Abstract
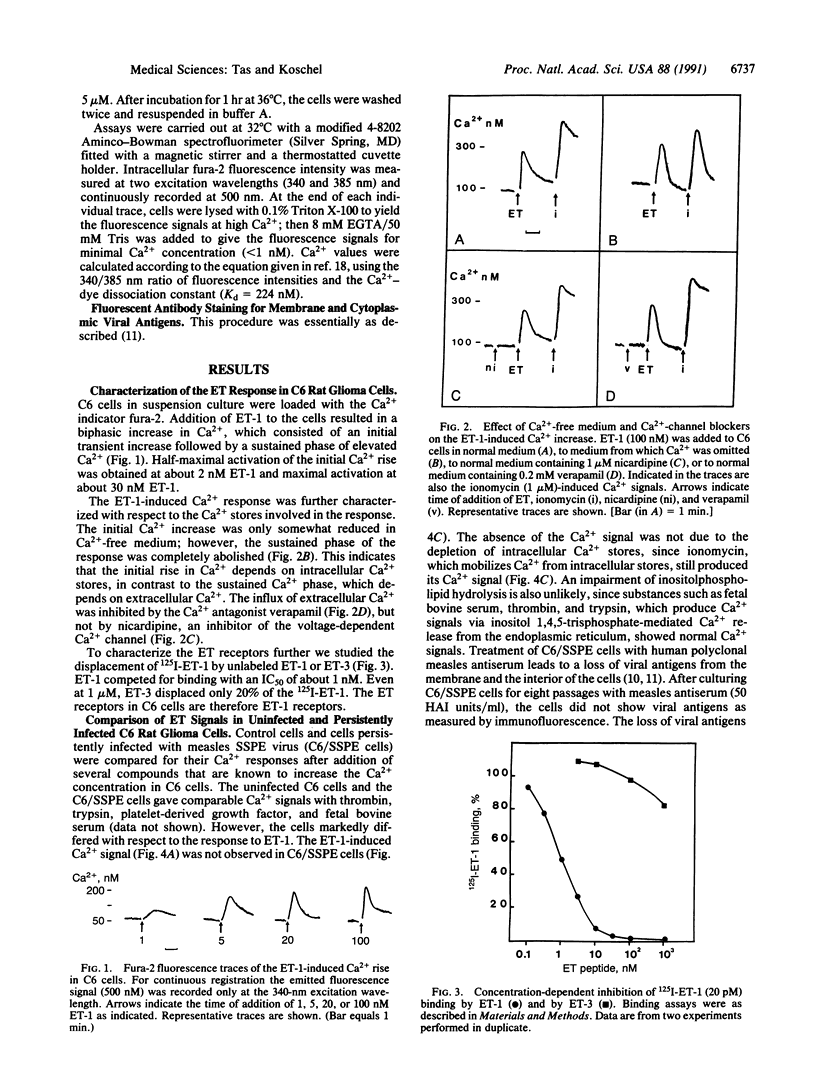
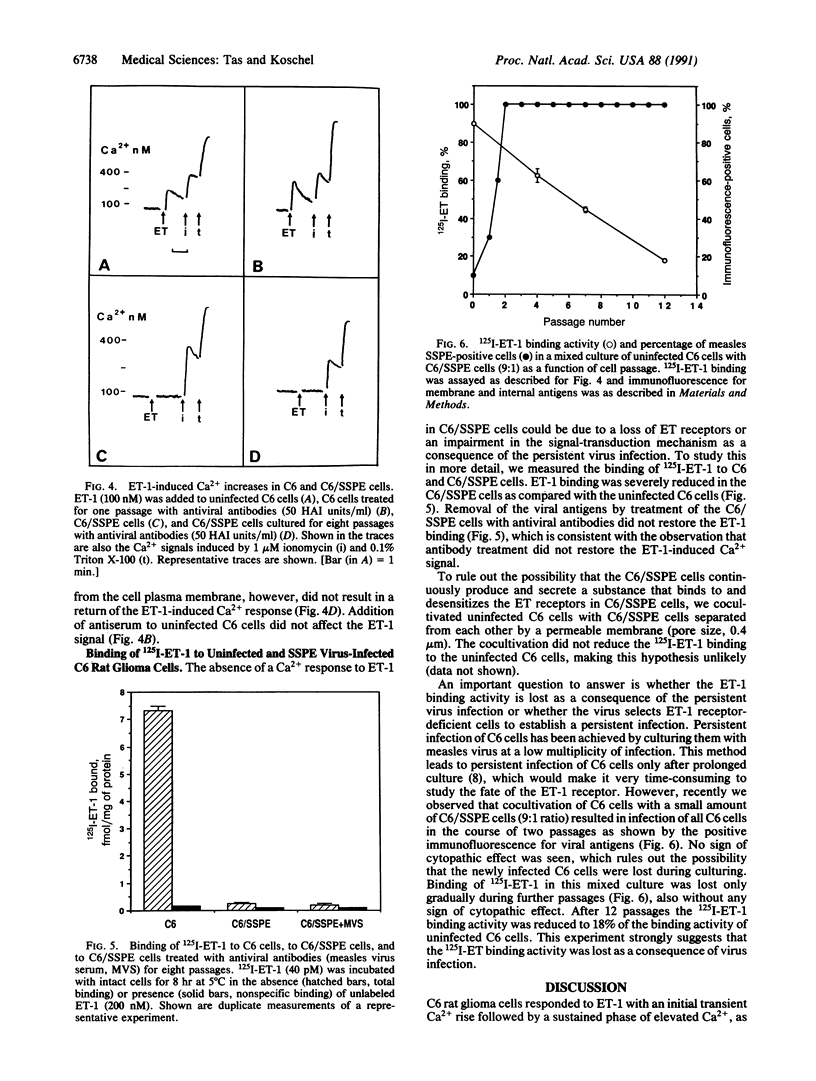
Endothelin 1 causes a strong Ca2+ signal in C6 rat glioma cells as measured by fura-2 fluorescence. This endothelin 1-induced Ca2+ signal was not observed when the cells were persistently infected with a measles virus strain of subacute sclerosing panencephalitis (SSPE, strain Lec). Binding of 125I-labeled endothelin 1 to the C6/SSPE cells was less than 5% of the binding to the C6 control cells, suggesting that the impairment in signal transduction was due to a loss of binding sites for endothelin 1. Treatment of the C6/SSPE cells with measles antiserum resulted in the loss of expression of viral proteins located in the membrane as well as inside the cells (antigenic modulation), but it restored neither the endothelin 1-induced Ca2+ rise nor the 125I-endothelin 1 binding. Cocultivation of uninfected C6 cells with C6/SSPE cells (9:1 ratio) resulting in contact-mediated transmission of measles virus showed that the 125I-endothelin 1 binding activity was gradually lost as a consequence of persistent virus infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baczko K., Liebert U. G., Billeter M., Cattaneo R., Budka H., ter Meulen V. Expression of defective measles virus genes in brain tissues of patients with subacute sclerosing panencephalitis. J Virol. 1986 Aug;59(2):472–478. doi: 10.1128/jvi.59.2.472-478.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. N., Koschel K., Carter M., ter Meulen V. Effect of measles virus antibodies on a measles SSPE virus persistently infected C6 rat glioma cell line. J Gen Virol. 1985 Jul;66(Pt 7):1411–1421. doi: 10.1099/0022-1317-66-7-1411. [DOI] [PubMed] [Google Scholar]
- Barrett P. N., Koschel K. Effect of antibody-induced modulation of measles (SSPE) virus membrane proteins on beta-adrenergic receptor-mediated adenylate cyclase activity. Virology. 1983 Jun;127(2):299–308. doi: 10.1016/0042-6822(83)90145-9. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R. Round cell variant of measles virus: neurovirulence and pathogenesis of acute encephalitis in newborn hamsters. Virology. 1986 Jan 30;148(2):349–359. doi: 10.1016/0042-6822(86)90331-4. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Rebmann G., Schmid A., Baczko K., ter Meulen V., Billeter M. A. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987 Mar;6(3):681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Halbach M., Koschel K. Impairment of hormone dependent signal transfer by chronic SSPE virus infection. J Gen Virol. 1979 Mar;42(3):615–619. doi: 10.1099/0022-1317-42-3-615. [DOI] [PubMed] [Google Scholar]
- Hänninen P., Arstila P., Lang H., Salmi A., Panelius M. Involvement of the central nervous system in acute, uncomplicated measles virus infection. J Clin Microbiol. 1980 Jun;11(6):610–613. doi: 10.1128/jcm.11.6.610-613.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y., Koprowski H. Cell to cell transmission of virus in the central nervous system. I. Subacute sclerosing panencephalitis. Lab Invest. 1974 Aug;31(2):187–196. [PubMed] [Google Scholar]
- Johnson K. P., Norrby E. Subacute sclerosing panencephalitis (SSPE) agent in hamsters. 3. Induction of defective measles infection in hamster brain. Exp Mol Pathol. 1974 Oct;21(2):166–178. doi: 10.1016/0014-4800(74)90087-2. [DOI] [PubMed] [Google Scholar]
- Koschel K., Muenzel P. Persistent paramyxovirus infections and behaviour of beta-adrenergic receptors in C-6 rat glioma cells. J Gen Virol. 1980 Apr;47(2):513–517. doi: 10.1099/0022-1317-47-2-513. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liebert U. G., Schneider-Schaulies S., Baczko K., ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J Virol. 1990 Feb;64(2):706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. W., Lee C. Y., Chuang D. M. Comparative studies of phosphoinositide hydrolysis induced by endothelin-related peptides in cultured cerebellar astrocytes, C6-glioma and cerebellar granule cells. Biochem Biophys Res Commun. 1990 Apr 30;168(2):512–519. doi: 10.1016/0006-291x(90)92351-y. [DOI] [PubMed] [Google Scholar]
- MacCumber M. W., Ross C. A., Snyder S. H. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2359–2363. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsault R., Vigne P., Breittmayer J. P., Frelin C. Astrocytes are target cells for endothelins and sarafotoxin. J Neurochem. 1990 Jun;54(6):2142–2144. doi: 10.1111/j.1471-4159.1990.tb04921.x. [DOI] [PubMed] [Google Scholar]
- Nambi P., Pullen M., Feuerstein G. Identification of endothelin receptors in various regions of rat brain. Neuropeptides. 1990 Aug;16(4):195–199. doi: 10.1016/0143-4179(90)90062-4. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Cellular signaling by peptides of the endothelin gene family. FASEB J. 1990 Sep;4(12):2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Vigne P., Barhanin J., Schmid-Alliana A., Frelin C., Lazdunski M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem Biophys Res Commun. 1988 Dec 30;157(3):977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sakai N., Yamada H., Fu T., Nozawa Y. Endothelin-1 induces intracellular calcium rise and inositol 1,4,5-trisphosphate formation in cultured rat and human glioma cells. Neurosci Lett. 1990 May 4;112(2-3):199–204. doi: 10.1016/0304-3940(90)90203-l. [DOI] [PubMed] [Google Scholar]



