Abstract
Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is a rare condition characterized by emotional and behavioral disturbances, dyskinesias, and extrapyramidal signs. It occurs in young women of reproductive age and is classically described as a paraneoplastic phenomenon. We present a 36-year-old, HIV-positive female who was admitted to the hospital in an acute confusional state, with a stiff posture, periods of motor agitation, and myoclonic jerks of the hands. Her mental state progressively deteriorated. Without evidence of infection, the presence of anti-NMDAR antibodies both in serum and cerebrospinal fluid clinched the diagnosis of autoimmune encephalitis. No evidence of neoplastic disease was found, and the beneficial response to immunosuppressive therapy was exceptional. This is the first report of anti-NMDAR encephalitis in an HIV-infected individual, reminding us that autoimmune encephalitis should be included in the differential diagnosis of a young patient presenting in an acute confusional state.
Keywords: HIV, Autoimmune encephalitis, Anti-N-methyl-D-aspartate receptor antibodies, Acute confusional state, Immunosuppressive therapy
Introduction
One of the most common types of autoimmune encephalitis (AE) associated with neuronal cell surface autoantibodies are mediated by antibodies against the N-methyl-D-aspartate receptor (NMDAR) [1]. Most of the patients with NMDAR encephalitis have prodromal headache, fever, or other symptoms that may resemble a viral process. Patients develop psychiatric manifestations such as anxiety, insomnia, agitation, hallucinations, or delusions. These symptoms are usually followed by orofacial and limb dyskinesias, choreoathetosis, dystonia, rigidity, and opisthotonic postures in the context of catatonia, coma, and autonomic instability [2]. This condition affects predominantly young females, in association with a tumor, most commonly an ovarian teratoma [1]. This is an immune-mediated disorder and despite its severity, patients often recover after tumor removal and immunotherapy (corticosteroids, intravenous immunoglobulin, or plasma exchange [PE]) [3]. Approximately 25% of the patients, mainly those without a tumor, have a tendency to relapse [1].
HIV patients have a higher incidence of systemic autoimmune diseases, even on highly active antiretroviral therapy (HAART) [4]. On the other hand, immunosuppressive therapy (IST) poses a not unreasonable fear of further immune dysfunction/immunodeficiency. In this case report, we describe the first known case of an HIV-positive individual with NMDAR encephalitis with a focus on therapeutic choices and a successful outcome.
Case Presentation
A 36-year-old female in an acute confusional state was admitted to the hospital in 2014. She had been in good health until 2 weeks prior to admission when she complained of acute-onset generalized severe headache, high fever, generalized myalgia, and anorexia. She had been prescribed analgesics to which she was unresponsive. Over the past week, her family had progressively noticed more frequent episodes of a stiff posture, blank stares, and tonic movements of her arms, alongside insomnia, anxiety, and confusion. She was known to be HIV-positive under anti-retroviral therapy for the past 16 years, with a normal CD4+ T-cell count, undetectable viral load, and no AIDS-defining diagnosis. There was no history of recreational drug use, alcohol abuse, toxin exposure, recent vaccination, or epidemiological risk factors. A general clinical examination was unremarkable. Initially, she was conscious and obeyed simple requests but was mostly noncooperative with the neurological examination, which failed to reveal any anomaly except for generalized stiffness and an absent reflexive blink. No meningism was elicited.
Laboratory investigations revealed a hemoglobin level of 11.6 g/dL, leucocytes 10,000 × 106/L (normal differential count), platelets 263,000 × 106/L, C-reactive protein <0.3 mg/dL, and no evidence of renal, hepatic, thyroid, or metabolic dysfunction. The antinuclear antibody test was positive (1/160) with a fine, granular pattern on HEp-2 cell indirect immunofluorescence. A brain CT scan excluded any structural abnormality. The cerebrospinal fluid (CSF) was clear, colorless with an increased number of cells (86/μL, predominantly mononuclear), a slight increase in protein concentration (64 mg/dL), normal glucose (45 mg/dL), negative Gram stain, and no evidence of Cryptococcus.
Treatment was empirically started with acyclovir, and 2 days later, ceftriaxone and ampicillin were added. In the next 48 h, she was in mutism, with periods of motor agitation, myoclonic jerks of the hands, and her mental state progressively deteriorated. She became comatose and faciobrachial seizures were also observed. Sodium valproate and levetiracetam reduced the seizure frequency. Her care was transferred to the Intensive Care Unit (ICU). She was sedated and subjected to endotracheal intubation with ventilatory support. The cerebral MRI showed a small number of focal T2 hyperintensities bilaterally, located in the frontal subcortical region. Electroencephalography revealed marked diffuse slow electrogenesis. Sedation was titrated for seizure control, reduced, and withdrawn on ICU day 8. On ICU day 10, she remained unreactive to painful stimuli, with episodes of involuntary eyelid contractions, chewing, and sucking motions. She completed 16 days of acyclovir and 14 days of antibiotics with no clinical improvement, remaining afebrile, hemodynamically stable, and without evidence of infection (Table 1). An autoimmune basis for her disorder was suspected, and the presence of anti-N-methyl-D-aspartate receptor (anti-NMDAR) antibodies (Euroimmun) both in serum and CSF clinched the diagnosis of AE. No evidence of neoplastic disease was found (actively searched for by abdominopelvic CT and MRI, thyroid and breast ultrasound, and a positron emission tomography scan).
Table 1.
Microbiological investigations
| CSF | Blood | |
|---|---|---|
| Sputum for direct acid-fast bacillus stain, mycobacteria culture, and PCR PCR virus: Herpes 1, 2, 6, 7, 8; Epstein Barr; Varicella; lymphocytic | negative | − |
| choriomeningitis virus; enterovirus; parvovirus, cytomegalovirus | negative | − |
| Venereal Disease Research Laboratory | negative | negative |
| Bacteriological and mycological cultural examinations | negative | negative |
| Hepatitis A, B, C | − | negative |
| Toxoplasma | − | negative |
| Coxiella | − | negative |
| Borrelia | negative | negative |
| RNA HIV 1 | <20 copies/mL | <20 copies/mL |
CSF, cerebrospinal fluid.
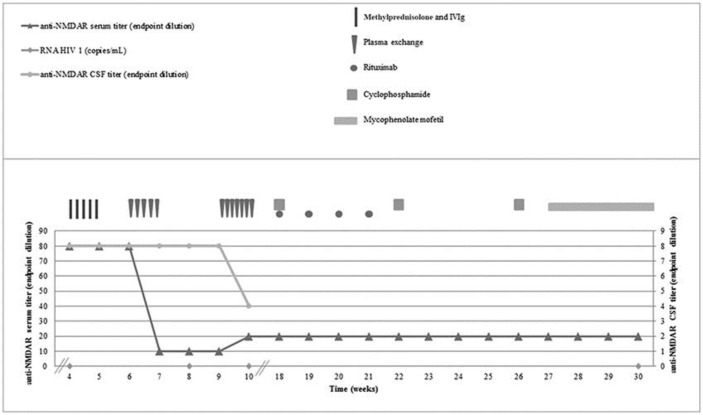
Further therapy was directed to AE (Fig 1). There was no response to steroids and intravenous immunoglobulin. Clinical improvement together with a reduction in the antibody titer was only apparent after 5 sessions of PE, on alternate days. PE was then performed daily over the next 7 days, after which the patient regained consciousness, there were no further seizures, and she was transferred back to the ward. She was then treated with rituximab and cyclophosphamide and maintained on mycophenolate mofetil (MMF). Motor status was fully normal when she was discharged after 147 days of hospital admission. At the 2-year follow-up, neuropsychiatric testing revealed moderate cognitive and memory impairment. Throughout this time, serial CD4+ T-cell counts remained persistently above 400 cells/μL, the HIV viral load was repeatedly undetectable, and there was no evidence of neoplastic disease or opportunistic infections.
Fig. 1.
Serologic response to the treatment. Shown are serum (triangles; left y-axis) and CSF (dots; right y-axis) titers of NMDAR antibodies, and RNA HIV 1 viral load (diamonds) measured over 30 weeks (x-axis). Week 4 corresponds to the diagnosis of anti-NMDAR encephalitis. The patient was given 5-day pulses of intravenous methylprednisolone (1 g per day) and intravenous immunoglobulin (0.2 g/kg per day; vertical rectangle), and 5 sessions of PE (inverted triangles) on alternate days. Despite a decrease in the serum anti-NMDAR titer at week 7, there was no clinical improvement, after which PE was performed daily over the next 7 days. Clinical improvement at week 10 corresponds to a decrease in the CSF anti-NMDAR titer. Rituximab (spheres) was given once a week for 4 weeks (375 mg/m2 per week), and cyclophosphamide (squares) was given once a month for 3 months (15 mg/kg/pulse). Mycophenolate mofetil (horizontal rectangle) 250 mg b.i.d. was increased by 500 mg on a weekly basis up to a maintenance dosage of 1,000 mg b.i.d. CSF, cerebrospinal fluid; NMDAR, anti-N-methyl-D-aspartate receptor; IVIg, intravenous immunoglobulin; PE, plasma exchange.
Discussion
AE is a rare condition, recently recognized and classically described in association with ovarian teratoma and as a paraneoplastic phenomenon [5]. It is mediated by anti-NMDAR antibodies, which likely result in the loss of receptor function [6]. Recent reports suggest that AE may be triggered by Herpes simplex and measles viral infection [7, 8].
There are several points we wish to emphasize. We recognize that there was a diagnostic delay as we actively sought to exclude a systemic infection or a metabolic cause for her illness (Table 1). The initial symptoms with acute-onset generalized severe headache, high fever, and neurologic symptoms (stiff posture, blank stares, tonic movements, insomnia, anxiety, and confusion) together with the CSF profile (normal glucose, a mild increase in the protein concentration, and the presence of mononuclear cells) were suggestive of a viral infection. Even though the HIV infection was controlled by HAART and Herpes simplex virus 1 and 2 are most frequently associated with psychiatric symptoms, an extensive search for several causes of viral encephalitis was undertaken, namely herpes 1, 2, 6, 7, 8; Epstein Barr; Varicella; lymphocytic choriomeningitis virus; enterovirus; parvovirus, and cytomegalovirus. There was also a concern as regards the need to exclude infection in the light of the need to institute IST. Other infections, such as Mycobacteria, Borrelia, Coxiella, Toxoplasma, and fungi, a space-occupying lesion, or ischemia were considered less likely but were still actively searched.
Our patient developed a prodromal illness, and her disease was further characterized by opisthotonic postures, rigidity, orofacial, and limb dyskinesia, confusion, seizures, and coma. The beneficial response to IST and the lack of improvement to the initial empirical viral and bacterial encephalitis treatment suggested an autoimmune condition.
As the HIV infection was controlled, it was not thought to contribute to the clinical picture, but detailed studies of immune function were not performed. Nevertheless, there is a recognized association between HIV infection and autoimmunity as patients with HIV infection managed with HAART may develop acute immune dysregulation collectively known as immune reconstitution inflammatory syndrome [9].
Once the diagnosis of AE was made, the therapy is yet another challenge because potent immunosuppression is required in a patient with an acquired immunodeficiency. The poor response to first-line treatments (high-dose intravenous corticosteroid, intravenous immunoglobulin, and PE) justified further therapy with rituximab and cyclophosphamide in combination [10]. The effectiveness of tumor removal should be highlighted in this condition. However, in our patient, despite active screening, no tumor was found. Further MMF therapy was justified by several studies suggesting that prolonged IST with mycophenolate MMF or azathioprine may prevent a relapse [6]. HIV has become a chronic disease after the advent of HAART [11], posing a dilemma as regards IST due to a documented risk of opportunistic infections and drug-drug interactions (HAART and IST) [12]. Of note, in our patient, there was no evidence of HIV reactivation despite IST, and she remained free of infections during the 2 years of follow-up.
The present case report illustrates that AE is a severe condition reversible with appropriate therapy. Importantly, the diagnosis can only be made if specific autoantibody reactivities are requested. This is the first report of anti-NMDAR encephalitis in an HIV-infected individual, reminding us that AE should be included in the differential diagnosis of any patient in an acute confusional state.
Ethical Statement
The patient has agreed to the publication by written consent.
Disclosure Statement
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77:179–189. doi: 10.1212/WNL.0b013e318224afde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramanathan S, Mohammad SS, Brilot F, Dale RC. Autoimmune encephalitis: recent updates and emerging challenges. J Clin Neurosci. 2014;21:722–730. doi: 10.1016/j.jocn.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Dessain SK, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yen Y-F, Chuang P-H, Jen I-A, Chen M, Lan Y-C, Liu Y-L, et al. Incidence of autoimmune diseases in a nationwide HIV/AIDS patient cohort in Taiwan, 2000–2012. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209815. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–772. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 6.Peery HE, Day GS, Dunn S, Fritzler MJ, Prüss H, De Souza C, et al. Anti-NMDA receptor encephalitis. The disorder, the diagnosis and the immunobiology. Autoimmun Rev. 2012;11:863–872. doi: 10.1016/j.autrev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis P, Papadopoulos G, Koufou E, Parissis D, Karacostas D. Anti-NMDA receptor encephalitis possibly triggered by measles virus. Acta Neurol Belg. 2015;115:801–802. doi: 10.1007/s13760-015-0468-2. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesan A, Benavides DR. Autoimmune Encephalitis and Its Relation to Infection. Curr Neurol Neurosci Rep. 2015;15:3. doi: 10.1007/s11910-015-0529-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson TP, Nath A. New insights into immune reconstitution inflammatory syndrome of the central nervous system. Curr Opin HIV AIDS. 2014;9:572–578. doi: 10.1097/COH.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastaldi M, Thouin A, Vincent A. Antibody-mediated autoimmune encephalopathies and immunotherapies. Neurotherapeutics. 2016;13:147–162. doi: 10.1007/s13311-015-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciuffreda D, Pantaleo G, Pascual M. Effects of immunosuppressive drugs on HIV infection: implications for solid-organ transplantation. Transpl Int. 2007;20:649–658. doi: 10.1111/j.1432-2277.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 12.Haas J, Singer T, Nowak K, Brust J, Göttmann U, Schnülle P, et al. Renal transplantation in HIV-positive renal transplant recipients: experience at the Mannheim University Hospital. Transplant Proc. 2015;47:2791–2794. doi: 10.1016/j.transproceed.2015.09.064. [DOI] [PubMed] [Google Scholar]