Abstract
Objective
We combined heads-up 3-dimensional (3D) 27-gauge microincision vitrectomy surgery (27GMIVS) with a very low-intensity illumination system.
Methods
This study was based on a retrospective, interventional case series of 6 eyes of 6 patients with macular disease. All patients underwent heads-up 3D 27GMIVS and the power of the intraocular illuminator was set to its minimum level, 1% (approximately 0.1 lm), throughout the surgery.
Results
We found that the procedure was easy when the heads-up 3D system was used, but not through the eyepiece of a microscope. All surgeries were successfully finished without any complications. Postoperative visual acuity was restored or maintained in all eyes during the follow-up period.
Conclusion
Heads-up, 3D system-assisted 27GMIVS with minimal illumination enabled excellent intraoperative visualization of retinal tissues, caused minimal phototoxicity to the macular retinal cells, and might therefore represent the next step in the development of an ideal, minimally invasive method of treating macular disease.
Key Words: Heads-up surgery, 27-Gauge vitrectomy, Macular hole, Epiretinal membrane, Phototoxicity
Introduction
Closed vitreous surgery for macular disease was introduced in the 1970s with 20-gauge pars plana vitrectomy [1]. Following this, 25-gauge instruments were developed, and then, in 2007, 27-gauge microincision vitrectomy surgery (27GMIVS) was described [2]. These smaller and less invasive instruments are safe and effective, with a reduced sclerotomy size, and their use for epiretinal membrane (ERM) is becoming common [3]. However, in addition to reducing sclerotomy size, it is also important to find ways to reduce intraoperative phototoxicity in the retina [4]. Heads-up 3-dimensional (3D) visualization during surgery promises to allow the use of greatly reduced illumination, thus reducing phototoxicity. Heads-up 3D surgery, which uses a large, 3D monitor rather than the usual intraoperative microscope [5], has been found to provide enhanced depth perception and increased CCD image sensitivity. Here, we combined heads-up 3D surgery with a very low-intensity illumination 27-gauge system and evaluated its efficacy in a case series of patients with macular disease. This is thus the first report to describe a technique combining heads-up, 3D system-assisted 27GMIVS and a very low-intensity illumination system to treat macular disease.
Technique
This study was based on a retrospective, interventional case series of 6 eyes of 6 patients with macular disease treated at a single center. All patients underwent 27GMIVS using Constellation instruments (Alcon Laboratories, Fort Worth, TX, USA) and a 3D heads-up system (MKC-700HD and CFA-3DL1; Ikegami, Tokyo, Japan). The procedures used an OPMI Lumera T surgical microscope with either a RESIGHT lens system (Carl Zeiss Meditec) or a vitrectomy contact lens (HHV Dispo, Type 1d; HOYA, Tokyo, Japan). During surgery, the surgeon visualized the macula with 3D polarized glasses and a high-resolution 3D display. The power of the Constellation intraocular illuminator was set to its minimum level, 1% (approximately 0.1 lm), during the vitreous surgery (Fig 1). The ERM or internal limiting membrane was removed and fluid air exchange was made if necessary. The heads-up 3D system was used exclusively throughout the procedure. All patients were followed for more than 1 month after surgery.
Fig. 1.
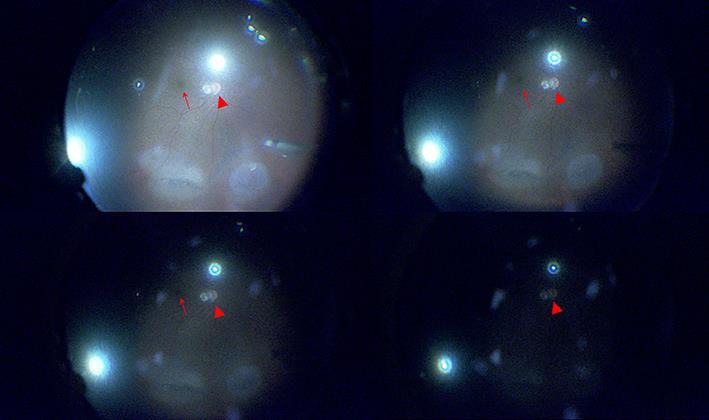
Intraoperative retinal images showing various illumination intensity levels during heads-up, 3D system-assisted 27-gauge microincision vitrectomy surgery. For ethical reasons, these example images were obtained from a patient with rhegmatogenous retinal detachment, rather than one of the macular disease patients included in this study. This patient underwent fluid-air exchange and cryoretinopexy. The arrowhead and arrow represent the optic nerve head and macula, respectively, in all images. Top left: moderate illumination power (39%, the level of the Constellation system; approximately 4 lm). The optic nerve head and macula are clearly visible. Top right: low illumination power (10%; approximately 1 lm). Bottom left: very low illumination power (5%; approximately 0.5 lm). Bottom right: lowest illumination power (1%; approximately 0.1 lm).
Heads-up 3D macular surgery was used to treat ERM in one eye, diabetic macular edema in two eyes, and macular hole in three eyes. Even though macular surgery was difficult when the intraocular illuminator was set to minimum and the macula was visualized through the eyepiece of a microscope, we found that the procedure was easy when the heads-up 3D system was used. All surgeries were successfully finished without any complications (Fig 2). Postoperative visual acuity was restored or maintained in all 6 eyes during the follow-up period.
Fig. 2.
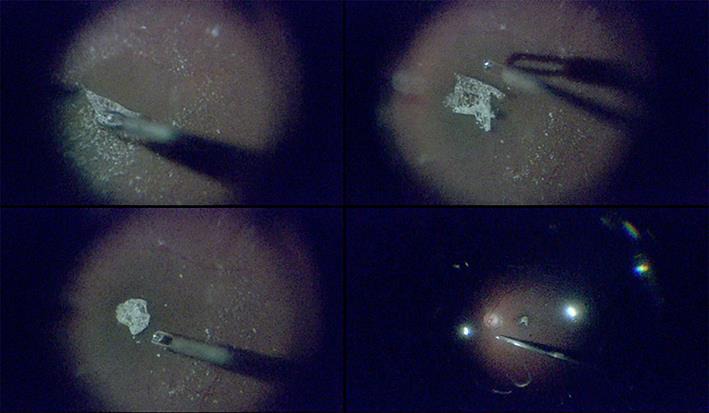
Representative intraoperative retinal images of eye with a 441-μm macular hole. Heads-up, 3D system-assisted 27-gauge microincision vitrectomy surgery with minimal illumination was used. The Constellation intraocular illuminator was set to its minimum level, 1%, in all images. Top left: after resecting the vitreal core, we performed triamcinolone acetonide-assisted internal limiting membrane (ILM) peeling. Top right: the ILM was peeled 360 degrees around the macular hole, with its edge attached, and carefully trimmed with a 27-gauge cutter. Bottom left: the ILM flap was inverted and placed over the macular hole. Bottom right: fluid-air exchange was performed with 27-gauge instruments. The macular hole closed completely postoperatively.
Discussion
This study set out to investigate the usability of heads-up 27GMIVS with minimal endoillumination. The manufacturers of modern endoilluminator systems do not provide a recommended illumination intensity level, leaving the surgeon free to set the intraoperative illumination intensity level according to individual preference. The 27GMIVS system in the current study uses values ranging from 1 to 100%, with a default level of 32%. In vitro research has shown that retinal cells undergo exposure time-dependent phototoxicity when they are exposed to a 25-gauge xenon illuminator, even when it is set to 50% intensity (approximately 3 lm) [4]. Clinically, however, it is difficult to assess retinal phototoxicity, despite the existence of retinal function tests such as perimetry or multifocal electroretinography, because of the weakness of the retinal phototoxicity experienced by patients undergoing conventional macular 27GMIVS with standard illumination. In the interest of maximizing patient safety, we therefore set out to determine the feasibility of performing heads-up 27GMIVS with the illuminator set at its lowest setting.
It is very important for vitreous surgeons to balance operational safety during macular surgery, which calls for sufficient intraoperative endoillumination, and achieving good postoperative visual outcomes, which calls for the suppression of retinal phototoxicity. The current study showed that heads-up 27GMIVS with the endoilluminator set at its lowest setting is a viable option to achieve this balance. Nevertheless, this technique cannot be considered routine and should only be chosen after taking into account the experience level of the surgeon. Moreover, endoillumination intensity is only one phototoxicity-inducing factor during vitreoretinal surgery. Other factors include light-exposure time (operation time), the distance between the macula and the light probe, and the type of light source. Taking these points into consideration, we thus consider that minimal illumination is most suitable for macular disease patients with good visual function (i.e., decimal visual acuity ≥1.0, or ≤0 logarithm of the minimal angle of resolution units) or patients with a combination of a retinal degenerative disease, such as retinitis pigmentosa, and surgically treatable macular disease, and that the surgery should only be performed by experienced vitreoretinal surgeons. Additionally, the nature of the retinal disease being treated also has bearing on the most appropriate illumination level. For example, rhegmatogenous retinal detachment surgery, as shown in Figure 1, does not require such careful control of the illumination level because during this procedure, illumination of the macular region is less important than overall illumination of the fundus with a chandelier system.
Our findings suggest that heads-up, 3D system-assisted 27GMIVS with minimal illumination enabled excellent intraoperative visualization of retinal tissues and should cause a minimal degree of phototoxicity in the macular retinal cells. This technique therefore promises to be the next step in the development of an ideal, minimally invasive method of treating macular disease. However, to confirm the efficacy of retinal surgery with minimal illumination, it will be necessary to perform a prospective study comparing heads-up macular 27GMIVS with minimal illumination and conventional macular 27GMIVS with standard illumination.
Statement of Ethics
This study followed the tenets of the Declaration of Helsinki. Informed consent was obtained for all treatments from each patient. The institutional review board of Tohoku University Graduate School of Medicine approved the research for this retrospective study.
Disclosure Statement
The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Acknowledgments
This paper was supported in part by grants for Scientific Research (C) (H.K. 26462629).
References
- 1.Michels RG. Vitreous surgery for macular pucker. Am J Ophthalmol. 1981;92:628–639. doi: 10.1016/s0002-9394(14)74654-9. [DOI] [PubMed] [Google Scholar]
- 2.Oshima Y, Awh CC, Tano Y. Self-retaining 27-gauge transconjunctival chandelier endoillumination for panoramic viewing during vitreous surgery. Am J Ophthalmol. 2007;143:166–167. doi: 10.1016/j.ajo.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Mitsui K, Kogo J, Takeda H, et al. Comparative study of 27-gauge vs 25-gauge vitrectomy for epiretinal membrane. Eye (Lond) 2016;30:538–544. doi: 10.1038/eye.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanagi Y, Iriyama A, Jang WD, Kadonosono K. Evaluation of the safety of xenon/bandpass light in vitrectomy using the A2E-laden RPE model. Graefes Arch Clin Exp Ophthalmol. 2007;245:677–681. doi: 10.1007/s00417-006-0428-x. [DOI] [PubMed] [Google Scholar]
- 5.Eckardt C, Paulo EB. Heads-up surgery for vitreoretinal procedures: an experimental and clinical study. Retina. 2016;36:137–147. doi: 10.1097/IAE.0000000000000689. [DOI] [PubMed] [Google Scholar]


