Abstract
Despite the clinical efficacy of anthracycline agents such as doxorubicin, dose-limiting cardiac toxicities significantly limit their long-term use. Here, we present the case of a 33-year-old female patient with extensive metastatic ER+/PR+/HER2– mucinous adenocarcinoma of the breast, who was started on doxorubicin/cyclophosphamide therapy after progressing on paclitaxel and ovarian suppressor goserelin with aromatase inhibitor exemestane. The patient was comanaged by cardiology, who carefully monitored measures of cardiac function, including EKGs, serial echocardiograms, and profiling of lipids, troponin, and pro-BNP every 2 months. The patient was treated with the cardioprotective agent dexrazoxane, and changes in cardiac markers [e.g. decreases in ejection fraction (EF)] were immediately addressed by therapeutic intervention with the ACE inhibitor lisinopril and beta-blocker metoprolol. The patient had a complete response to doxorubicin therapy, with a cumulative dose of 1,350 mg/m2, which is significantly above the recommended limits, and to our knowledge, the highest dose reported in literature. Two and a half years after the last doxorubicin cycle, the patient is asymptomatic with no cardiotoxicity and an excellent quality of life. This case highlights the importance of careful monitoring and management of doxorubicin-mediated cardiotoxicity, and that higher cumulative doses of anthracyclines can be considered in patients with ongoing clinical benefit.
Keywords: Anthracycline, Doxorubicin, Cardiotoxicity, Cardio-oncology, Metastatic breast cancer, Complete response
Introduction
Anthracyclines, such as doxorubicin (adriamycin), are a class of drugs that have been used in cancer chemotherapy regimens in a large number of tumor types since their discovery in the 1970s [1]. These agents exert anticancer effects by binding topoisomerase-II (topo2) and intercalating with DNA to inhibit macromolecular biosynthesis [2]. In breast cancer, anthracyclines are a mainstay of adjuvant breast cancer treatment either alone or in combination with other cytotoxic therapy [3]. However, these drugs are commonly associated with cardiac toxicity which significantly limits their long-term use, even in the case of ongoing response [4].
Current guidelines are limited in that they fail to address high-risk patient populations, where the benefit of higher cumulative doses of anthracyclines has the potential to outweigh the risks. In this case report, we describe a patient with extensive metastatic estrogen receptor-positive (ER+) breast cancer, who achieved a complete response on well-managed prolonged doxorubicin treatment with a total cumulative dose of 1,350 mg/m2. Here, we demonstrate that higher cumulative doses of anthracyclines can be considered in patients with ongoing clinical benefit, and that a multidisciplinary approach to early detection and treatment of asymptomatic cardiac dysfunction can maximize the risk-benefit ratio for anthracycline-based therapy.
Case Report
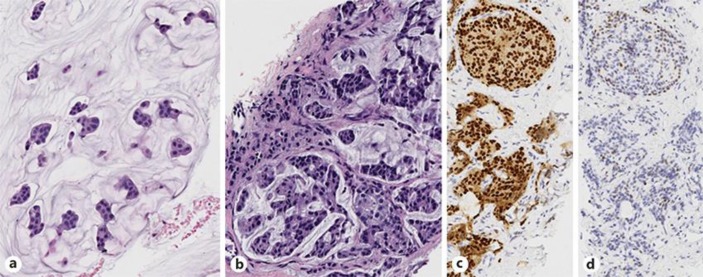
In December 2010, a 33-year-old woman presented with a 2-week history of moderate left hip pain and a lump in her stomach. On exam, a mildly tender palpable mass was noted in her epigastrium, with pain upon flexion, abduction and rotation of her left hip without atrophy or weakness. An abdominal CT was performed showing a large heterogeneous mass replacing the majority of the left lobe of the liver. CT of the chest and pelvis showed 4 indeterminate pulmonary nodules, all smaller than 5 mm, and an osseous metastasis in the anterior left acetabulum. The patient was 4 months postpartum and still lactating but a possibly 4-cm mass was noted in the left breast with bilateral lobular irregularities consistent with lactation. PET scan confirmed hypermetabolic breast, liver, and acetabular lesions, with additional detection of small hypermetabolic masses in the sacrum, inferior pubic ramus, and bilateral ovaries. Biopsy of left breast mass and metastatic liver lesion revealed histologically similar ER+/PR+/HER2– mucinous adenocarcinoma (Fig 1).
Fig. 1.
a Biopsy of the left breast reveals an extracellular mucin pool containing nests of neoplastic epithelial cells, classic features of mucinous (colloid) carcinoma (HE. ×20). b–d The tumor cells in the CT-guided biopsy of the metastatic liver lesion shows similar morphology to the left breast lesion (b; HE. ×20). A total of 80–90% of tumor cells are strongly positive for ER (c; HE ×20) and ∼5% are positive for PR with low intensity (d; HE ×20). HER2 fluorescence in situ hybridization (FISH) study performed on liver biopsy shows negative HER2/NEU amplification (not shown).
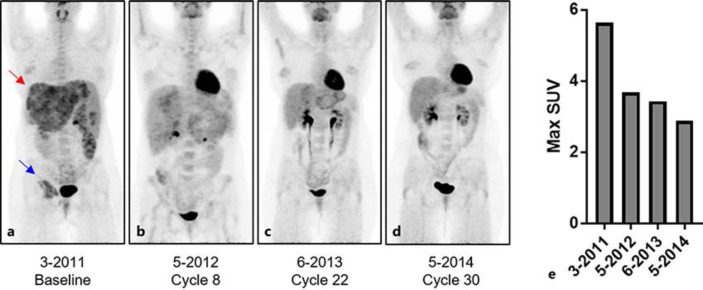
In January 2011, the patient was started on chemotherapy with weekly paclitaxel (80 mg/m2) and monthly zoledronic acid (4 mg), which led to initial stable disease with clinical improvement. After 4 cycles (July 2011), restaging CT demonstrated progressive disease, leading to the substitution of paclitaxel with ovarian suppression by goserelin and the aromatase inhibitor exemestane plus the bone-targeted agent denosumab. After 3 months on hormone therapy, the patient progressed further. In November 2011, the decision was made to pursue doublet therapy consisting of 60 mg/m2 doxorubicin and 600 mg/m2 cyclophosphamide with continuation of denosumab (120 mg monthly). At baseline, PET scan revealed extensive hypermetabolic regions in the liver and right acetabulum (Fig 2a). After 8 cycles (May 2012), with a cumulative doxorubicin dose of 444 mg/m2 (20% dose reduction for cycles 6–8), PET scan revealed no residual disease in the left breast, 75% reduction of activity in the liver, minimal residual activity in the acetabulum, and no new areas of disease (Fig 2b). In a PET scan taken on cycle 22 (June 2013; cumulative doxorubicin dose of 1,116 mg/m2), the patient achieved nearly complete resolution of disease by PET (Fig 2c). Complete response was achieved on cycle 24 (August 2013; cumulative dose of 1,200 mg/m2) and maintained through cycle 30 (May 2014; a cumulative dose of 1,350 mg/m2) (Fig 2d), after which a multidisciplinary tumor board recommended the patient be switched to second-line anti-estrogen fulvestrant with continuation of denosumab monthly.
Fig. 2.
a Initial PET scan prior to treatment with doxorubicin shows multiple FDG-avid metastases throughout the liver (red arrow) and a large FDG-avid metastasis in the right acetabulum (blue arrow). b–d PET scans during therapy show decreasing metabolic tumor burden in the liver and in the right acetabulum, achieving a complete metabolic response at both sites by the end of X cycles of doxorubicin. e The maximum standardized uptake value (SUV) of liver metastases decreases from the baseline pretreatment value of 5.6 to an end of treatment maximum SUV of 2.9 which is not significantly different than normal liver SUV.
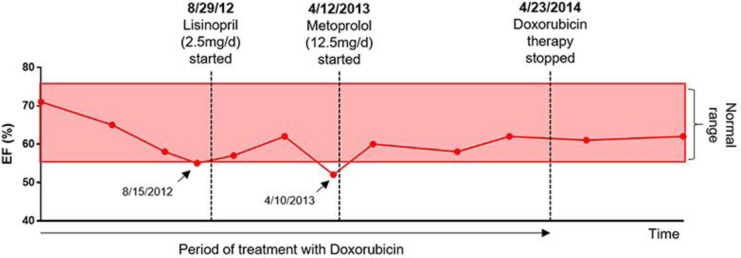
As cardiotoxicity is the primary concern for doxorubicin-based therapy, the patient was closely comanaged by cardiology over the course of treatment. Cardioprotective iron chelator dexrazoxane was initiated with cycle 6 (March 2012; cumulative anthracycline dose of 300 mg/m2). Baseline EKG, lipid profile, cardiac biomarkers troponin and pro-BNP, and serial echocardiograms every 2–3 months were also recommended. Two decreases in ejection fraction (EF) to below normal limits (55% on August 2012 and 52% on April 2013), were managed by addition of lisinopril (2.5 mg daily) and metoprolol (12.5 mg daily), respectively, which reversed the drop in EF (Fig 3).
Fig. 3.
Image showing EF (%) over time as measured by echocardiogram. Two points at the lower limit of normal or below the normal EF limit (arrows) were treated immediately with therapeutic intervention by cardiology, each of which was able to restore the EF to normal limits. The EF remained stable over the course of doxorubicin treatment and afterwards. Each point represents the date at which the patient had an echocardiogram. EF, ejection fraction.
In 2016, 6 years after diagnosis, nearly 5 years after initial AC treatment, and 2.5 years after termination of AC therapy, the patient remains minimally symptomatic on capecitabine, working full time with no cardiotoxicity and an excellent quality of life with her husband and 6-year old daughter.
Discussion
Here, we describe a patient who achieved a complete response after cumulative doses of anthracyclines of 1,350 mg/m2, with well-managed normal cardiac function (as measured by echocardiogram) through the entire duration of treatment and for >2.5 years of follow-up. While we cannot rule out future late-onset cardiotoxicity (>2 years after treatment), most cardiotoxicity after anthracycline-containing therapy occurs within the first year [5]. Here, we will discuss what is currently known about anthracycline-induced cardiotoxicity, including risk factors, molecular mechanisms, genetics, management, and limitations of current guidelines.
The strongest predictor of anthracycline-induced cardiotoxicity is cumulative dose. In the most recent analysis of 630 patients from 3 prospective studies, the estimated cumulative percentage of patients with doxorubicin-related cardiotoxicity (congestive heart failure) was 5% at a cumulative dose of 400 mg/m2, 26% at 550 mg, 48% at 700 mg/m2, and 100% above 800 mg/m2 [6]. In light of this analysis, the well-managed cardiotoxicity in our patient is remarkable. Additional well-characterized risk factors of anthracycline-induced cardiotoxicity include coadministration of other cardiotoxic chemotherapeutic agents (e.g. trastuzumab), prior radiation to the mediastinum, older age, and pre-existing cardiovascular disease (e.g. hypertension, vascular disease, diabetes) [7, 8].
Multiple hypotheses exist for the mechanism of anthracycline-mediated toxicity. The predominant hypothesis is treatment-induced increased production of toxic oxygen free radicals and oxidative stress, which leads to irreversible damage to myocytes and fibrosis of tissues [9]. Alternative hypotheses include myocyte damage due to (1) anthracycline inhibition of the beta isoform of topoisomerase-II (Top2), (2) calcium overload, (3) disruption of adrenergic function, (4) release of anthracycline metabolites, (5) release of vasoactive amines, and/or (6) release of proinflammatory cytokines [9, 10]. The lack of clear consensus despite a wealth of evidence suggests that the mechanism of anthracycline-induced cardiotoxicity is likely multifactorial, and may differ on a case-by-case basis.
Significant interindividual variability in the development of anthracycline-mediated cardiotoxicity has recently been attributed to genetics. Early data describes significant associations between the incidence of cardiotoxicity and inherited polymorphisms in genes involved in anthracycline metabolism (e.g. carbonyl reductase CBR1, CBR3), drug efflux (e.g. MRP1, MRP2), and reactive oxygen species generation (e.g. NAPD[H] oxidase), which have been further confirmed in functional animal studies [11, 12]. Genes involving cell survival (Hsp27, TLR2/4), DNA damage and repair (p53, Rac1), as well as transcriptional regulation (GATA4, STAT3, and AMPK) have also been implicated [13]. In this patient, molecular analyses (DNA sequencing of custom 196-gene panel, chromosomal microarray) were performed on tumor and plasma DNA, which revealed no known genetic alteration that could account for cardioprotection. However, we cannot rule out the possible contribution of an uncharacterized or undetected genetic mechanism.
The management of anthracycline-mediated cardiotoxicity consists of constant monitoring and treatment with cardioprotective agents. Several cardiac tests used successfully to detect or predict early changes in cardiac function or health include echocardiogram, radionuclear cardiac imaging, cardiac MRI, electrocardiogram, and serum biomarkers such as troponin-T and NT pro-BNP [7]. Dexrazoxane has been clinically proven to significantly reduce the incidence of clinical and subclinical cardiotoxicity, and is currently recommended in metastatic patients who have received a cumulative dose of doxorubicin ≥300 mg/m2 [14]. Additional cardioprotective therapeutics used in preventative treatment include beta-blocker (e.g. carvedilol) and ACE inhibitor (e.g. elanapril), which have also been used in combination to decrease the incidence of death, heart failure, or a final LVEF <45% in patients treated with anthracyclines [15]. Additional strategies to combat cardiotoxicity include the use of less toxic structural analogs of doxorubicin, such as epirubicin and mitoxantrone, or incorporation of anthracyclines into liposomes [7].
Several guidelines are available regarding the monitoring and management of anthracycline-related cardiotoxicity [5]. These guidelines generally provide an upper limit for cumulative dosages of anthracyclines based on cardiotoxicity data from retrospective analyses, generally recommending cumulative doses of less than 450–500 mg/m2 [7]. However, these guidelines are limited in that they fail to address high-risk patient populations where the benefit of higher cumulative doses of anthracyclines has the potential to outweigh the costs. This case thus highlights the need to establish evidence-based clinical guidelines for the monitoring and management of cardiotoxicity in these high-risk patient populations who respond to doxorubicin therapy.
Conclusion
Anthracyclines are an extremely effective chemotherapeutic agent, but concerns of cardiotoxicity at higher doses prevents maximal therapeutic benefit of anthracycline treatment. Our case demonstrates that higher cumulative doses of anthracycline can be considered in high-risk patients with ongoing clinical benefit, and that maximum benefit-cost ratio from anthracyclines can be achieved through vigilant monitoring and managing of small changes in cardiac function.
Statement of Ethics
The authors received informed consent from the patient prior to writing of the case report.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, et al. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969;11:1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- 2.Momparler RL, Karon M, Siegel SE, Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res. 1976;36:2891–2895. [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 6.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 7.Floyd JMJ, Berg S. Cardiotoxicity of anthracycline-like chemotherapy agents. In: Drews REPD, Gottlieb SS, editors. UpToDate. Waltham, MA: UpToDate; (accessed September 7, 2016). [Google Scholar]
- 8.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 9.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 11.Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes – a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojnowski L, Kulle B, Schirmer M, Schluter G, Schmidt A, Rosenberger A, et al. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin-induced cardiotoxicity. Circulation. 2005;112:3754–3762. doi: 10.1161/CIRCULATIONAHA.105.576850. [DOI] [PubMed] [Google Scholar]
- 13.Jensen BC, McLeod HL. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics. 2013;14:205–213. doi: 10.2217/pgs.12.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensley ML, Hagerty KL, Kewalramani T, Green DM, Meropol NJ, Wasserman TH, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- 15.Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (Prevention of left Ventricular dysfunction with Enalapril and Carvedilol in patients submitted to intensive Chemotherapy for the treatment of Malignant Hemopathies) J Am Coll Cardiol. 2013;61:2355–2362. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]