundation (grant numbers 20150600 and 20150683) and the Stockholm County Council (grant number 20140222). Funding to pay the Open Access publication charges for this article was provided by the Swedish Research Council.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, Hofmann-Bowman MA, Mortlock DP, Rogers MB, Sadeghi MM, Aikawa E. Calcific aortic valve disease: a consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler Thromb Vasc Biol 2014;34:2387–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Michel J-B, Martin-Ventura JL, Nicoletti A, Ho-Tin-Noé B. Pathology of human plaque vulnerability: mechanisms and consequences of intraplaque haemorrhages. Atherosclerosis 2014;234:311–319. [DOI] [PubMed] [Google Scholar]
- 4. Akahori H, Tsujino T, Naito Y, Matsumoto M, Lee-Kawabata M, Ohyanagi M, Mitsuno M, Miyamoto Y, Daimon T, Hao H, Hirota S, Masuyama T. Intraleaflet haemorrhage is associated with rapid progression of degenerative aortic valve stenosis. Eur Heart J 2011;32:888–896. [DOI] [PubMed] [Google Scholar]
- 5. Akahori H, Tsujino T, Naito Y, Yoshida C, Lee-Kawabata M, Ohyanagi M, Mitsuno M, Miyamoto Y, Daimon T, Masuyama T. Intraleaflet haemorrhage as a mechanism of rapid progression of stenosis in bicuspid aortic valve. Int J Cardiol 2013;167:514–518. [DOI] [PubMed] [Google Scholar]
- 6. Nyström-Rosander C, Lindh U, Friman G, Lindqvist O, Thelin S, Ilbäck N-G. Trace element changes in sclerotic heart valves from patients are expressed in their blood. Biometals 2004;17:121–128. [DOI] [PubMed] [Google Scholar]
- 7. Bories G, Colin S, Vanhoutte J, Derudas B, Copin C, Fanchon M, Daoudi M, Belloy L, Haulon S, Zawadzki C, Jude B, Staels B, Chinetti-Gbaguidi G. Liver X receptor activation stimulates iron export in human alternative macrophages. Circ Res 2013;113:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pang JH, Jiang MJ, Chen YL, Wang FW, Wang DL, Chu SH, Chau LY. Increased ferritin gene expression in atherosclerotic lesions. J Clin Invest 1996;97:2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu S, Liu AC, Gotlieb AI. Wnt3a/β-catenin increases proliferation in heart valve interstitial cells. Cardiovasc Pathol 2013;22:156–166. [DOI] [PubMed] [Google Scholar]
- 10. Brookes MJ, Boult J, Roberts K, Cooper BT, Hotchin NA, Matthews G, Iqbal T, Tselepis C. A role for iron in Wnt signalling. Oncogene 2008;27:966–975. [DOI] [PubMed] [Google Scholar]
- 11. Bunda S, Kaviani N, Hinek A. Fluctuations of intracellular iron modulate elastin production. J Biol Chem 2004;280:2341–2351. [DOI] [PubMed] [Google Scholar]
- 12. Hinton RB, Adelman-Brown J, Witt S, Krishnamurthy VK, Osinska H, Sakthivel B, James JF, Li DY, Narmoneva DA, Mecham RP, Benson DW. Elastin haploinsufficiency results in progressive aortic valve malformation and latent valve disease in a mouse model. Circ Res 2010;107:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helske S, Syväranta S, Lindstedt KA, Lappalainen J, Oörni K, Mäyränpää MI, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol 2006;26:1791–1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.