Abstract
Background
Valvular heart disease (VHD) is expected to become more common as the population ages. However, current estimates of its natural history and prevalence are based on historical studies with potential sources of bias. We conducted a cross-sectional analysis of the clinical and epidemiological characteristics of VHD identified at recruitment of a large cohort of older people.
Methods and results
We enrolled 2500 individuals aged ≥65 years from a primary care population and screened for undiagnosed VHD using transthoracic echocardiography. Newly identified (predominantly mild) VHD was detected in 51% of participants. The most common abnormalities were aortic sclerosis (34%), mitral regurgitation (22%), and aortic regurgitation (15%). Aortic stenosis was present in 1.3%. The likelihood of undiagnosed VHD was two-fold higher in the two most deprived socioeconomic quintiles than in the most affluent quintile, and three-fold higher in individuals with atrial fibrillation. Clinically significant (moderate or severe) undiagnosed VHD was identified in 6.4%. In addition, 4.9% of the cohort had pre-existing VHD (a total prevalence of 11.3%). Projecting these findings using population data, we estimate that the prevalence of clinically significant VHD will double before 2050.
Conclusions
Previously undetected VHD affects 1 in 2 of the elderly population and is more common in lower socioeconomic classes. These unique data demonstrate the contemporary clinical and epidemiological characteristics of VHD in a large population-based cohort of older people and confirm the scale of the emerging epidemic of VHD, with widespread implications for clinicians and healthcare resources.
Keywords: Epidemiology, Valvular heart disease, Echocardiography, Health policy and outcome research
Introduction
Valvular heart disease (VHD) is an important cause of reduced functional capacity, heart failure, arrhythmia, recurrent hospital admission, and early mortality. The combination of an ageing population, earlier diagnosis, and greater availability of surgical and percutaneous interventions heralds a major increase in the healthcare resources required for its optimal management.1,2
There is therefore a pressing need to better understand the contemporary clinical and epidemiological characteristics of VHD, particularly in the elderly population. Previous studies have either been hospital based3 or retrospective in design4,5 and thus subject to significant selection biases. Broader epidemiological studies provide limited data and there are no contemporary prospective large-scale population-based studies specific to VHD in developed countries. Available retrospective data demonstrate an increasing prevalence with age, predominantly as a result of degenerative pathophysiology.3–5 A major increase in the prevalence of VHD would therefore seem an inevitable consequence of the anticipated increase in the number of older people in the population. Reliable contemporary data demonstrating the prevalence of VHD are an essential requirement for researchers and clinicians to improve understanding of its underlying pathophysiology, risk factors, and natural history; and for policy makers and economists to plan the provision of health services for both surgical and percutaneous interventions and long-term medical care.
Accordingly, we established a large prospective study to provide a unique population-based evaluation of the contemporary clinical and epidemiological characteristics of VHD and create well defined and carefully phenotyped cohorts with individual valve lesions for future study. Herein, we present a cross-sectional analysis of the population prevalence of undiagnosed and known VHD in the first 2500 participants and quantify the community prevalence of milder forms of VHD for the first time.
Methods
The OxVALVE Population Cohort Study (OxVALVE-PCS) is an ongoing prospective cohort study conducted in Oxfordshire, UK. Methodological details have been previously reported elsewhere.6 In brief, subjects aged 65 years and older without known VHD who were registered with one of five primary care medical centres were invited to participate. Younger subjects were excluded since (i) previous retrospective studies have demonstrated that the prevalence of VHD is low in those aged <65 years4 and (ii) uptake of community screening was likely to be low in those with work or family commitments. The participating medical centres were representative of the local population demographics and selected for the availability of accurate patient databases with comprehensive search facilities. Subjects with a previous diagnosis of VHD (identified using relevant National Health Service diagnostic Read codes) were not included in the echocardiographic study but their diagnostic data were collected to derive total prevalence. Exclusion criteria included terminal illness and immobility or general frailty precluding attendance (as judged by the general practitioner/family physician). Eligible subjects received an initial study invitation letter with a single follow-up reminder to non-responders—further contact with potential study participants was not permitted by the local research ethics committee which approved the study. All participants provided written informed consent.
Participant assessment
An investigating physician or British Society of Echocardiography (BSE) accredited sonographer undertook clinical and transthoracic echocardiographic assessment in the participant's local medical centre. Socioeconomic class (SEC) was determined using Index of Multiple Deprivation (IMD) scores based upon home address postcode. Each SEC corresponds to 20% of the national population (with SEC1/SEC5 denoting the least/most deprived quintiles, respectively).
Diagnostic criteria
The primary outcome was a finding of mild or more severe left-sided VHD or moderate or more severe right-sided VHD. Valve anatomy, physiology, and severity of VHD were defined according to BSE criteria7 and international guidelines.8,9 Aortic sclerosis (AoScl) was defined according to 2009 European Association of Echocardiography/American Society for Echocardiography guidelines9—thickening and focal calcification of the aortic valve leaflets, with normal or near normal cusp mobility and maximum aortic transvalvular velocity ≤2.5 m/s. Aortic stenosis (AS) was defined as aortic valve thickening or calcification with a maximum aortic transvalvular velocity >2.5 m/s.
Statistical analysis
Participants were stratified by age and gender to explore the prevalence of VHD and examine demographic and clinical characteristics. Descriptive statistics for the study cohort are presented using means and standard deviations (SD) for continuous variables, and counts (percentages) for categorical variables. Student's t-test and Chi-squared test or Fisher's exact test were used to explore associations between VHD and quantitative and categorical variables, respectively. Logistic regression models were used to assess associations of newly diagnosed VHD. For multivariate regression, all variables with initial univariate regression P-value < 0.10 were included and subsequently removed if the Wald test P-value was >0.05. All results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs) and a two-tailed P-value ≤ 0.05 considered significant (χ2 distribution P-value with 1 degree of freedom). Total prevalence of clinically significant (moderate or severe) VHD was obtained by combining data on newly diagnosed and pre-existing VHD (assuming that moderate or severe VHD would result in a clinical diagnosis). To derive population projections for significant VHD, we applied our age-specific prevalence data to Office of National Statistics projections (based upon the 2012 UK census) using gender-specific 5-year age-bands.
Results
Demography
Initial screening of primary healthcare records in study centres demonstrated that 2.9% of potential participants aged 65 years and older were deceased, 3.1% were no longer registered with the practice, and 4.9% had a pre-existing diagnosis of VHD. A further 13.2% were excluded from the echocardiographic study for other reasons (e.g. cognitive decline, terminal non-cardiac disease, or severe immobility). Study uptake among the remaining potential study population in the first practice to complete recruitment was 53%, consistent with previous community-based echocardiographic screening studies for cardiovascular disease (Supplementary material online, Figure S1).10,11
In the first 2500 enrolled participants, the mean age (SD) was 73 (6) years and 51.5% were female (Table 1), consistent with the demographics of the wider community population—UK 2011 Census data indicate that the mean age of the entire Oxfordshire population aged 65 years and older is 75.3 years (55.8% female). Almost all the study participants were of White ethnic background (99%) and relatively affluent, reflecting the demography of England and Wales, where 95% aged ≥65 years are of White ethnicity.12 Similarly, the socioeconomic status of the study cohort (assessed by mean IMD score) was comparable with that of Oxfordshire as a whole (OxVALVE cohort 11.68, Oxfordshire 12.26).13
Table 1.
Demographics of study participants with and without valvular heart disease
| No VHD (SD or % of those with condition) | VHD (SD or % of those with condition) | P-value | |
|---|---|---|---|
| Number of participants | 1231 (49.2%) | 1269 (50.8%) | |
| Age (years) | 71.8 (5.3) | 74.2 (6.5) | <0.001 |
| Gender | |||
| Male (%) | 612 (50.5%) | 600 (49.5%) | |
| Female (%) | 619 (48.1%) | 669 (51.9%) | 0.239 |
| Smoking status | |||
| Non-smoker | 586 (47.6%) | 646 (52.4%) | |
| Ex-smoker | 544 (49.7%) | 550 (50.3%) | |
| Smoker | 101 (58.0%) | 73 (42.0%) | 0.032 |
| Socioeconomic class | |||
| 1 (least deprived) | 647 (51.7%) | 605 (48.3%) | |
| 2 | 280 (50.1%) | 279 (49.9%) | |
| 3 | 207 (47.9%) | 225 (52.1%) | |
| 4 or 5 (most deprived) | 97 (37.9%) | 159 (62.1%) | <0.001 |
| NYHA class | |||
| I/II | 1196 (49.1%) | 1240 (50.9%) | |
| III/IV | 35 (54.7%) | 29 (45.3%) | 0.449 |
| History | |||
| Diabetes mellitus | 143 (50.7%) | 139 (49.3%) | 0.645 |
| Hypertension | 508 (45.2%) | 615 (54.8%) | <0.001 |
| Hyperlipidaemia | 413 (46.3%) | 479 (53.7%) | 0.032 |
| Atrial fibrillation | 35 (32.1%) | 74 (67.9%) | <0.001 |
| Myocardial infarction | 51 (38.9%) | 80 (61.1%) | 0.02 |
| Coronary angiography | 86 (38.4%) | 138 (61.6%) | <0.001 |
| Percutaneous coronary intervention | 42 (43.3%) | 55 (56.7%) | 0.276 |
| Coronary artery bypass grafting | 15 (31.9%) | 32 (68.1%) | 0.024 |
| Angina | 101 (47.4%) | 112 (52.6%) | 0.628 |
| Stroke/TIA | 60 (38.7%) | 95 (61.3%) | 0.009 |
| Rheumatic fever | 20 (44.4%) | 25 (55.6%) | 0.618 |
| Examination | |||
| Heart rate (bpm) | 73.9 (11.9) | 71.7 (11.7) | <0.001 |
| Systolic blood pressure (mmHg) | 138.5 (18.9) | 141.5 (19.9) | <0.001 |
| Diastolic blood pressure (mmHg) | 79.9 (11.3) | 79.1 (11.2) | 0.099 |
| Ankle oedema | 163 (53.4%) | 142 (46.6%) | 0.132 |
| Body mass index (kg/m2) | 27.9 (4.9) | 27.3 (5.1) | 0.002 |
Results are presented as mean (SD) for continuous variables and numbers (%) for categorical variables, with percentage by row for each category. P-value is for t-test (continuous) and χ2 or Fisher's exact test (categorical) to assess independence of VHD from each characteristic/condition. AF, current or previous atrial fibrillation; NYHA, New York Heart Association; TIA, transient ischaemic attack; VHD, valvular heart disease.
The vast majority (97.4%) of the study cohort (excluding those with known VHD) had minimal or no symptoms with only 2.6% (65/2500) in New York Heart Association Class III/IV.
Prevalence of valvular heart disease
Overall
Newly detected (predominantly mild) VHD was identified in just over half (50.8%) of this large asymptomatic population (Table 2, Figure 1). The most common valve lesion was AoScl (34% of those with newly detected VHD), followed by mitral regurgitation (MR; mild 19.8%, moderate/severe 2.3%) and aortic regurgitation (AR; mild 13.6%, moderate/severe 1.6%). Aortic stenosis (AS), the most prognostically significant manifestation of VHD, was newly diagnosed in 1.3% of participants at a mean age of 77.3 (7.0) years in men and 75.6 (7.2) years in women. A bicuspid aortic valve (BAV) was found in only 8 (0.3%) participants, reflecting the age of the study cohort, and the fact that clinical manifestations of this condition usually present in the fifth or sixth decades.
Table 2.
New diagnosis of valvular heart disease
| None/trivial | Mild | Significant (moderate/severe) | |
|---|---|---|---|
| Any VHD | 1231 (49.2%) | 1110 (44.4%) | 159 (6.4%) |
| Left-sided VHD | |||
| Mitral regurgitation | 1948 (77.9%) | 494 (19.8%) | 58 (2.3%) |
| Mitral stenosis | 2491 (99.6%) | 7 (0.3%) | 2 (0.1%) |
| Aortic regurgitation | 2118 (84.7%) | 341 (13.6%) | 41 (1.6%) |
| Calcific aortic valve disease—AoScl and stenosis | 1617 (64.7%) | 866 (34.6%)a | 17 (0.7%) |
| None/Trivial/Mild | Significant (moderate/severe) | ||
| Right-sided VHD | |||
| Tricuspid regurgitation | 2433 (97.3%) | 67 (2.7%) | |
| Pulmonary regurgitation | 2493 (99.7%) | 7 (0.3%) | |
Number of study participants (% of total cohort) with newly diagnosed VHD (there were no cases of tricuspid or pulmonary stenosis). VHD, valvular heart disease.
a Mild calcific aortic valve disease refers to the combined number with AoScl and mild aortic stenosis.
Figure 1.
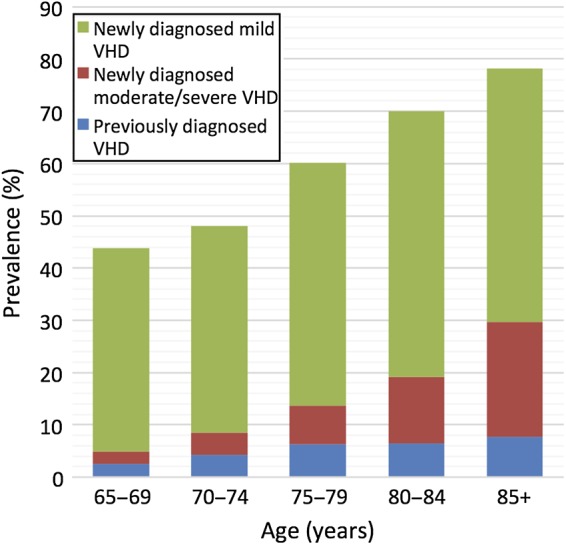
Population prevalence of valvular heart disease according to age.
Clinically significant valvular heart disease
Clinically significant (moderate or severe) VHD was newly diagnosed in 6.4% of participants. Addition of the further 4.9% of subjects with pre-existing VHD from the overall study cohort (assuming that moderate or severe VHD would result in a clinical diagnosis) created a derived total population prevalence of moderate or severe VHD of 11.3%.
Right-sided valvular heart disease
Significant right-sided VHD was less common. In addition to moderate–severe tricuspid regurgitation (2.7%), moderate pulmonary regurgitation was found in 7/2500 participants (0.3%). There were none with tricuspid or pulmonary stenosis.
Multivalve disease
Multiple valve lesions were identified in over one-third (38.5%) of the study population, affecting 47% of those with AoScl, 58% with MR, and 81% with AR (Figure 2).
Figure 2.
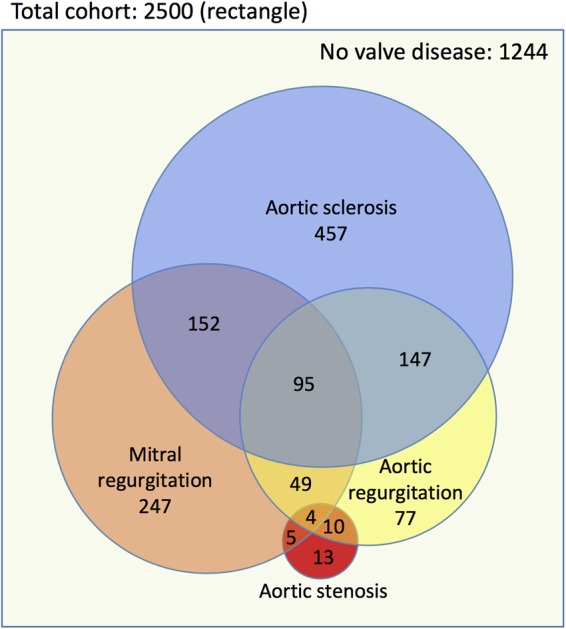
Venn diagram demonstrating the distribution of single and multiple left-sided valve abnormalities in OxVALVE participants with newly diagnosed valvular heart disease. The outer rectangle represents the full cohort (n = 2500) and the area of each circle is proportionate to the number of participants with different manifestations of left-sided valvular heart disease. Numbers denote the number of participants in each group.
Association with clinical variables and socioeconomic class
Participants with newly detected VHD were on average 2.4 years older than those without (Table 1) and prevalence increased linearly with age, from 42.4% (379/894) in those aged 65–69 years to 76.3% (103/135) in those aged 85–95 years (Figure 1). The proportion with moderate or severe VHD was 3.3% (54/1621) amongst those aged 65–74 years, rising to 11.9% (105/879) in those aged ≥75 years.
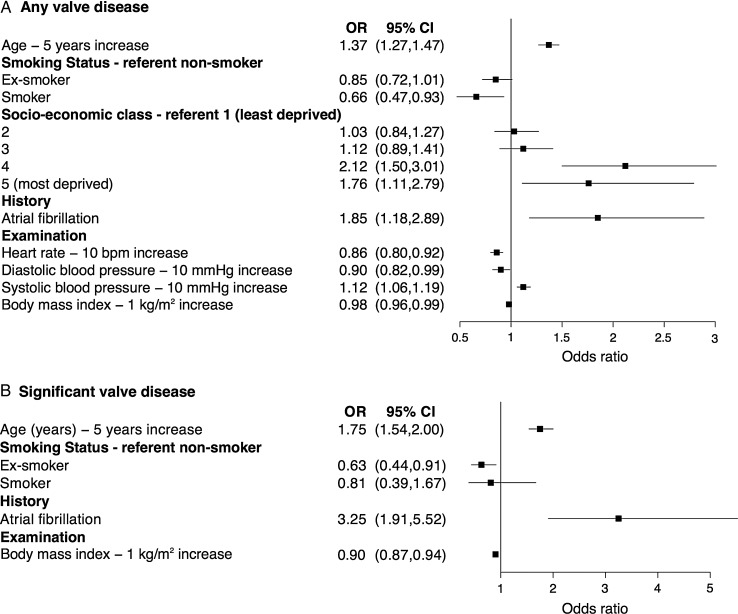
We explored associations with clinical characteristics using multiple regression (Figure 3A, Supplementary material online, Table S1). After removal of non-significant variables, VHD was associated with older age, more deprived SEC, current or previous atrial fibrillation (AF), and higher systolic blood pressure. There was no association with gender. Conversely, current smoking, higher heart rate, diastolic blood pressure, and body mass index were associated with a lower prevalence of VHD.
Figure 3.
Clinical variables associated with a new diagnosis of valvular heart disease. Forest plot of odds ratios from multiple regression analysis of clinical associations with (A) any valvular heart disease, and (B) significant (moderate/severe) valvular heart disease. Only statistically significant variables are shown. Dots represent the odds ratio and whiskers represent the 95% confidence interval. CI, confidence interval; OR, odds ratio; SEC, socioeconomic class, according to national quintile.
Restricting this analysis to subjects with newly diagnosed clinically significant (moderate or severe) VHD, there was an association with older age, current or previous AF, prior smoking, and lower body mass index (Figure 3B). In this smaller subset, there was no SEC association.
A history of current or previous AF was present in 109 participants (4.4% of the total study population) and independently associated with both a new diagnosis of VHD of any severity (OR 1.85, 95% CI 1.18–2.89) and clinically significant (moderate or severe) VHD (OR 3.25, 95% CI 1.91–5.52, P < 0·001; Figure 3). Moreover, participants with AF had a much higher prevalence of VHD than those in sinus rhythm (newly diagnosed VHD 67.9 vs. 32.1%; clinically significant [moderate or severe] VHD 21.1 vs. 5.7%), principally related to increased frequency of MR (mild 33.9 vs. 19.1%; moderate or severe 5.5 vs. 2.2%) and AR (mild 22.0 vs. 13.3%; moderate or severe 3.7 vs. 1.6%). By comparison, there was little difference in the frequency of AoScl (38.8 vs. 34.7%) or aortic stenosis (1.9 vs. 1.2%) between these groups. There were no participants with both AF and mitral stenosis in this Western European population.
Population projections
Combining our data concerning pre-existing and newly diagnosed VHD (and assuming no change in age and gender-specific prevalence), we predict a substantial rise in the clinical impact (and financial consequences) of clinically significant (moderate or severe) VHD within the rapidly expanding elderly population (Figure 4). The OxVALVE-PCS data suggest that the number of individuals in the UK aged 65 years or older with moderate or severe VHD will increase from 1.5 million in 2015 to 3.3 million in 2056 (122% increase), with a doubling in prevalence by 2046.
Figure 4.
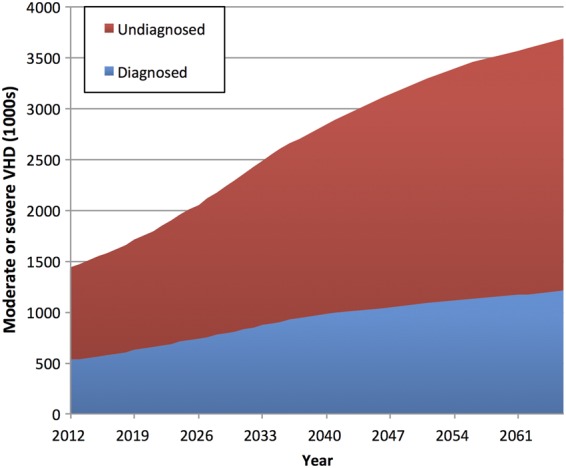
UK population projections of diagnosed and undiagnosed significant valvular heart disease. Diagnosed estimates are based on the number excluded from participation in the present study due to a prior diagnosis of valvular heart disease. Undiagnosed estimates are based on the number with newly diagnosed significant valvular heart disease in OxVALVE-PCS.
Discussion
The OxVALVE Population Cohort Study is the first population-based study of VHD worldwide and demonstrates both the prevalence of undiagnosed and known VHD in older people and the contemporary clinical and epidemiological characteristics of VHD. Previously undetected VHD was detected in just over half of 2500 participants and prevalence increased linearly with age. The total prevalence of clinically significant VHD was 11.3% when we included subjects with known VHD from the overall population cohort.
Previous studies addressing the prevalence of VHD have either been retrospective,4,5 hospital-based,3 or developed primarily to examine non-VHD.14 Consistent with these, we demonstrated a significant increase in the prevalence of VHD with age.3–5,15–17 A previous North American meta-analysis demonstrated that the prevalence of moderate or severe left-sided VHD was <1, 9.9, and 13.2% in those aged 18–44 years, >65 years, and >75 years, respectively.4 Similarly, the prevalence of undiagnosed moderate/severe VHD in those aged ≥75 years in OxVALVE-PCS was 11.9%. Moreover, we excluded 4.9% of the overall potential community cohort (6.8% of those aged ≥75 years) from our echocardiographic study on account of a previous diagnosis of VHD. Although ethical constraints restricted access to more detailed information concerning this group, we can reasonably assume that most (if not all) of this VHD was clinically significant, providing an 11.3% total prevalence of significant VHD in the elderly population aged 65 years or older (18.7% of those aged ≥75 years). These figures are significantly higher than estimated in a previous meta-analysis.4 A new diagnosis of moderate or severe VHD remains clinically important and should prompt specialist referral for evaluation, treatment, and/or long-term surveillance.
Aortic sclerosis, defined as thickening and focal calcification of the aortic valve leaflets without obstruction to flow, is a frequent incidental echocardiographic finding. Prevalence increases linearly with age and is estimated at 20–40% in the population aged >65 years.18 Consistent with this, AoScl was detected in one-third of participants in the present study and was the most common manifestation of VHD. Although progression of AoScl to AS is slow and affects only a minority of individuals,19,20 it is now accepted that AoScl is associated with adverse outcome over long-term follow-up and an independent marker of cardiovascular risk.21,22 While on-going research is addressing underlying mechanisms, vigorous vascular risk factor management combined with scheduled clinical and echocardiographic follow-up to monitor potential progression to significant AS is appropriate for individuals with AoScl identified in the context of cardiovascular screening or other echocardiographic assessment.
Aortic stenosis is associated with reduced life expectancy and carries a poor prognosis following the onset of symptoms. Aortic valve replacement and transcatheter aortic valve implantation are associated with significant reduction in mortality and morbidity, even in the very elderly.23 Early identification of AS remains an important priority to avoid excess morbidity and mortality, even in the oldest population cohorts, and we were able to detect AS in 1.3% of our study population. Although BAV was historically reported in 1–2% of live births, more recent studies suggest that the prevalence is lower at 0.5–0.8%,24–27 consistent with the prevalence of 0.3% in OxVALVE-PCS (particularly since those with a known BAV would have been excluded from our echocardiographic study).
Multivariate analysis confirmed a number of expected findings, such as the association of VHD with increasing age, current or previous AF, and elevated systolic blood pressure. While initially counterintuitive, lower BMI has previously been associated with higher prevalence of regurgitant valve lesions, perhaps due the increased ease of echocardiographic imaging in thin individuals.17 Although current smoking was associated with less VHD, a concurrent history of coronary heart disease (CHD) may have been an important confounding variable. Both current and ex-smokers were more likely to have CHD (Supplementary material online, Table S2) and previous echocardiography demonstrating associated VHD would have led to exclusion of this group from our echocardiographic study.
Variation in the prevalence and outcomes of coronary artery disease and stroke according to SEC is well described28 but OxVALVE-PCS demonstrates an association between SEC and VHD for the first time. While this finding should be interpreted with caution given the relatively small sample sizes, the rate of VHD in the two most deprived groups (SEC 4/5) was approximately double that in the least deprived group (SEC 1, Figure 3). This association is not readily explained but could reflect a higher burden of unmeasured risk factors, such as exposure to passive smoking, or the small but measureable association between SEC and markers of ageing.29 Consistent with local demographics, SEC 1 accounted for 50% of our cohort—larger, more diverse studies to analyse inequalities in the prevalence of VHD are warranted. At a practical level, there is a need to devise strategies to enhance access to specialist care in less affluent communities to facilitate the earlier diagnosis of VHD.
We identified current or previous AF as the clinical variable most strongly associated with newly diagnosed clinically significant (moderate or severe) VHD. These participants had a more than three-fold increase in the likelihood of newly diagnosed clinically significant VHD. Although international guidelines indicate the need for initial echocardiography in all patients with AF,30,31 recent recommendations from the UK National Institute for Health and Care Excellence reaffirm previous guidance that routine echocardiography is unnecessary.32 Our data indicate that AF is an important and easily identifiable marker of silent VHD in asymptomatic individuals in primary care and that auscultation and routine echocardiography are appropriate. Anticoagulation is also of potential importance in this cohort, although none of the OxVALVE-PCS participants had a mechanical valve or mitral stenosis with associated AF (the two conditions known to independently increase thromboembolic risk in the setting of AF,33 and for which novel oral anticoagulants are unlicensed).
The unique OxVALVE-PCS cohort will provide a platform for future studies examining long-term outcomes and cross-sectional associations of VHD in its earliest stages, enabling accurate assessment of the rate of progression in the community, exploration of genetic and biomarker associations, and elucidation of factors involved in the pathogenesis of this increasingly common condition.
Limitations
The present study has inevitable limitations. Although uptake was over 50% (despite ethical constraints permitting mail only contact with potential participants) and compatible with similar community-based studies,10,11 selection bias with over-representation of motivated healthy individuals is possible.34 Thus, although it is likely that the pragmatic exclusion of the infirm or those with cognitive decline will have skewed the data to an indefinable extent, this will, if anything, have led to an underestimation of the considerable population burden of VHD. Indeed, previous studies amongst nursing home residents have demonstrated very high levels of VHD.35,36
The Oxfordshire population is relatively homogeneous and our study population lacks ethnic and socioeconomic diversity, which makes it difficult to generalise our findings to other communities. This is, of course, a problem faced by other similar clinical studies. For example, a recent US study demonstrated a far lower rate of aortic stenosis in African Americans compared with Caucasians but equal rates of severe mitral regurgitation.37 However, other key epidemiological series5,17 have been similarly homogeneous despite being population-based, while other important studies16 were limited to single ethnic groups. Although there have been numerous changes in European population dynamics in recent decades, a traditional ethnic structure prevails in older cohorts. For example, UK 2011 Census data demonstrate an equivalent or higher prevalence of White ethnicity compared with the OxVALVE cohort (98.8%) in 71/174 counties in England and Wales and prevalence of 95% or more in 120/174 counties.12 Comparison with other European nations is difficult, although Dutch data indicate that 97% of those aged 65 and over in Holland are of Western origin,38 suggesting that the Oxfordshire population is reasonably representative of other elderly Western European populations.
We also recognize that our study cohort (mean IMD score 11.68) and the overall Oxfordshire population (mean IMD score 12.26) is relatively affluent in comparison with the wider UK South East region (mean IMD score 14.75) or large cities such as London (mean IMD score 25.22) where multi-ethnicity and immigrant communities are likely to contribute to a greater prevalence of rheumatic valve disease. Comparison of our cohort with other UK populations would be of value and such collaborative studies are planned within our future research programme.
Conclusions
The OxVALVE Population Cohort Study is the first population-based study worldwide aimed specifically at the community detection of VHD. In a large cohort of 2500 elderly subjects we demonstrate a high prevalence of previously unidentified VHD and infer that the number of individuals with clinically significant VHD will increase substantially over the next five decades. The novel finding of increased VHD in more deprived socioeconomic groups (even in a high-income country such as the UK) places VHD among the group of diseases that disproportionately affects the poor. Furthermore, we demonstrate that clinically significant VHD is three times more common in individuals with current or previous AF, which provides an important and easily identifiable marker of silent VHD in asymptomatic individuals. These data are important for researchers and clinicians in enabling improved understanding of the pathophysiology, risk factors, and natural history of VHD, and for healthcare policy-makers and economists as the size of the elderly population and their health expectations increase, and expensive percutaneous treatment options emerge as an evidence-based alternative to conventional valve surgery.39,40
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Institute of Health Research (NIHR) Thames Valley Comprehensive Local Research Network (UKCRN ID 6086) and the NIHR Oxford Biomedical Research Centre, with initial support coming from the NIHR School for Primary Care Research. A.F. is an NIHR Senior Investigator.
Supplementary Material
Acknowledgements
The authors acknowledge Benjamin Cairns, Peter Rothwell, and Robert Clarke for their helpful comments on earlier drafts of this manuscript.
Conflict of interest: none declared.
The OxVALVE-PCS group
Principal Investigators: Bernard Prendergast, Saul Myerson
Primary Investigators: Joanna L d'Arcy, Sean Coffey, Margaret A Loudon
Co-Investigators: Harald Becher, David Ebbs, Andrew Farmer, Peter Grimwade, Richard Hobbs, Louise Locock, David Mant, Jonathon Pearson-Stuttard
Statistical support: Jacqueline Birks, Eleni Frangou, Abdelouahid Tajar.
Echocardiographers: Linda Arnold, Cassandra Hammond, Claudio Eduardo Lima, Claire Mabbett, Nadia Pinheiro, Rebecca Reynolds.
Administration: Hema Collappen, Sabrina Harris, Andrew Kennedy, Lee Potiphar, Jo Wilson.
References
- 1. d'Arcy JL, Prendergast BD, Chambers JB, Ray SG, Bridgewater B. Valvular heart disease: the next cardiac epidemic. Heart 2011;97:91–93. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers H-J, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde J-L, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–1243. [DOI] [PubMed] [Google Scholar]
- 4. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 5. Eveborn GW, Schirmer H, Heggelund G, Lunde P, Rasmussen K. The evolving epidemiology of valvular aortic stenosis. the Tromsø study Heart 2013;99:396–400. [DOI] [PubMed] [Google Scholar]
- 6. Coffey S, D'Arcy JL, Loudon MA, Mant D, Farmer AJ, Prendergast BD. The OxVALVE population cohort study (OxVALVE-PCS) – population screening for undiagnosed valvular heart disease in the elderly: study design and objectives. Open Hear 2014;1:e000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steeds R, Wharton G, Allen J, Chambers J, Graham J, Jones R, Bushra R, Masani N. Echocardiography: Guidelines for Valve Quantification http://www.bsecho.org.uk/education/postersguides/ (11 January 2016).
- 8. Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1–25. [DOI] [PubMed] [Google Scholar]
- 10. Davies M, Hobbs F, Davis R, Kenkre J, Roalfe AK, Hare R, Wosornu D, Lancashire RJ. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 2001;358:439–444. [DOI] [PubMed] [Google Scholar]
- 11. Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 12. Office for National Statistics. 2011 Census Data for England and Wales on Nomis http://www.nomisweb.co.uk/census/2011 (11 January 2016).
- 13. The English Indices of Deprivation 2010: County Summaries http://www.communities.gov.uk/documents/statistics/xls/1981199.xls (11 January 2016).
- 14. Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Wood EA, Howard BV, Welty TK, Lee ET, Fabsitz RR. Relations of Doppler stroke volume and its components to left ventricular stroke volume in normotensive and hypertensive American Indians: the Strong Heart Study. Am J Hypertens 1997;10:619–628. [DOI] [PubMed] [Google Scholar]
- 15. Lindroos M, Kupari M, Heikkilä J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 1993;21:1220–1225. [DOI] [PubMed] [Google Scholar]
- 16. Lebowitz NE, Bella JN, Roman MJ, Liu JE, Fishman DP, Paranicas M, Lee ET, Fabsitz RR, Welty TK, Howard BV, Devereux RB. Prevalence and correlates of aortic regurgitation in American Indians: the Strong Heart Study. J Am Coll Cardiol 2000;36:461–467. [DOI] [PubMed] [Google Scholar]
- 17. Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897–902. [DOI] [PubMed] [Google Scholar]
- 18. Coffey S, Cox B, Williams MJA. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol 2014;63:2852–2861. [DOI] [PubMed] [Google Scholar]
- 19. Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. The risk of the development of aortic stenosis in patients with ‘benign’ aortic valve thickening. Arch Intern Med 2002;162:2345–2347. [DOI] [PubMed] [Google Scholar]
- 20. Novaro GM, Katz R, Aviles RJ, Gottdiener JS, Cushman M, Psaty BM, Otto CM, Griffin BP. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: the Cardiovascular Health Study. J Am Coll Cardiol 2007;50:1992–1998. [DOI] [PubMed] [Google Scholar]
- 21. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 22. Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med 2014;371:744–756. [DOI] [PubMed] [Google Scholar]
- 23. Dunning J, Gao H, Chambers J, Moat N, Murphy G, Pagano D, Ray S, Roxburgh J, Bridgewater B. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use-an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg 2011;142:776–782. [DOI] [PubMed] [Google Scholar]
- 24. Basso C, Boschello M, Perrone C, Mecenero A, Cera A, Bicego D, Thiene G, De Dominicis E. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol 2004;93:661–663. [DOI] [PubMed] [Google Scholar]
- 25. Nistri S, Basso C, Marzari C, Mormino P, Thiene G. Frequency of bicuspid aortic valve in young male conscripts by echocardiogram. Am J Cardiol 2005;96:718–721. [DOI] [PubMed] [Google Scholar]
- 26. Movahed M-R, Hepner AD, Ahmadi-Kashani M. Echocardiographic prevalence of bicuspid aortic valve in the population. Heart Lung Circ 2006;15:297–299. [DOI] [PubMed] [Google Scholar]
- 27. Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J 2005;150:513–515. [DOI] [PubMed] [Google Scholar]
- 28. Pearson-Stuttard J, Bajekal M, Scholes S, O'Flaherty M, Hawkins NM, Raine R, Capewell S. Recent UK trends in the unequal burden of coronary heart disease. Heart 2012;98:1573–1582. [DOI] [PubMed] [Google Scholar]
- 29. Carroll JE, Diez-Roux AV, Adler NE, Seeman TE. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA). Brain Behav Immun 2013;28:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 31. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J-Y, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 32. National Institute for Health and Care Excellence. Atrial fibrillation: the management of atrial fibrillation (clinical guideline 180) http://www.nice.org.uk/guidance/cg180 (11 January 2016).
- 33. De Caterina R, Camm AJ. What is ‘valvular’ atrial fibrillation? A reappraisal. Eur Heart J 2014;35:3328–3335. [DOI] [PubMed] [Google Scholar]
- 34. Jones A, Cronin PA, Bowen M. Comparison of risk factors for coronary heart disease among attenders and non-attenders at a screening programme. Br J Gen Pract 1993;43:375–377. [PMC free article] [PubMed] [Google Scholar]
- 35. Aronow WS, Ahn C, Kronzon I. Prevalence of echocardiographic findings in 554 men and in 1,243 women aged >60 years in a long-term health care facility. Am J Cardiol 1997;79:379–380. [DOI] [PubMed] [Google Scholar]
- 36. Aronow WS, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am J Cardiol 1991;67:776–777. [DOI] [PubMed] [Google Scholar]
- 37. Patel DK, Green KD, Fudim M, Harrell FE, Wang TJ, Robbins MA. Racial differences in the prevalence of severe aortic stenosis. J Am Heart Assoc 2014;3:e000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centraal Bureau voor de Statistiek. StatLine http://statline.cbs.nl/Statweb/ (11 January 2016).
- 39. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 40. Thyregod HGH, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: one-year results from the all-comers nordic aortic valve intervention (NOTION) randomized clinical trial. J Am Coll Cardiol 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.