Abstract
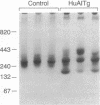
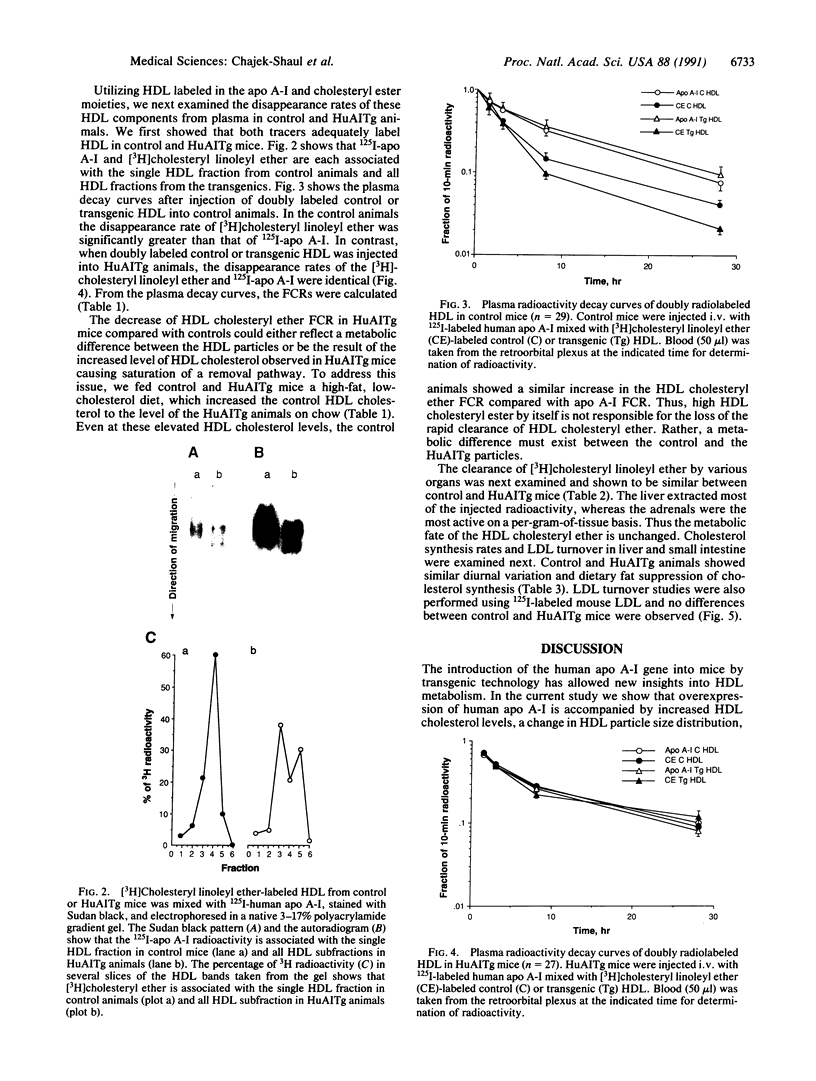
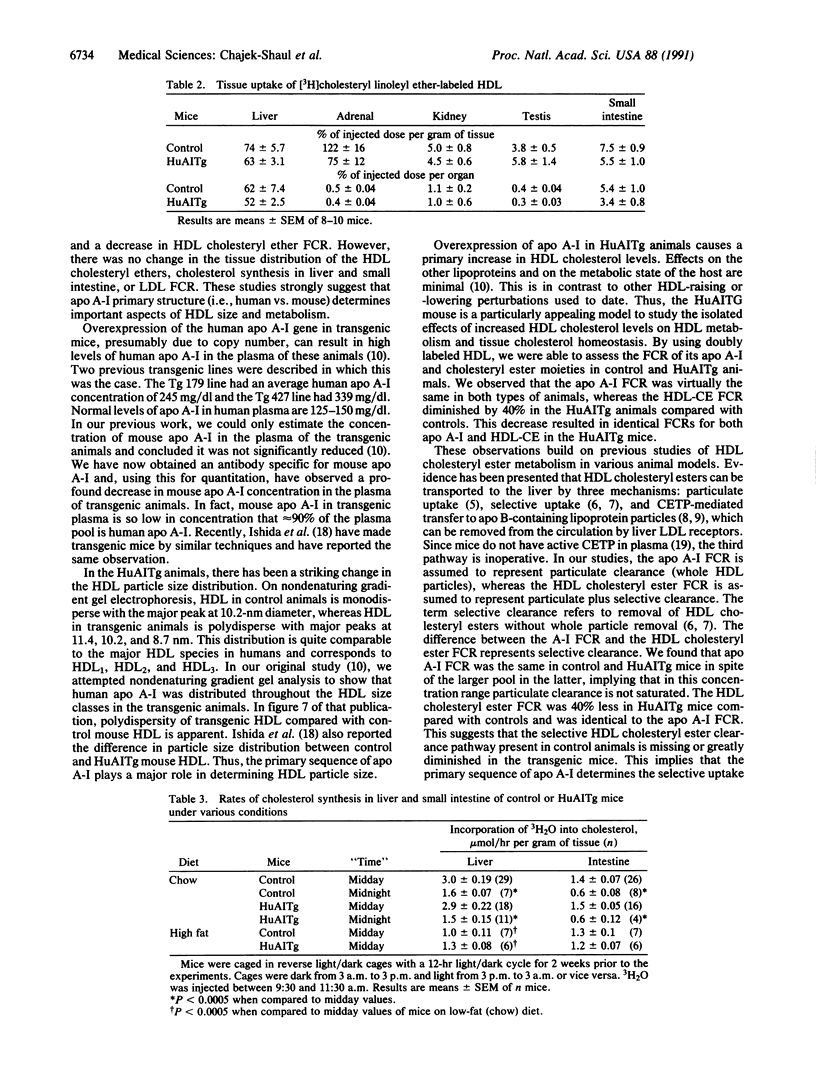
Transgenic mice carrying the human apolipoprotein (apo) A-I gene (HuAITg mice) were used to examine the effects of overexpression of the human gene on high density lipoprotein (HDL) particle size distribution and metabolism. On a chow diet, control mice had HDL cholesterol and apo A-I levels of 49 +/- 2 and 137 +/- 12 mg/dl of plasma, respectively. HuAITg mice had HDL cholesterol, human apo A-I, and mouse apo A-I levels of 88 +/- 2, 255 +/- 19, and 16 +/- 2 mg/dl, respectively. Nondenaturing gradient gel electrophoresis revealed control mouse plasma HDL to be primarily monodisperse with a particle diameter of 10.2 nm, whereas HuAITg mouse plasma HDL was polydisperse with particles of diameter 11.4, 10.2, and 8.7 nm, which correspond in size to human HDL1, HDL2, and HDL3, respectively. In vivo turnover studies of HDL labeled with [3H]cholesteryl linoleyl ether (representing the cholesteryl ester pool) and 125I-apo A-I were performed. In control animals, the fractional catabolic rate (FCR) for HDL cholesteryl ester (0.197 +/- 0.010 pool/hr) was significantly (P less than 0.0005) more than the apo A-I FCR (0.118 +/- 0.006 pool/hr). In the HuAITg mice, the HDL cholesteryl ester FCR (0.124 +/- 0.008 pool/hr) was the same as the apo A-I FCR (0.126 +/- 0.010 pool/hr). There were no significant differences between control and HuAITg animals in the sites of tissue removal of HDL cholesteryl ester, with the liver extracting most of the injected radioactivity. Control and HuAITg animals had comparable liver and intestinal cholesterol synthesis and LDL FCR. In conclusion, HuAITg mice have principally human and not mouse apo A-I in their plasma. This apparently causes a change in HDL particle size distribution in the transgenic mice to one resembling the human pattern. The replacement of mouse by human apo A-I also apparently causes the loss of the selective uptake pathway of HDL cholesteryl esters present in control mice. These data imply that apo A-I primary structure has a profound influence on HDL particle size distribution and metabolism.
Full text
PDF

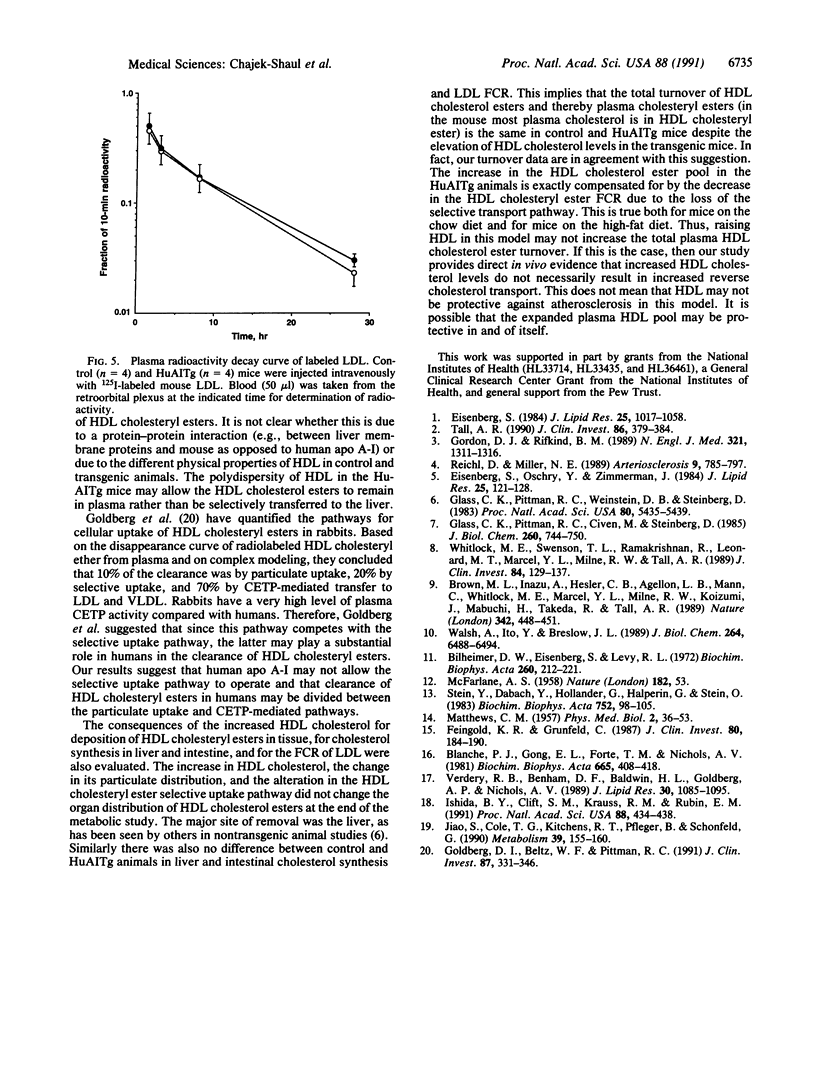
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Blanche P. J., Gong E. L., Forte T. M., Nichols A. V. Characterization of human high-density lipoproteins by gradient gel electrophoresis. Biochim Biophys Acta. 1981 Sep 24;665(3):408–419. doi: 10.1016/0005-2760(81)90253-8. [DOI] [PubMed] [Google Scholar]
- Brown M. L., Inazu A., Hesler C. B., Agellon L. B., Mann C., Whitlock M. E., Marcel Y. L., Milne R. W., Koizumi J., Mabuchi H. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins. Nature. 1989 Nov 23;342(6248):448–451. doi: 10.1038/342448a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. High density lipoprotein metabolism. J Lipid Res. 1984 Oct;25(10):1017–1058. [PubMed] [Google Scholar]
- Eisenberg S., Oschry Y., Zimmerman J. Intravascular metabolism of the cholesteryl ester moiety of rat plasma lipoproteins. J Lipid Res. 1984 Feb;25(2):121–128. [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C., Pittman R. C., Civen M., Steinberg D. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J Biol Chem. 1985 Jan 25;260(2):744–750. [PubMed] [Google Scholar]
- Glass C., Pittman R. C., Weinstein D. B., Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. I., Beltz W. F., Pittman R. C. Evaluation of pathways for the cellular uptake of high density lipoprotein cholesterol esters in rabbits. J Clin Invest. 1991 Jan;87(1):331–346. doi: 10.1172/JCI114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Rifkind B. M. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989 Nov 9;321(19):1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- Jiao S., Cole T. G., Kitchens R. T., Pfleger B., Schonfeld G. Genetic heterogeneity of lipoproteins in inbred strains of mice: analysis by gel-permeation chromatography. Metabolism. 1990 Feb;39(2):155–160. doi: 10.1016/0026-0495(90)90069-o. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Reichl D., Miller N. E. Pathophysiology of reverse cholesterol transport. Insights from inherited disorders of lipoprotein metabolism. Arteriosclerosis. 1989 Nov-Dec;9(6):785–797. doi: 10.1161/01.atv.9.6.785. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. Expression of human apolipoprotein A-I in transgenic mice results in reduced plasma levels of murine apolipoprotein A-I and the appearance of two new high density lipoprotein size subclasses. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):434–438. doi: 10.1073/pnas.88.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein Y., Dabach Y., Hollander G., Halperin G., Stein O. Metabolism of HDL-cholesteryl ester in the rat, studied with a nonhydrolyzable analog, cholesteryl linoleyl ether. Biochim Biophys Acta. 1983 Jun 16;752(1):98–105. doi: 10.1016/0005-2760(83)90237-0. [DOI] [PubMed] [Google Scholar]
- Tall A. R. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990 Aug;86(2):379–384. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdery R. B., Benham D. F., Baldwin H. L., Goldberg A. P., Nichols A. V. Measurement of normative HDL subfraction cholesterol levels by Gaussian summation analysis of gradient gels. J Lipid Res. 1989 Jul;30(7):1085–1095. [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]
- Whitlock M. E., Swenson T. L., Ramakrishnan R., Leonard M. T., Marcel Y. L., Milne R. W., Tall A. R. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J Clin Invest. 1989 Jul;84(1):129–137. doi: 10.1172/JCI114132. [DOI] [PMC free article] [PubMed] [Google Scholar]