Abstract
Primary pancreatic lymphoma is an unlikely malignancy accounting for less than 0.5% of pancreatic tumors. Clinical presentation is often nonspecific and may be clinically misdiagnosed as pancreatic adenocarcinoma. Here we present an Iranian case of primary pancreatic lymphoma in a 47-year-old male suffering from jaundice and 20% weight loss. Endoscopic ultrasound revealed a mixed echoic mass lesion at the head of pancreas. The patient underwent endoscopic ultrasound-guided fine needle aspiration of solid pancreatic mass and histopathologic diagnosis revealed granuloma. Computed tomography-guided core needle biopsy was performed and eventually histological examination showed granuloma that was coherent with the diagnosis of primary pancreatic lymphoma. Primary pancreatic lymphoma is a rare entity presenting with nonspecific symptoms, laboratory and radiological findings. Computed tomography results in combination with clinical and radiological studies generally provide guidance for appropriate investigation.
Keywords: Primary pancreatic lymphoma, Endoscopic ultrasound, Endoscopic ultrasound-guided fine needle aspiration, Computed tomography-guided core needle biopsy
Introduction
Primary pancreatic lymphoma (PPL) is a rare but treatable malignancy accounting for less than 0.5% of pancreatic tumors [1]. Clinical presentation is often nonspecific and may be clinically misdiagnosed as pancreatic adenocarcinoma [2, 3].
PPLs are usually of B-cell lineage and show the following features: (1) a mass located in the pancreas with peripancreatic lymphadenopathies; (2) absence of hepatic or splenic involvement; (3) normal peripheral leukocyte count and bone marrow; (4) absence of palpable superficial lymphadenopathy and mediastinal lymph node enlargement on chest radiography [4]. The most common presenting symptoms include abdominal pain, weight loss, vomiting, and more rarely jaundice, small-bowel obstruction, diarrhea, night sweats, and fever [4, 5, 6, 7]. Lactate dehydrogenase elevation is not necessarily a feature of PPL, while the serum carbohydrate antigen 19-9 (CA19-9) level is usually normal unless biliary obstruction is present [8].
Since the therapeutic approaches and prognosis of pancreatic lymphoma differ significantly from those for pancreatic adenocarcinoma, it is crucial to differentiate PPL from pancreatic carcinoma [1]. However, due to its nonspecific clinical presentation and radiographic findings, cytohistological diagnosis is mandatory for final diagnosis and treatment planning. Tissue sampling can be obtained by endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) or computed tomography (CT)-guided core needle biopsy. Here we present a case of PPL in a 47-year-old male suffering from jaundice, pruritus, and 20% weight loss.
Case Report
A 47-year-old Iranian man presented with a history of jaundice, pruritus from 2 weeks ago and mild right upper quadrant pain, and 20% involuntary weight loss from 3 months ago (the weight of patient dropped from 75 to 60 kg during the 3 months before our visit). There was no family history of cancer. On admission, physical examination revealed sclera icterus and scratch marks on the arms, legs, and abdomen, with no tenderness or organomegaly. Initial laboratory values showed the following: white blood cell, 7,400 cells/μL (reference range: 4,000–11,000 cells/μL) with 67% neutrophils (reference range: 40–70%); hemoglobin, 11.2 g/dL (reference range: 12–16 g/dL); platelet, 274,000/μL (reference range: 150,000–350,000/μL); total bilirubin, 17 mg/dL (0.2–1.3 mg/dL); direct bilirubin, 12.6 mg/dL (normal range: <0.3 mg/dL); alanine transaminase [5], 357 U/L (range: <40 U/L); aspartate transaminase [4], 320 U/L (range: <40 U/L); serum alkaline phosphatase, 257 IU/L (reference range: 80–270 IU/L); and CA19-9, 23 U/mL (reference range: 0–37 U/mL).
Chest X-ray was normal. EUS was done using a linear array echoendoscope (PENTAX EG-3870 UTK) under conscious sedation and appropriate cardiopulmonary monitoring. EUS revealed dilation of intra- and extrahepatic biliary ducts. The diameter of the common bile duct was 18 mm at the distal part, with no mass lesion and no ascites. EUS demonstrated a mixed echoic mass lesion (26–23 mm) at the head of pancreas with adhesion to the duodenal wall (D1-D2), portal vein, superior mesenteric vein, and superior mesenteric artery [9]. The mass occluded the gastroduodenal artery. Common bile duct diameter was 19 mm. The diameter of the Wirsung duct at the body was 6 mm and the tail 5 mm, with no stigmata of chronic pancreatitis. Some lymph nodes (hyperechoic, maximum size 12–10 mm) were seen adjacent to the lesion.
EUS-FNA was performed using a 22-gauge needle (1–22 Echotip®; Wilson-Cook Medical, Winston-Salem, NC, USA) to further investigate the lesion and establish a more definitive diagnosis (Fig 1, Fig 2). A to-and-fro rapid jabbing movement within the pancreatic mass was done for 45 s with suction. Three subsequent passes were achieved similarly. The material within the needle was expressed onto four ethanol 70% filled glass tubes. Cytopathology showed chronic inflammatory cells and granuloma with no evidence of any malignancy (Fig 3a, b). Because of negative purified protein derivative, normal chest X-ray, and no evidence of acid-fast bacilli, pancreatic tuberculosis was ruled out. Since serum IgG4 level was normal (36 mg/dL, normal values: 8–140 mg/dL), a diagnosis of autoimmune pancreatitis was not made.
Fig. 1.

EUS-FNA from pancreatic head mass lesion by 22-gauge needle: dilated common bile duct (CBD).
Fig. 2.

EUS-FNA from pancreatic head mass lesion by 22-gauge needle.
Fig. 3.

a Histocytic aggregation consistent with granulomatous reaction (hematoxylin-eosin stain). b CD68-positive histocytes detected by immunohistochemistry method. Histopathological examination. c, d Low-grade B-cell lymphoma (hematoxylin-eosin stain; original magnification, ×200). e, f CD20-BCL2-CD10-CD43-CD3-positive atypical lymphocytic cells (immunohistochemistry; magnification, ×25).
Since clinical and imaging findings could not exclude malignancy, we considered CT-guided core needle biopsy of the pancreatic head mass (Fig 4). Histopathological finding showed granuloma and atypical small lymphoid cells with immunohistochemical pattern consistent with a low-grade B-cell lymphoma (Fig 3c–f). The patient was then referred to oncologists and started chemotherapy. Because of increased serum bilirubin, ERCP was performed and a plastic stent was inserted before chemotherapy (Fig 5, Fig 6, Fig 7).
Fig. 4.
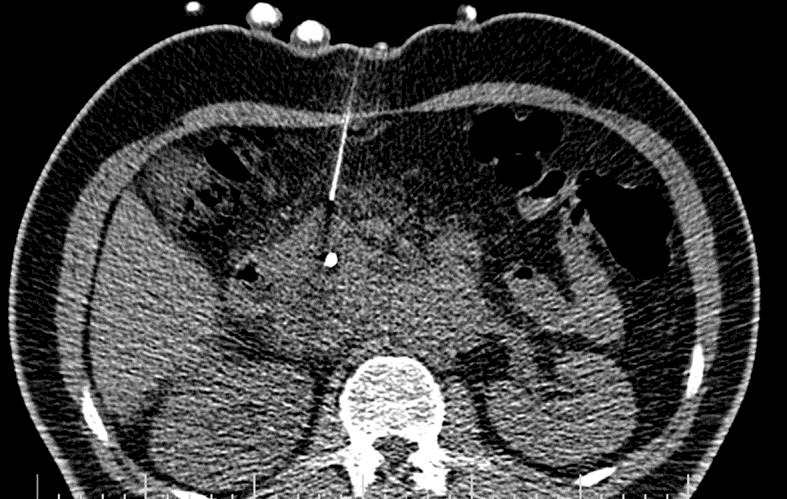
CT-guided biopsy of pancreatic mass.
Fig. 5.

Cannulation was impossible, so needle knife fistulotomy was done.
Fig. 6.

Cannulation was performed through accessory path.
Fig. 7.
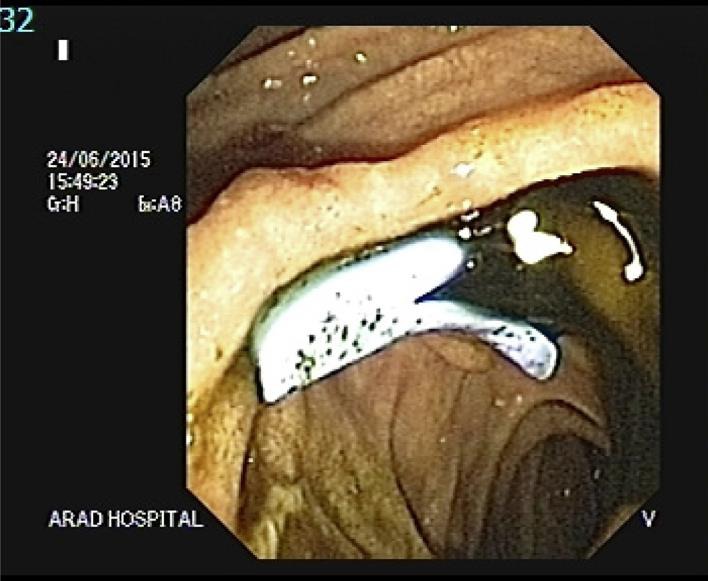
One biliary plastic stent (7 cm, 10 Fr) was inserted inside the common bile duct.
Discussion
We report a 47-year-old male patient presenting with jaundice. EUS revealed a mixed echoic mass lesion at the head of pancreas. The patient underwent EUS-FNA of solid pancreatic mass and histopathologic diagnosis revealed granuloma. This diagnosis was not convincing because we could not find evidence of tuberculosis, inflammatory and autoimmune disease of the pancreas. Therefore, to obtain tissue samples and rule out malignancy, CT-guided core needle biopsy was performed, and the diagnosis of lymphoma was confirmed.
PPL is an unlikely nonepithelial tumor (<1% incidence) [10] of the pancreas which may mimic pancreatic adenocarcinoma. Under 0.5% of all pancreatic masses and less than 2% of all extranodal malignant lymphomas are PPLs [11]. Diagnostic criteria of PPL are: (1) mass predominantly within the pancreas with peripancreatic lymphadenopathies; (2) no thoracic or superficial adenopathies, no hepatic or splenic involvement; (3) normal leukocyte count and bone marrow biopsy [12]. Most PPLs are intermediate or high-grade non-Hodgkin's lymphomas, with diffuse large B-cell lymphoma presenting the most common subtype [13]. PPLs are frequently found in the pancreatic head (80% of cases) [14], although this tumor may also occur in the body and tail [15, 16].
The clinical presentation of PPL is often nonspecific [17]. In the literature, abdominal pain is the most common presenting symptom of PPL (83%), followed by (in order of frequency) abdominal mass (58%), weight loss (50%), jaundice (37%), acute pancreatitis (12%), small bowel obstruction (12%), and diarrhea (12%) [7, 13, 15]. In terms of laboratory findings, levels of the aminotransferases, alkaline phosphatases, and direct bilirubin may be elevated [11]. Elevated level of the serum tumor marker CA19-9 is uncommon; however, it may occur when biliary obstruction is present [18].
Percutaneous ultrasound, EUS, and CT scan are well-established modalities for the diagnosis of pancreatic cancer [3].
There are some reliable signs in radiological findings which have been found to be helpful in the diagnosis and staging of pancreatic masses and to distinguish PPL from the more common pancreatic adenocarcinoma [3]. A bulky localized tumoral mass in the pancreas without dilation of the main pancreatic duct would suggest the diagnosis of pancreatic lymphoma. Furthermore, in patients with enlargement of the lymph nodes below the level of renal veins, the radiologist should be alert to the possibility of pancreatic lymphoma. Invasive tumor growth not respecting anatomic boundaries and infiltrating retroperitoneal or upper abdominal organs strengthens the diagnosis of pancreatic lymphoma over adenocarcinoma [19]. Imaging modalities could suggest the suspicion of PPL, but histopathologic examination is mandatory for the final diagnosis of PPL [3].
EUS-FNA, ultrasound- and CT-guided biopsy techniques can be used to obtain sufficient diagnostic tissue in patients who are suspected of having pancreatic lymphoma or are nonoperative candidates.
This patient highlights some important points. Our case presented with jaundice, a less frequent symptom in PPL than adenocarcinoma [7, 13, 20]. In addition, the level of lactate dehydrogenase and β2-microglobulin was normal. This is different from other reports, in which elevated levels of both lactate dehydrogenase and β2-microglobulin have been noted in diagnosing PPL [4]. These findings would not favor a diagnosis of PPL over adenocarcinoma and autoimmune pancreatitis. However, there was no splenic, hepatic, or vascular involvement and serum IgG4 and CA19-9 levels were normal. According to the literature, CA19-9 level >200 U/mL is strongly suggestive of pancreatic adenocarcinoma [9]. IgG4 levels can be useful in differentiating pancreatic masses; high IgG4 levels are seen in autoimmune pancreatitis [21].
Histologically, the most common type of PPL is high-grade diffuse large B-cell lymphoma [22], but in this report, we presented a pancreatic lymphoma case presented with low-grade B-cell lymphoma.
Conclusion
PPL is a rare entity presenting with nonspecific symptoms, laboratory and radiological findings. We describe an extremely rare case of low-grade B-cell lymphoma. Although it is often difficult to diagnose low-grade B-cell lymphoma, CT results in combination with clinical and radiological studies generally provide guidance for appropriate investigation, which helps avoid unnecessary major operation. Tissue sample is a necessity for mass lesions in the pancreas.
Authors’ Contribution
N.R. and A.H. gathered the patient's information and followed up on him. A.H.M.-A. was the patient's physician and supervised the study. S.S. prepared the images. A.N. was responsible for the pathologic assessment of the samples. All authors have contributed in writing of the article. All authors have read and approved the content of the manuscript.
Statement of Ethics
All procedures and data gathering were performed by the patient's informed consent.
Disclosure Statement
The authors declare that they have no competing interests.
References
- 1.Yoon WJ, Yoon YB, Kim YJ, et al. Primary pancreatic lymphoma in Korea – a single center experience. J Korean Med Sci. 2010;25:536–540. doi: 10.3346/jkms.2010.25.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orootan FH, Mansour Ghanaie F, Ghofrani H. Primary pancreatic lymphoma: a case report and literature review. Med J Islam Repub Iran. 2001;15:117–121. [Google Scholar]
- 3.Lin H, Li S-D, Hu X-G, et al. Primary pancreatic lymphoma: report of six cases. World J Gastroenterol. 2006;12:5064–5067. doi: 10.3748/wjg.v12.i31.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Zhang S, Vasdani N, et al. Clues for diagnosing primary pancreatic lymphoma. Case Rep Gastroenterol. 2012;6:438–445. doi: 10.1159/000339968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haji AG, Sharma S, Majeed KA, et al. Primary pancreatic lymphoma: report of three cases with review of literature. Indian J Med Paediatr Oncol. 2009;30:20–23. doi: 10.4103/0971-5851.56331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: gastrointestinal, cutaneous and genitourinary lymphomas. Skin. 1997;19:20. doi: 10.1023/a:1008282818705. [DOI] [PubMed] [Google Scholar]
- 7.Saif MW. Primary pancreatic lymphomas. JOP. 2006;7:262–273. [PubMed] [Google Scholar]
- 8.Anderloni A, Genco C, Ballarè M, et al. A case of primary pancreatic non-Hodgkin B-cell lymphoma mimicking autoimmune pancreatitis. J Gastrointestin Liver Dis. 2015;24:245–248. doi: 10.15403/jgld.2014.1121.hdk. [DOI] [PubMed] [Google Scholar]
- 9.Forsmark CE, Lambiase L, Vogel SB. Diagnosis of pancreatic cancer and prediction of unresectability using the tumor-associated antigen CA19-9. Pancreas. 1994;9:731–734. doi: 10.1097/00006676-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Rose JF, Jie T, Usera P, et al. Pancreaticoduodenectomy for primary pancreatic lymphoma. Gastrointest Cancer Res. 2012;5:32–34. [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Hua R, Zhang J, et al. First report of primary pancreatic natural killer/T-cell nasal type lymphoma. Eur Rev Med Pharmacol Sci. 2013;17:318–322. [PubMed] [Google Scholar]
- 12.Fukita Y, Asaki T, Adachi S, et al. Non-Hodgkin lymphoma mimicking pancreatic adenocarcinoma and peritoneal carcinomatosis. J Clin Oncol. 2013;31:e373–e376. doi: 10.1200/JCO.2012.45.2904. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura R, Takakuwa T, Hoshida Y, et al. Primary pancreatic lymphoma: clinicopathological analysis of 19 cases from Japan and review of the literature. Oncology. 2001;60:322–329. doi: 10.1159/000058528. [DOI] [PubMed] [Google Scholar]
- 14.Haaga JR, Boll D. Computed Tomography and Magnetic Resonance Imaging of the Whole Body. Philadelphia: Elsevier Health Sciences; 2008. [Google Scholar]
- 15.Nayer H, Weir EG, Sheth S, et al. Primary pancreatic lymphomas: a cytopathologic analysis of a rare malignancy. Cancer. 2004;102:315–321. doi: 10.1002/cncr.20488. [DOI] [PubMed] [Google Scholar]
- 16.Islam S, Callery MP. Primary pancreatic lymphoma – a diagnosis to remember. Surgery. 2001;129:380–382. doi: 10.1067/msy.2001.113284. [DOI] [PubMed] [Google Scholar]
- 17.Salvatore J, Cooper B, Shah I, et al. Primary pancreatic lymphoma: a case report, literature review, and proposal for nomenclature. Med Oncol. 2000;17:237–247. doi: 10.1007/BF02780536. [DOI] [PubMed] [Google Scholar]
- 18.Alexander RE, Nakeeb A, Sandrasegaran K, et al. Primary pancreatic follicle center-derived lymphoma masquerading as carcinoma. Gastroenterol Hepatol. 2011;7:834–838. [PMC free article] [PubMed] [Google Scholar]
- 19.Merkle EM, Bender GN, Brambs H-J. Imaging findings in pancreatic lymphoma: differential aspects. Am J Roentgenol. 2000;174:671–675. doi: 10.2214/ajr.174.3.1740671. [DOI] [PubMed] [Google Scholar]
- 20.Battula N, Srinivasan P, Prachalias A, et al. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. 2006;33:192–194. doi: 10.1097/01.mpa.0000227910.63579.15. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni G, Capelli P, Bogina G, et al. Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:439–453. doi: 10.5858/133.3.439. [DOI] [PubMed] [Google Scholar]
- 22.Grimison PS, Chin MT, Harrison ML, et al. Primary pancreatic lymphoma – pancreatic tumours that are potentially curable without resection, a retrospective review of four cases. BMC Cancer. 2006;6:1. doi: 10.1186/1471-2407-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]


