Abstract
Squamous cell carcinoma of the skin (SCCS) is a common malignancy with potentially devastating consequences in patients with locally advanced or metastatic disease. Its rising incidence, primarily a result of an aging population and increased ultraviolet (UV) radiation exposure, characterize an emerging unmet need. A firm understanding of the biology of this disease, likely distinct from that of other squamous malignancies because of the influence of UV radiation, is necessary in the evaluation of treatment paradigms. Careful recognition of high-risk features pertaining to tumor and host characteristics is paramount to proper management. However, a lack of standardization in guidelines in this regard creates a challenge for physicians. Questions persist regarding additional evaluation and treatment for advanced disease such as the roles for sentinel lymph node biopsy and the adjuvant use of radiation and chemotherapy. With respect to advanced disease, multiple combinations of chemotherapy have been tested with variable success, but no rigorous randomized studies have been conducted. In addition, EGFR inhibitors such as cetuximab and erlotinib have displayed antitumor activity and as such, warrant further investigation. In sum, the treatment of locally advanced and metastatic SCCS is a ripe area for clinical investigation. This article summarizes the current understanding of disease biology and emerging questions in the management of this disease.
Squamous cell carcinoma of the skin (SCCS) is the second most common human cancer in the United States. However, its true incidence remains elusive given its exclusion from national registries. Because of the high incidence and overall good prognosis, the costs of tracking SCCS have been considered too high.1 However, as the incidence of this malignancy climbs, ultimately more than 4,000 patents die from this disease every year in the United States, with the associated direct cost of nonmelanoma skin cancer treatment surpassing $1.5 billion.2 A 2012 study estimated the incidence among white individuals in the United States to be between 186,157 and 419,543, with 5,604 to 2,572 reported developments of nodal metastasis and 3,932 to 8,791 deaths from the disease.1 The same authors estimate that in the central and southern United States, which are areas of high sun exposure, the mortality from SCCS may be actually comparable to common cancers such as melanoma, leukemia, non-Hodgkin lymphoma, renal, and bladder cancer.1 Perhaps because the vast majority of cases are localized and highly curable, locally advanced and metastatic SCCS, which can be associated with devastating local destruction and mortality, is still an under-recognized health issue. As such, rigorous randomized clinical trials that may inform treatment standards for this disease have not been conducted. With the number of cases consistently rising over the past 20 years because of increased ultraviolet (UV) radiation and tanning bed exposure and the aging of the U.S. population,3 advanced SCCS is sure to remain a major unmet niche in need of further treatment advances. This article focuses on current knowledge and treatment practices as well as emerging questions and new strategies in the evolving landscape of advanced SCCS.
Overview of Disease Biology and Etiology
Treatment decisions for advanced SCCS are often made in line with paradigms for squamous cancers of the head and neck or anogenital regions because of their similar histologies and anatomic locations. However, these diseases likely feature distinct biologies and etiologies. The pathogenesis of SCCS in the general population is thought to be the result of several acquired genetic events beginning with initiation by UV radiation and associated with various genetic alterations that continue to be investigated and debated. UV radiation, the most important teratogen in this disease, is directly absorbed by DNA and can result in DNA damage leading directly to malignant transformation (Fig. 1). Indeed, patients with fair skin, albinism, exposure to radiation, or xeroderma pigmentosum, a genetic disorder resulting in defects in DNA repair, are at increased risk for SCCS. Although UVB radiation from cumulative sun exposure is the most important environmental cause of SCCS, UVA also plays a role as demonstrated by an increased incidence of SCCS with PUVA (psoralen and UVA light therapy) and tanning beds.3 SCCS arises within precursor lesions called actinic keratoses (AK). Mutations inactivating the TP53 suppressor gene can be induced by UVB radiation, are found in up to 45% to 60% of SCCS and are also found in AK. The mutation of TP53 is thought to be an early event proceeding tumor formation as it occurs in clusters of keratinocytes in sun-exposed skin4-6 (Fig. 1). Other genes of interest implicated in the development of SCCS include CDKN2A and RAS. Inactivation of CDKN2A through mutation or hypermethylation occurs frequently and has been found to predict poor outcome in a study of 35 primary and metastatic tumors.7 RAS mutations have also been identified in AK and SCCS6 at a frequency of 3% to 30%8 in sporadic SCCS.9 Recent studies have reported an incidence of SCCS in up to 4% to 31% of those taking ve-murafenib, dabrafenib, or sorafenib.10 Mutations in RAS are frequent in these SCCS in BRAF-inhibitor treated patients; the molecular mechanism is driven by paradoxical activation of the MAPK pathway leading to accelerated growth of SCCS lesions.8 The noted higher frequency of RAS mutations in these lesions on sun-exposed areas suggests that these drugs may contribute to an existing oncogenic process rather than induce one de novo.8 In general, genetic alterations may allow keratinocytes to resist apoptosis that would normally be induced by radiation,6 although further elucidation of the contribution of genetic alterations is required.
Fig. 1.
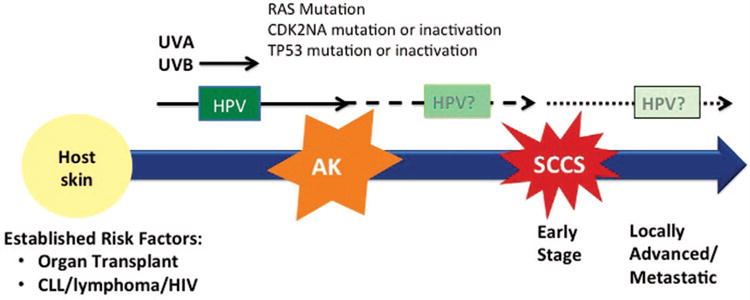
Disease biology of SCCS. The disease biology of SCCS has yet to be fully elucidated. Immunosuppression of the host is a strong risk factor for the development of SCCS, e.g., lymphoma, organ transplant. Ultraviolet (UV) radiation has an established role in the development of precancerous lesions, i.e., actinic keratosis (AK). TP53 mutation or inactivation whether de novo or induced by UVB may aid in malignant transformation. RAS mutations and CDK2NA mutations/inactivation may also play a role. HPV may play a role in the early stages of carcinogenesis, but its link to disease maintenance or progression has not been proven.
Unlike head and neck and anogenital malignancies, where human papillomaviruses (HPV) play an important role in the development of malignancy, there is considerable debate as to whether an actual association between HPV and SCCS exists (Fig. 1). Multiple studies have reported an association including a case control study of 252 patients with SCCS and 461 age-matched controls in which HPV antibodies were more frequently detected in the SCCS cohort.11 A subsequent study found a higher prevalence rate of each individual with HPV assayed than with the controls, and the odds ratio for SCCS but not basal cell carcinoma increased with increasing numbers of beta types positive.12 Another case-control study examined HPV prevalence using polymerase chain reaction detection assays in 85 patients who were immunocompetent with confirmed SCCS and 95 age-matched controls. This report found that HPV DNA from beta-HPV species 2 was more likely to be present in tumors than in adjacent healthy tissue.13 Because the detection rate of HPV DNA among controls was very high, this finding may suggest that only one or few subtypes are actually linked to the progression of SCCS. A more recent study from Arron et al, however, refutes this association. Utilizing ultra-high-throughput sequencing of the cancer transcriptome to assess the presence of papilloma-virus in the cancer cells, they found no difference in viral load between tumor and normal tissue.14 Finally, a recent retrospective study from Toll et al analyzed 35 patients with primary SCCS where DNA from beta HPV types was detected in a subset or primary tumors and metastatic lesions; they found no correlation in HPV DNA. Although this may suggest that beta HPV types do not play a vital role in the pathogenesis of advanced stages of squamous cell carcinogenesis, it does not argue against the initial role HPV may play in car-cinogenesis. This is supported by the fact that multiple studies have shown a higher prevalence of beta HPV in actinic keratosis, a well-established precursor lesion, than in the actual invasive SCCS.15 Further study is needed to conclusively determine the contribution of HPV to SCCS pathogenesis.
Finally, the role of immunosuppression in SCCS development deserves special consideration. Immunosuppressed patients, through conditions such as HIV, CLL, or organ transplant, are at increased risk for aggressive disease and development of parotid or cervical nodal metastases as well as distant metastatic disease in comparison with immunocom-petent SCCS patients (Fig. 1).16,17 It is thought that the local immunodeficiency induced by UV radiation inhibits Lang-erhans cells and decreases immune surveillance, an effect that is compounded by the use of immunosuppressive agents.18 Interestingly, genotype investigations among allo-geneic organ transplant recipients have found an increased risk of SCCS in those who carried the MTHFR 677T allele, suggesting it may be worth further investigating the role that folate-sensitive pathways play in the development of SCCS. Further, studies among patients that have undergone renal transplant indicate that switching the immunosuppressant agent to sirolimus, an mTOR inhibitor, can not only decelerate the incidence of new nonmelanoma skin lesions, but also induce regression of pre-existing malignancies because of the known antiproliferative effects of this class of agents.19 This population requires close surveillance and early biopsy of suspicious lesions, in addition to aggressive prevention, especially because treatment of advanced disease is often challenging in the face of concomitant treatment of the condition requiring or producing immunosuppression.
Recognition of High-Risk Features for Progression to Locally Advanced and Metastatic Disease
The vast majority of SCCS cases are localized and require minor surgical interventions, but in contrast to BCC, the biology of SCCS can be more aggressive with a higher chance of local extension and/or metastasis. Tumors may manifest with in-transit metastasis, satellitosis, discontinuous tumor growth pattern, and extensive local infiltration or as local recurrences within flaps and grafts near tumor beds that have been previously resected or irradiated. The physician's challenge lies in properly assessing a patient's risk for aggressive disease based on tumor or host characteristics. Generally accepted high-risk features include: tumor diameter ≥ 2 cm; poorly differentiated histology; perineural invasion; invasion beyond fat, ear, temple, or anogenital location; desmoplastic growth pattern; and immunosuppressed host status.20 Like most malignancies, the prognosis changes from excellent to poor once the tumor has become locally advanced with regional lymph node involvement and worse still with distant metastases. These characteristics can guide decisions on surgical, radiation, and medical management. However, it is unclear which factor or combination of factors has the greatest prognostic significance, and the current American Joint Committee on Cancer (AJCC) staging system demonstrates limitations. The difficulty inherent in identifying high-risk SCCS was recognized and addressed by the AJCC in 2010, with a revision of the prior staging system that allowed SCCS to be staged separately from other nonmelanoma skin cancers and considers tumor location, depth, histology, and perineural invasion. However, this revised system is also not ideal for identifying high-risk SCCS, with the bulk of poor outcomes occurring in stage T2 cases. Thus, an alternative staging system to stratify T2 tumors based on the number of four prognostic factors observed (poor differentiation, perineural invasion, tumor diameter ≥ 2 cm, and invasion beyond subcutaneous fat) was developed.21 Without widely available prognostic information, high-risk cases may continue under-recognized and variability in treatment will persist. Until accurate and validated staging systems are available, consideration of the proposed alternative staging criteria is reasonable in management decisions.
Surgical Management
Although many low-risk squamous cancers are readily managed with methods such as cryotherapy and electrodessication and curettage, this approach does not permit histologic confirmation of clear margins and is generally contraindicated for recurrent, large, poorly defined, and other high-risk tumors as well as tumors that invade into or beyond the subcutaneous fat. In these instances, conventional surgical excision may be considered. In general, low-risk tumors defined by size smaller than or equal to 2 cm, well or moderately differentiated, thinner than 2 mm, and located on trunk or extremities, can be safely resected with 4 mm margins. Tumors with any of these high-risk features warrant 6 mm margins.22 Mohs surgery is the standard of care, with the highest reported cure rate for SCCS and the ability to intraoperatively analyze 100% of the excision margin. An important alternative to Mohs is excision with complete margin assessment, which is useful when continuous removal of layers of tissue during the Mohs procedure is not ideal, for example, when surgical preservation of major vessels or nerves is desired.
In cases of locally advanced and metastatic SCCS of the head and neck that clearly involve lymph nodes, surgery and adjuvant radiation therapy have generally been considered best practice and the best chance for achieving locoregional control.23 The evaluation of subclinical metastases in the nodes, however, is not yet subject to standard guidelines. The presence of cervical lymph node metastases carries the most prognostic weight in terms of rates of recurrence and overall survival for SCCS of the head and neck. Thus, early identification of nodal disease is essential as it affects decisions regarding lymphadenectomy and adjuvant radiation. When there is histologic confirmation of lymph node involvement, the preferred treatment is regional lymph node dissection.24 However, the controversy lies in the management of high-risk SCCS with no clinical or radiological evidence of metastatic disease (clinical N0 disease). Elective lymphadenectomy is not advocated given its high morbidity to benefit ratio.25 The utility of sentinel lymph node biopsy (SLNB), a common practice in melanoma, is not well defined in the setting of SCCS. Criteria that have been proposed for consideration of SLNB include size >2 cm, clinical evidence of deep invasion >8 mm, immunosuppression, development in a scar from burn or trauma, high grade pathologic features such as perineural, lymphatic, or vascular differentiation or poor differentiation, and locations such as the ear, nasal vestibule or lip.26 When two or more high-risk features, as defined by Schmults et al,20 are present, the rate of lymph node metastases as assessed by a sentinel lymph node biopsy is 29.4%. This number rises to 50.0% when four risk factors are present.27 In a recent systematic review, the authors successfully demonstrated the feasibility and reliability of SLNB for accurate staging of head and neck SCCS patients with a false omission rate of only 4.76%, similar to the experience with melanoma.2 Indeed, SLNB use may improve survival rates in SCCS, not only by identifying subclinical lymph node involvement but by locating the correct draining nodal basin, which would guide directed therapy at an earlier and potentially more curable point of regional metastatic spread. In comparison to melanoma, there may be more potential for control of metastatic spread in SCCS considering the propensity to metastasize predominantly to regional lymph nodes and the overall radiosensitivity of the disease.25 Sentinel lymph node biopsy could ultimately identify the 20% to 30% of patients within the high-risk SCCS group that will progress to develop locoregional disease and potentially die.21 It could then be used to guide further treatments. In conclusion, SLNB could be an important tool in the staging and management of SCCS, although data do not exist to recommend this routinely. Prospective clinical trials are needed for further evaluation of which patients will benefit most from this procedure.
Radiation Therapy Options
Given advances in oncologic and reconstructive techniques, many patients with low-risk or early-stage SCCS can be treated with definitive surgical resection, and typically no adjuvant therapy is needed. However, in select patients who may need to consider their cosmetic and functional outcome, medical operability, and patient preference, radiation may play a role. Definitive radiation therapy (RT) offers comparable results to surgery for smaller lesions, but may be sub-optimal for larger lesions.29 Nonetheless, radiation may be the only modality available for medically inoperable patients.
Postoperative RT is utilized in SCCS patients in whom the likelihood of microscopic disease is relatively high or the probability of a successful salvage resection of a local recurrence is not feasible.30 Typical clinical indications for postoperative RT include large tumor size, rapid growth, recurrent disease, and neurological compromise at presentation. Typical pathologic indications for postoperative RT include grossly positive margins, extensive perineural invasion, bone invasion, and the presence of risk factors such as satellitosis and poorly differentiated histology.30 Adjuvant radiotherapy is also usually recommended for patients with parotid-area node metastases as well as to involved nodal regions within the axilla and groin because the risk of nodal relapse is high with surgery alone.23 Relative contraindications to RT are recurrent tumor in a previously irradiated field, genetic syndromes predisposing to skin cancer, connective tissue diseases, and patients of younger age.31
Many specialized radiation therapy techniques are used to treat skin cancer including orthovoltage RT, electron beam, external beam RT, and interstitial brachytherapy. The variety of techniques available is a confounding factor in attempted comparison of results across reports. The preferred modality and fractionation depend on the size, depth, and anatomic location of the lesion, ability to achieve setup reproducibility on a daily basis, and the technical capability to shield surrounding critical organs at risk (eye, lens, etc). For example, customized surface applicators using high-dose-rate 192Ir sources may be useful when protracted daily treatment fractionation is inconvenient or for treatment of lesions on sloping surfaces, where dose inhomogeneity using external beam RT may be clinically relevant. Higher doses per fraction may cause greater cutaneous and subcutaneous effects leading to poor cosmesis, so the fractionation scheme used must weigh concerns about tumor control, cosmesis, and late tissue damage, along with considerations for patient age and functional status30 (Table 1).
Table 1. Radiotherapy Fractionation Schemes for SCC.
| Tumor Characteristics | Dose Fractionation |
|---|---|
| <2 cm tumor or debilitated patient | 64 Gy in 32 fractions |
| 55 Gy in 20 fractions | |
| 50 Gy in 15 fractions | |
| 35 Gy in 5 fractions | |
| 2–4 cm or cosmetic concerns | 66 Gy in 33 fractions |
| 55 Gy in 20 fractions | |
| Postoperative | 50 Gy in 20 fractions |
| 60 Gy in 30 fractions |
From NCCN guidelines.30
Systemic Treatment
Systemic treatments in the adjuvant, neoadjuvant, or metastatic disease settings are not well studied. Extrapolations for SCCS therapy have been made, with varying degrees of success, from regimens utilized in head and neck squamous cell carcinomas, but there is lack of information from prospective trials to formulate specific guidelines. Active agents usually include platinum compounds, 5-fluorouracil (5-FU), or biologic response modifiers such as interferon–alpha or retinoic acids. With respect to adjuvant therapy, one phase III prospective, randomized study of 13cRA and interferon-alpha was conducted by Brewster et al.32 The trial enrolled 66 patients with aggressive SCCS who were randomly assigned to treatment versus placebo. Patients with high-risk features also received adjuvant radiotherapy. Improvement was not detected in time to recurrence or prevention of secondary cancers. Based on studies of mucosal SCCS, NCCN guidelines indicate that chemoradiotherapy with cisplatin could be considered in select patients, particularly if extracapsular extension and microscopic invasion of margins are noted.30 Investigation of targeted therapies such as epidermal growth factor receptor (EGFR) inhibitors with radiation is also currently ongoing. A small study published by Heath et al demonstrated an acceptable toxicity profıle using adjuvant erlotinib in combination with RT in patients with locally advanced SCCS.33 The authors reported that dermatitis, mu-cositis and diarrhea were common and the two-year overall survival and disease-free survival rate were 65% and 60%, respectively, with the disease-free survival rate improved compared with historic controls. A phase II trial is currently underway to evaluate the efficacy of this potential regimen. Future studies exploring targeted therapy and/or chemotherapy and RT in this space are necessary to advance treatment standards.
In the locally advanced or metastatic setting, patients with advanced SCCS have historically been treated with platinum-based chemotherapy regimens. A major limitation of these studies is the heterogeneity of the populations reported, with both locally advanced and metastatic patients undergoing therapy with or without concomitant RT often reported within the same study, a confounding factor since patients with locally advanced disease often have a better prognosis.34 As well, the presence of many anecdotal case reports and few randomized studies can be confusing. Multiple regimens have been reported to active in case reports of seven patients or less.35 With regard to larger phase II trials, all studies are small and nonrandomized (Table 2). A phase II study by Lip-pman et al treated 32 patients that were inoperable with oral isotretinoin and interferon alfa-2a, showing an overall 68% response rate and 5-month response duration, with better outcomes in patients with less extensive disease.34 Shin et al examined the combination of these agents plus cisplatin in 39 patients with a 34% response rate, again with better outcomes in patients with less extensive disease.36 Prospective observational studies ranging in size from 12 to 14 patients in pretreated SCCS suggest activity of oral 5-FU, cisplatin/5-FU/bleomycin, or cisplatin/doxorubicin (Table 235,37-39). However, it is important to note that a recent report that attempted to analyze data regarding patients with locally advanced disease only using platinum agents as monotherapy and in combination with taxanes, antimetabolites, and anthra-cyclines, as well as novel approaches such as intra-arterial chemotherapy or hyperthermic isolated limb perfusion, concluded that although chemotherapy often induces responses that can occasionally be durable, disease is still not amenable to local cure,40 underscoring the need for additional rigorous investigation in this arena.
Table 2. Prospective Clinical Trials in Advanced Squamous Cell Carcinoma of the Skin.
| Phase | Completion Date | Results | |
|---|---|---|---|
| Chemotherapy Trials | |||
| Prospective observational study of cisplatin, 5-fluorouracil, and bleomycin in pretreated SCCS (n = 14)35 | II | 1990 | Four CR (30%), seven PR (54%). Local control after definitive XRT and/or surgery achieved in seven patients. |
| Prospective observational study of cisplatin and doxorubicin (n = 12)35 | III | 1990 | Four CR (33%), three PR (25%), 42% with progressive disease at time of report, both treated and untreated patients included |
| 13-cis-retinoic acid and IFN alpha-2a: effective combination therapy for advanced SCCS (n = 32)34 | II | 1992 | 28 evaluable; seven CR (25%), 12 PR (43%), 5-mo response duration |
| Oral 5-fluorouracil in SCCS (n = 14, pretreated patients)35 | II | 2000 | Therapy-induced measurable improvement in nine patients (64.3%): two PR, three minimal remissions, and four arrests of disease with median duration of 30+ months |
| Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced SCCS36 | II | 2002 | Six CR (17%), six PR (17%), median survival 14.6 mo, 67% RR in locally advanced and 17% RR in metastatic disease |
| Targeted Therapy Trials | |||
| Phase II study of cetuximab as first-line single-drug therapy in patients with unresectable SCCS42 (n = 36) | II | 2011 | Disease control rate at 6 wk in 69% of pts (both locally advanced and metastatic). Best responses were eight partial responses and 2 CRs. No cetuximab-related deaths. Three related serious adverse events: two grade 4 infusion reactions and one grade 3 interstitial pneumopathy. Grades 1–2 acne-like rash occurred in 78% of patients and was associated with prolonged PFS. EGFR expression required for study entry. |
| A phase II study of gefitinib for aggressive SCCS of the head and neck (n = 23)45 | II | 2011 | Neoadjuvant approach followed by surgery or radiation; tolerable side effect profile; all patients received planned definitive treatment; 18% CR rate; Two-year OS 72.1%, PFS 63.6%. No EGFR mutations in 10 patients studied. Results led to a trial of erlotinib in this setting. |
| Phase 1 study of erlotinib plus radiation therapy in patients with advanced SCCS (n = 15)33 | I | 2012 | Treatment was felt to be tolerable. Most common toxicity attributed to erlotinib was grades 2–3 dermatologic reaction in 100% of patients, followed by mucositis (87%), and diarrhea (20%). Two-year recurrence rate was 26.7%, and mean time to cancer recurrence was 10.5 mo. Two-year OS was 65%, and DFS was 60%. |
| Ongoing Trials | |||
| Sirolimus in Kidney Transplant Patients With New or Recurrent SCCS Currently on Calcineurin-based Immunosuppression | II | Anticipated Dec-14 | n/a |
| Study of Erlotinib in the Treatment of Recurrent or Metastatic SCCS | II | Anticipated Mar-15 | n/a |
| Capecitabine in Treating Patients With Advanced or Recurrent SCCS | II | Anticipated Mar-16 | n/a |
Abbreviations: SCCS, squamous cell carcinoma of the skin; CR, complete response; PR, partial response; mo, months; RR, relative risk; pts, patients; PFS, progression-free survival; OS, overall survival; DFS, disease-free survival.
Antagonists of EGFR Signaling
In recent years, the approval of cetuximab, a monoclonal antibody that inhibits the activity of the EGFR receptor, has opened another possible avenue for systemic treatment of patients with advanced SCCS. Cetuximab has shown activity in squamous carcinomas of the head and neck41 and has also shown activity in SCCS in a small number of publications. Multiple published case and retrospective reports have documented tumor responses in patients with SCCS treated with cetuximab42-45 with one from our own institution demonstrating an impressive complete response in an elderly patient with SCCS. The largest existing study is a phase II trial of 36 patients, median age 79, which demonstrated eight partial responses, two complete responses, and a 69% disease control rate at 6 weeks and median progression-free survival of 4.1 months.46 Therapy was well tolerated, with the most common toxicities being grade 1–2 acneiform rash, which was associated with prolonged progression-free survival. EGFR overexpression was required for entry into this study; however, tumor EGFR expression levels were not associated with efficacy. No KRAS or NRAS mutations were found, and only one HRAS mutation was found in a nonresponder. The authors thus concluded that the lack of RAS mutations makes SCCS tumors attractive targets for EGFR inhibition by cetuximab. These data indicate that cetuximab is an agent with an-titumor activity in advanced SCCS that is also tolerable in this patient population. However, it is important to note that although impressive responses have been seen with cetuximab, the majority of patients who receive this therapy do not respond, suggesting that clinical trials to determine: (a) which patients maybe most likely to respond and (b) if combination strategies may produce superior outcomes are warranted.
Erlotinib and gefıtinib are EGFR tyrosine-kinase inhibitors that have also shown promise in the treatment of advanced SCCS. Erlotinib has anecdotally demonstrated activity as a single agent in chemotherapy refractory disease.47 Although the best response in an abstract report of 17 patients with pretreated SCC was stable disease,48 gefıtinib showed activity when evaluated by Lewis et al in a recent phase II trial in the neoadjuvant setting for the treatment of high-risk SCCS. Although they were not able to reach their primary endpoint, they did show an overall response rate to induction therapy of 45.5% without increased toxicity above that expected for radiation alone. Of the four patients who had a clinical complete response, three had a pathologic complete response as well. These results have encouraged a follow-up clinical trial of erlotinib in the neoadjuvant setting for the treatment of aggressive SCCS, as gefitinib is no longer available in the United States.49
Future Directions
The future is unchartered in this disease with few current standards of care. However, it is interesting to note advances in the development of immune checkpoint inhibitors which blockade the programmed death protein (PD)-1 and programmed death ligand (PD-L)-1, which have demonstrated intriguing responses in advanced melanoma and squamous tumors of the lung.50 An interesting preclinical report suggests that cetuximab-activated NK cells promote maturation of dendritic cells and CD8 T-cell priming, leading to tumor antigen spreading and TH1 cytokine release through “NK-DC cross-talk.” The authors concluded that EGFR-specific T cells arising after cetuximab treatment for patients with head and neck cancer may contribute to clinical response.51 An additional report of preclinical SCCS animal models demonstrated that immune neutralization of PD-1+ cells resulted in decreased papilloma incidence which was associated with CD4+ and CD8+ T cell infiltrates, as well as increased interferon-γ and decreased transforming growth factor-β production within tumors, supporting the role of PD-1 blockade in SCCS.52 As more trials are created to evaluate the role of novel immunotherapeutics in solid tumors, their use alone and possibly in combinatorial therapy with EGFR blockade is a rational consideration in the treatment of SCCS.
Conclusion
The advanced SCCS patient population is likely to grow in the foreseeable future. As such, unraveling the biology behind this disease merits further scrutiny. Although patients with advanced SCCS disease have historically been offered few very options for a meaningful cure, there is renewed hope with advances in surgery and radiation which need to be explored in prospective clinical trials. As well, exciting opportunities exist in the study of targeted therapies and new immunotherapies. In sum, few standards exist for the treatment of advanced SCCS; as this patient population increases, this niche represents an exciting area for clinical investigation.
Key Points.
Given the increasing incidence of SCCS worldwide, there is a rising unmet need for new and effective treatments of locally advanced and metastatic disease.
The disease biology of SCCS is likely distinct from other squamous malignancies and as such, trials specific for this tumor type are warranted to evaluate best clinical practices.
Guidelines to aid the evaluation, surgical staging, and adjuvant treatment of high-risk disease need to be solidified with data from prospective trials.
Studies conducted in the locally advanced and metastatic settings have been small, heterogeneous, and nonrandomized. As such, the opportunity exists to conduct rigorous studies to develop standards of care in this disease.
Early reports of success with cetuximab and other EGFR inhibitors represent an exciting new opportunity for clinical trials, which are currently underway. Strategies for patient selection to optimize treatment response remain to be identified and are the subject of current and future studies.
Acknowledgments
The authors would like to thank Sharad Goyal, MD, of Radiation Oncology, for his review and helpful comments with this article.
Footnotes
Disclosures of Potential Conflicts of Interest: The author(s) indicated no potential conflicts of interest.
References
- 1.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: Estimated incidence of disease, nodal metastasis, and deaths from disease in the United States. J Am Acad Dermatol. 2013;68:957–966. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed MM, Moore BA, Schmalbach CE. Utility of Head and Neck Cutaneous Squamous Cell Carcinoma Sentinel Node Biopsy: A Systematic Review. Otolaryngol Head Neck Surg. 2014;150:180–187. doi: 10.1177/0194599813511949. [DOI] [PubMed] [Google Scholar]
- 3.Stern RS. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J Am Acad Dermatol. 2012;66:553–562. doi: 10.1016/j.jaad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Brash DE, Rudolph JA, Simon JA, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hameetman L, Commandeur S, Bavinck JN, et al. Molecular profiling of cutaneous squamous cell carcinomas and actinic keratoses from organ transplant recipients. BMC Cancer. 2013;13:58. doi: 10.1186/1471-2407-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai KY, Tsao H. The genetics of skin cancer. Am J Med Genet C Semin Med Genet. 2004;131C:82–92. doi: 10.1002/ajmg.c.30037. [DOI] [PubMed] [Google Scholar]
- 7.Kusters-Vandevelde HV, Van Leeuwen A, Verdijk MA, et al. CDKN2A but not TP53 mutations nor HPV presence predict poor outcome in metastatic squamous cell carcinoma of the skin. Int J Cancer. 2010;126:2123–2132. doi: 10.1002/ijc.24871. [DOI] [PubMed] [Google Scholar]
- 8.Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207–215. doi: 10.1056/NEJMoa1105358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer JM, Kahn SM, Jiang W, et al. Activated ras genes occur in human actinic keratoses, premalignant precursors to sqaumous cell carcinomas. Arch Dermatol. 1995;13:796–800. [PubMed] [Google Scholar]
- 10.Belum VR, Fischer A, Choi JN, et al. Dermatological adverse events from BRAF inhibitors: A growing problem. Curr Oncol Rep. 2013;15:249–259. doi: 10.1007/s11912-013-0308-6. [DOI] [PubMed] [Google Scholar]
- 11.Karagas MR, Nelson HH, Sehr P, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–395. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- 12.Karagas MR, Waterboer T, Li Z, et al. Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: Population based case-control study. BMJ. 2010;341:c2986. doi: 10.1136/bmj.c2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asgari MM, Kiviat NB, Critchlow CW, et al. Detection of human papillomavirus DNA in cutaneous squamous cell carcinoma among immunocompetent individuals. J Invest Dermatol. 2008;128:1409–1417. doi: 10.1038/sj.jid.5701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arron ST, Ruby JG, Dybbro E, et al. Transcriptome sequencing demonstrates that human papillomavirus is not active in cutaneous squamous cell carcinoma. J Invest Dermatol. 2011;131:1745–1753. doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toll A, Lloveras B, Masferrer E, et al. Human beta papillomavirus DNA study in primary cutaneous squamous cell carcinomas and their corresponding metastases. Arch Dermatol Res. 2014;306:93–95. doi: 10.1007/s00403-013-1424-8. [DOI] [PubMed] [Google Scholar]
- 16.Martinez JC, Otley CC, Stasko T, et al. Defining the clinical course of metastatic skin cancer in organ transplant recipients:A multicenter collaborative study. Arch Dermatol. 2003;139:301–306. doi: 10.1001/archderm.139.3.301. [DOI] [PubMed] [Google Scholar]
- 17.Mehrany K, Weenig RH, Lee KK, et al. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53:1067–1071. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 18.Laing ME, Dicker P, Moloney FJ, et al. Association of methylenetetra-hydrofolate reductase polymorphism and the risk of squamous cell carcinoma in renal transplant patients. Transplantation. 2007;84:113–116. doi: 10.1097/01.tp.0000266069.41882.28. [DOI] [PubMed] [Google Scholar]
- 19.Salgo R, Gossmann J, Schöfer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: Reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant. 2010;10:1385–1393. doi: 10.1111/j.1600-6143.2009.02997.x. [DOI] [PubMed] [Google Scholar]
- 20.Schmults CD, Karia PS, Carter JB, et al. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: A 10-year, single-institution cohort study. JAMA Dermatol. 2013;149:541–547. doi: 10.1001/jamadermatol.2013.2139. [DOI] [PubMed] [Google Scholar]
- 21.Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402–410. doi: 10.1001/jamadermatol.2013.2456. [DOI] [PubMed] [Google Scholar]
- 22.Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1992;27(2 Pt 1):241–248. doi: 10.1016/0190-9622(92)70178-i. [DOI] [PubMed] [Google Scholar]
- 23.Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope. 2005;115:870–875. doi: 10.1097/01.MLG.0000158349.64337.ED. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien CJ, McNeil EB, McMahon JD, et al. Significance of clinical stage, extent of surgery, and pathologic findings in metastatic cutaneous squamous carcinoma of the parotid gland. Head Neck. 2002;24:417–422. doi: 10.1002/hed.10063. [DOI] [PubMed] [Google Scholar]
- 25.Reschly MJ, Messina JL, Zaulyanov LL, et al. Utility of sentinel lymph-adenectomy in the management of patients with high-risk cutaneous squamous cell carcinoma. Dermatol Surg. 2003;29:135–140. doi: 10.1046/j.1524-4725.2003.29035.x. [DOI] [PubMed] [Google Scholar]
- 26.Bumpous J. Metastatic cutaneous squamous cell carcinoma to the parotid and cervical lymph nodes: Treatment and outcomes. Curr Opin Otolaryngol Head Neck Surg. 2009;17:122–125. doi: 10.1097/MOO.0b013e32832924e0. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt AR, Brewer JD, Bordeaux JS, et al. Staging for Cutaneous Squamous Cell Carcinoma as a Predictor of Sentinel Lymph Node Biopsy Results: Meta-analysis of American Joint Committee on Cancer Criteria and a Proposed Alternative System. JAMA Dermatol. 2014;150:19–24. doi: 10.1001/jamadermatol.2013.6675. [DOI] [PubMed] [Google Scholar]
- 28.Lovett RD, Perez CA, Shapiro SJ, et al. External irradiation of epithelial skin cancer. Int J Radiat Oncol Biol Phys. 1990;19:235–242. doi: 10.1016/0360-3016(90)90529-s. [DOI] [PubMed] [Google Scholar]
- 29.Mendenhall WM, Amdur RJ, Hinerman RW, et al. Radiotherapy for cutaneous squamous and basal cell carcinomas of the head and neck. Laryngoscope. 2009;119:1994–1999. doi: 10.1002/lary.20608. [DOI] [PubMed] [Google Scholar]
- 30.Basal Cell and Squamous Cell Skin Cancers. [Accessed March 10, 2014]; National Comprehensive Cancer Network website. www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf.
- 31.Samarasinghe V, Madan V, Lear JT. Management of high-risk squamous cell carcinoma of the skin. Expert Rev Anticancer Ther. 2011;11:763–769. doi: 10.1586/era.11.36. [DOI] [PubMed] [Google Scholar]
- 32.Brewster AM, Lee JJ, Clayman GL, et al. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alfa for patients with aggressive skin squamous cell carcinoma. J Clin Oncol. 2007;25:1974–1978. doi: 10.1200/JCO.2006.05.9873. [DOI] [PubMed] [Google Scholar]
- 33.Heath CH, Deep NL, Nabell L, et al. Phase 1 study of erlotinib plus radiation therapy in patients with advanced cutaneous squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2013;85:1275–1281. doi: 10.1016/j.ijrobp.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippman SM, Parkinson DR, Itri LM, et al. 13-cis-retinoic acid and interferon alpha-2a: Effective combination therapy for advanced squamous cell carcinoma of the skin. J Natl Cancer Inst. 1992;84:235–241. doi: 10.1093/jnci/84.4.235. [DOI] [PubMed] [Google Scholar]
- 35.Cranmer LD, Engelhardt C, Morgan SS. Treatment of unresectable and metastatic cutaneous squamous cell carcinoma. Oncologist. 2010;15:1320–13208. doi: 10.1634/theoncologist.2009-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin DM, Glisson BS, Khuri FR, et al. Phase II and biologic study of interferon alfa, retinoic acid, and cisplatin in advanced squamous skin cancer. J Clin Oncol. 2002;20:364–370. doi: 10.1200/JCO.2002.20.2.364. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie TH, Jr, Porubsky ES, Luxenberg MN, et al. Cisplatin-based chemotherapy in advanced basal and squamous cell carcinomas of the skin: Results in 28 patients including 13 patients receiving multimodality therapy. J Clin Oncol. 1990;8:342–346. doi: 10.1200/JCO.1990.8.2.342. [DOI] [PubMed] [Google Scholar]
- 38.Cartei G, Cartei F, Interlandi G, et al. Oral 5-fluorouracil in squamous cell carcinoma of the skin in the aged. J Clin Oncol. 2000;23:181–184. doi: 10.1097/00000421-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Sadek H, Azli N, Wendling JL, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer. 1990;66:1692–1696. doi: 10.1002/1097-0142(19901015)66:8<1692::aid-cncr2820660807>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 40.Behshad R, Garcia-Zuazaga J, Bordeaux JS. Systemic treatment of locally advanced nonmetastatic cutaneous squamous cell carcinoma: A review of the literature. Br J Dermatol. 2011;165:1169–1177. doi: 10.1111/j.1365-2133.2011.10524.x. [DOI] [PubMed] [Google Scholar]
- 41.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 42.Bauman JE, Eaton KD, Martins RG. Treatment of recurrent squamous cell carcinoma of the skin with cetuximab. Arch Dermatol. 2007;143:889–892. doi: 10.1001/archderm.143.7.889. [DOI] [PubMed] [Google Scholar]
- 43.Kalapurakal SJ, Malone J, Robbins KT, et al. Cetuximab in refractory skin cancer treatment. J Cancer. 2012;3:257–261. doi: 10.7150/jca.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S, Eleff M, Nicolaou N. Cetuximab as primary treatment for cutaneous squamous cell carcinoma to the neck. Head Neck. 2011;33:286–288. doi: 10.1002/hed.21299. [DOI] [PubMed] [Google Scholar]
- 45.Suen JK, Bressler L, Shord SS, et al. Cutaneous squamous cell carcinoma responding serially to single-agent cetuximab. Anticancer Drugs. 2007;18:827–829. doi: 10.1097/CAD.0b013e32809ef9e0. [DOI] [PubMed] [Google Scholar]
- 46.Maubec E, Petrow P, Scheer-Senyarich I, et al. Phase II study of cetuximabas first-line single-drug therapy inpatients with unresectable squamous cell carcinoma of the skin. J Clin Oncol. 2011;29:3419–3426. doi: 10.1200/JCO.2010.34.1735. [DOI] [PubMed] [Google Scholar]
- 47.Engelhardt C, Curiel-Lewandrowski C, Warneke J, et al. Metastatic cutaneous squamous cell carcinoma responding to erlotinib therapy. J Am Acad Dermatol. 2011;65:237–238. doi: 10.1016/j.jaad.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 48.Glisson BS, Kim ES, Kies MS, et al. Phase II study of gefitinib in patients with metastatic/recurrent squamous cell carcinoma of the skin. J Clin Oncol. 2006;24:287s. suppl; abstr 5531. [Google Scholar]
- 49.Lewis CM, Glisson BS, Feng L, et al. A phase II study of gefitinib for aggressive cutaneous squamous cell carcinoma of the head and neck. Clin Cancer Res. 2012;18:1435–4146. doi: 10.1158/1078-0432.CCR-11-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Belai EB, de Oliveira CE, Gasparoto TH, et al. PD-1 blockage delays murine squamous cell carcinoma development. Carcinogenesis. 2014;35:424–431. doi: 10.1093/carcin/bgt305. [DOI] [PubMed] [Google Scholar]


