ABSTRACT
The presentation and delivery of antigens are crucial for inducing immunity and, desirably, lifelong protection. Recombinant viral vectors—proven safe and successful in veterinary vaccine applications—are ideal shuttles to deliver foreign proteins to induce an immune response with protective antibody levels by mimicking natural infection. Some examples of viral vectors are adenoviruses, measles virus, or poxviruses. The required attributes to qualify as a vaccine vector are as follows: stable insertion of coding sequences into the genome, induction of a protective immune response, a proven safety record, and the potential for large-scale production. The need to develop new vaccines for infectious diseases, increase vaccine accessibility, reduce health costs, and simplify overloaded immunization schedules has driven the idea to combine antigens from the same or various pathogens. To protect effectively, some vaccines require multiple antigens of one pathogen or different pathogen serotypes/serogroups in combination (multivalent or polyvalent vaccines). Future multivalent vaccine candidates are likely to be required for complex diseases like malaria and HIV. Other novel strategies propose an antigen combination of different pathogens to protect against several diseases at once (multidisease or multipathogen vaccines).
KEYWORDS: multidisease vaccine, multipathogen vaccine, multivalent vaccine, polyvalent vaccine, viral vector vaccine
INTRODUCTION
In the last 20 years, many new human diseases have emerged, and worryingly, diseases previously presumed to be under control, such as diphtheria, plague, and polio, have resurged (1, 2). Vaccines are recognized to be one of the most cost-effective interventions for the prevention of infectious diseases. Effective and safe vaccines capable of undergoing mass production provide the prospect of eradication of certain diseases. In most cases, a large proportion of vaccination scheme costs arises from maintaining cold chains, storage, and transport as well as the salaries of medical and paramedical staff rather than the costs of the vaccines themselves. Despite this, some recently introduced advanced vaccines are markedly more costly. The expenditure on vaccination programs can be minimized by the well-established practice of combining individual vaccines (e.g., diphtheria/tetanus/pertussis or mumps/measles/rubella), but each component has to be manufactured separately and the method of combination can be complex. With the advent of genetically engineered viral vaccines, it has become feasible to combine multiple protective antigens into a single viral vector, e.g., a complex filovirus-vesicular stomatitis virus (VSV) recombinant (3). This is especially true of larger viral vectors (e.g., herpesviruses, poxviruses, and adenoviruses [AdVs]), where there are few restrictions imposed by gene packaging limits. It is increasingly recognized that viral vectors may be deployed to protect against a broad range of infectious diseases, for example, protozoal (e.g., malaria [4]) and mycobacterial (e.g., tuberculosis [5, 6]) infections. This allows the prospect of multipathogen vaccines, in which a single vaccine agent can be envisaged to simultaneously protect against several common global pathogens, employing well-established and safe vectors in a cost-effective and unified program. Further, the underlying principles of genetic engineering and vaccine design may be applied to the prevention of infections, where the problem of protection of the host from multiple serotypes or genotypes has to be addressed. It is also important to recognize that eliciting protective cytotoxic T lymphocyte responses to epitopes within conserved viral proteins is another means to the same end (7).
Viral vector vaccines have been applied extensively in veterinary medicine (although these will not be discussed in detail in this minireview). An outstanding example of this is Raboral V-RG (Merial), the first oral live vaccinia virus (VacV) vector vaccine expressing the glycoprotein (GP) of Evelyn-Rokitnicki-Abelseth rabies virus. Before its introduction, rabies control in wildlife relied mostly on depopulation and the vaccination of individual animals. Raboral V-RG allowed oral vaccination on a large scale using vaccine-containing baits. Several countries have used Raboral V-RG safely without any adverse effects and have achieved complete rabies control (8, 9). The hurdles that have to be taken for introducing viral vector vaccine candidates into humans are appreciably higher than those for animals. Thus, the development of human viral vaccines and their licensing remain behind those achieved with animal vaccines. The pressure to find vaccines for diseases causing widespread epidemics, such as the recent Ebola outbreak, has accelerated efforts to fast track viral vaccine candidates in humans. Promising examples like the Ebola vaccine based on a recombinant adenovirus and modified vaccinia Ankara (MVA) may mark the advent of the first of a new generation of viral vaccines used in humans (10–13).
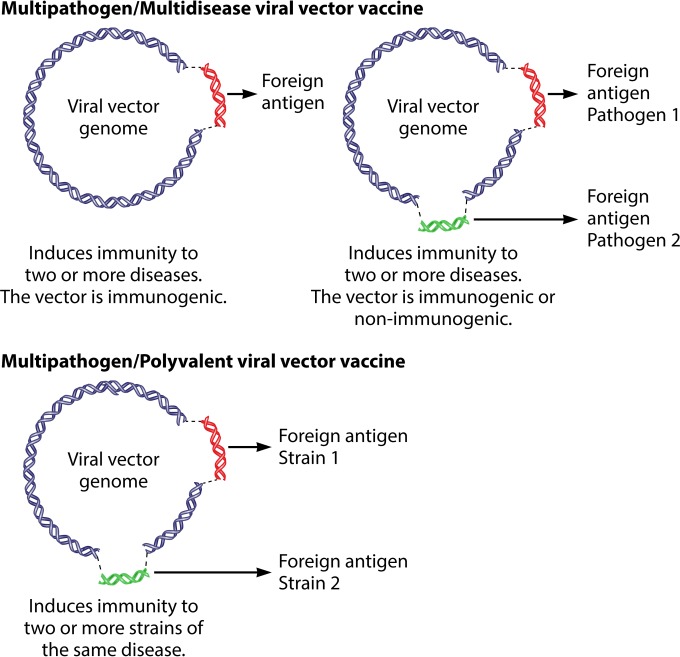
The development of new combined vaccines requires reflection on the terminology that is currently used to describe this new class of chimeric vaccines. A review of relevant literature reveals a problematic ambiguity in definition. The term “multivalent/polyvalent vaccine” is ambiguously used to describe either a vaccine candidate with the ability to protect against several diseases or a vaccine candidate that can protect against several strains of a single pathogen. In general parlance, “multivalent/polyvalent” refers to an agent that is effective against different types of the same organism. In accordance with this terminology, an infection consisting of multiple pathogens is generally described as a multipathogen disease or simply multidisease. To avoid confusion, the following nomenclature is proposed to distinguish the different types of combined vaccines.
DEFINITIONS
Multivalent/polyvalent vector vaccine.
Combined antigens from different strains (serotypes/serogroups) of one pathogen in a single vector to immunize against one disease.
Multidisease/multipathogen vector vaccine.
Key protective antigens from two or more pathogens in a single vector to immunize against several diseases.
Figure 1 shows a schematic overview of a multivalent/polyvalent and a multipathogen/multidisease viral vector.
FIG 1.
Schematic overview of multipathogen/multidisease and multivalent/polyvalent viral vector vaccines.
In this minireview, we will detail existing multipathogen and multivalent vaccines derived from viral vectors. We will draw a distinction between simple multipathogen vaccines where the vector itself forms a part of the protective agent (for example, recombinant vaccinia viruses with the theoretical but currently irrelevant potential to induce immunity to smallpox) and those where the vector backbone is a vehicle to deliver protection to two or more additional pathogens.
Another possibility to generate multipathogen or multivalent vaccines is based on vectors where protective antigens have been replaced by antigens derived from heterologous viruses. While these vaccines are likely to yield protection to the heterologous virus, the protection yielded against the vector agent is likely to be impaired. These vaccine candidates will not be discussed further in this minireview. We will also compare and contrast the larger viral vectors that are likely to serve as backbones for future broad-spectrum multipathogen and multivalent vaccines and address the technological hurdles remaining before such human vaccines become widely distributed. Table 1 provides an overview of the current multivalent and multipathogen viral vector vaccines that are under investigation.
TABLE 1.
Overview of multivalent/polyvalent and multipathogen/multidisease viral vector vaccine candidates
| Vector | Disease(s)a | Immunogenic insert(s) | Insertion site | Animal model | Administrationg | Cellular immune response | Humoral immune response | Challenge(s)g | Protective immunitya | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Adenovirus | Marburg virus, Ebola virus | NP ZEBOV, GP EBOV, GP SEBOV, GP Ci67, GP Ravn, GP Musoke, NP Musoke | Between the ITRf | Cynomolgus macaques | i.m., 4 × 1010 total PFU (day 0, day 63) | ✓ | 1st challenge (wk 0): s.c. MARV Musoke or i.m. ZEBOV (1 × 103 PFU); 2nd challenge (wk 10): i.m. SEBOV or s.c. MARV Ci67 (1 × 103 PFU) | 100% of animals protected | 36 | |
| Ebola virus* | GP SEBOV, GP ZEBOV | Between the ITR | Mice (C57BL/6; BALB/c) | i.p. or s.c., 108 PFU (wk 0, wk 16, wk 24) | ✓ | ✓ | i.p., 1 × 103 PFU mouse-adapted ZEBOV-derived Ebola virus | 100% of animals protected | 32 | |
| Influenza* | HA of A/Indonesia/05/05 and A/Vietnam/1203/04 | Into the E1 region | Mice (BALB/c) | i.m., 1 × 108 PFU | ✓ | ✓ | i.n., 100 MID50 of influenza virus Egypt/08, TK/VA, G1/99, pH1N1, or X-31 | Provided the same HA subtype was included in vaccine, the challenge virus was neutralized | 82 | |
| HA of A/Netherlands/219/03 and A/Chicken/Hong Kong/G9/97 | Into the E1 region | Mice (BALB/c) | i.m., 1 × 108 PFU | ✓ | ✓ | i.n., 100 MID50 of influenza virus Egypt/08, TK/VA, G1/99, pH1N1, or X-31 | Provided the same HA subtype was included in vaccine, the challenge virus was neutralized | |||
| Marburg virus* | GP fusion protein of MARV Ci67, Ravn, and Musoke strains | Between the ITR | CD-1 mice | i.p., 1 × 108 PFU (wk 0, wk 8) | ✓ | 83 | ||||
| Guinea pigs | s.c.; low dose: 5 × 106 PFU; medium dose: 5 × 107 PFU; high dose: 5 × 108 PFU (wk 0, wk 4) | ✓ | s.c. with MARV Musoke, Ravn, or Ci67 (2 × 103 LD50) | Low dose group: 83.3%; medium/high dose group: 100% of animals protected | ||||||
| Coxsackievirus group B | Myocarditis | Hexon protein L1 loop of AdV2 | Between capsid protein P-1D and protease P-2A | Mice (BALB/c) | i.p., 5 × 105 TCID50 (a: day 0; b: day 0, day 14; c: day 0, day 14, day 28) | ✓ | 55 | |||
| Human parainfluenza virus type 3 | Measles, respiratory tract infection | HA of MV Edmonston | Between N/P, P/M, or HN/L genes | Golden Syrian hamster | i.n., 1 × 106 PFU | ✓ | Wild-type PIV3 (1 × 106 PFU) | 100- to 1,000-fold reduction of PIV3 virus titers | 49 | |
| Measles virus | Measles, hepatitis B | HBsAg and/or HBcAg (ayw type) | Between M/P genes (HBsAg), H/L genes (HBcAg) | Mice (IFNAR−, CD46) | MV-HBsAg i.n. or i.p., 5 × 105 PFU | ✓ | 84 | |||
| Measles, HPV-associated disease | L1 protein | Between P/M genes | Mice (IFNAR−, CD46) | i.p., 1 × 105 PFU (day 0, day 28) | ✓ | 20 | ||||
| Measles, HIV | Env gene gp140/gp160 (HIV-1 isolate, 89.6), V3 loop deletion | Between P/M or H/L | Mice (IFNAR−, CD46) | i.p., 15 × 106 TCID50 | ✓ | ✓ | 85 | |||
| Measles, HIV | F4 antigen (= p24 [BH10], RT [HXB2], Nef [Bru-Lai], and p17 [BH10]) of HIV-1 subtype B | Between P/M genes | Mice (IFNAR−, CD46) | i.p., 1 × 104 or 1 × 107 TCID50 | ✓ | ✓ | 86 | |||
| Cynomolgus monkeys | i.m., 1.6 × 104 CCID50/ml (a: day 1; b: day 1, day 29, day 57 | ✓ | ✓ | |||||||
| Measles, HIV | Gag-Pol fusion protein and gp140dCFI (e1), or Gag-Pol fusion protein and gp140dV1/2dCFI (e2) of HIV-1 clade B | G-P between H/L gene and e1/e2 between P/M | Mice (IFNAR−, CD46) | i.p., 1 × 104 or 1 × 105 PFU (wk 0, wk 4) | ✓ | ✓ | i.n. pseudochallenge using 5 × 106 PFU recombinant vaccinia virus-HIV-gag | Immunized animals were less susceptible to weight loss | 87 | |
| Measles, HIV | F4 antigen (= p17, p24, RT and Nef) of HIV-1C | Cynomolgus macaques | i.m., 1 × 104 TCID50 and 1 × 105 TCID50 MV1-F4 (day 0, day 84) | ✓ | ✓ | 88 | ||||
| Measles, West Nile virus | WNVc E protein (IS-98-STI) | Between P/M genes | Mice (IFNAR−, CD46) | 1 × 104 or 1 × 106 TCID50 (wk 0, wk 4) | ✓ | i.p., LD50 of WNV IS-98-ST1 | 100% of animals protected | 89 | ||
| Mice (BALB/c) | 2 μl pooled immune sera from IFNAR− CD46 mice | ✓ | i.p., 10 × LD50 of WNV IS-98-ST1 | 100% of animals protected | ||||||
| Measles, SARSb | Spike protein (SARS-CoVd, specimen no. 031589) | Mice (IFNAR−, CD46) | i.p., 1 × 105 TCID50 (day 0, day 28) | ✓ | i.n., 1 × 105 PFU SARS-CoV | 100% of animals protected | 90 | |||
| Measles, SARS | Nucleocapsid protein, spike glycoprotein (SARS-CoV Urbani) | Between P/M genes | Mice (IFNAR−, CD46) | i.p., 1 × 104 PFU (wk 0, wk 4, or wk 8) | ✓ | 91 | ||||
| Measles, dengue fever | EDIII from E protein (aae 295 to 394) and the ectodomain of the M protein (aa 1 to 40) from strain FGA/89 French Guiana for serotype DV1, Jamaica/N.1409 for DV2, DV3 H87, 63632/76 Burma for DV4 | Between P/M genes | Mice (IFNAR−, CD46) | i.p., 1 × 105 TCID50 (wk 0, wk 4) | ✓ | 92 | ||||
| Measles, chikungunya fever | C, E3, E2, 6K, E1 (La Reunion strain 06-49) | Mice (IFNAR−, CD46) | i.p., 1 × 103, 1 × 104, or 1 × 105 TCID50 (wk 0, wk 4) | ✓ | i.p., 1 × 102 PFU CHIKV 06-49 (= 33 times LD50) | 100% survival (immunized with 104 or 105 TCID50); 83% survival (immunized with 103 TCID50)** | 26, 27 | |||
| Modified vaccinia Ankara | Influenza H5N1 strains* | HA of A/Vietnam/1203/04, A/Indonesia/CDC669/06, and A/Anhui/01/05 | Deletion site III | Mice (BALB/c) | i.m., 100 μl 8 × 107 TCID50 (day 0, day 28) | ✓ | i.n., 10 MLD50 of A/Vietnam/1203/04 or A/chicken/Shanxi/2/06 | 100% of animals protected | 61 | |
| Varicella-zoster virus | Mumps, chickenpox/shingles | HN and F protein (or F protein with serine 195 replaced with tyrosine) | Unique long domain | Guinea pigs | s.c., 2 × 106 to 5 × 106 MRC-5 cells infected with VZV vOka-HN-F (day 0, day 14, day 28, day 42) | ✓ | 42 |
An asterisk indicates a multivalent/polyvalent vaccine candidate. Two asterisks indicate a vaccine candidate that advanced to phase 1 clinical studies.
SARS, severe acute respiratory syndrome.
WNV, West Nile virus.
SARS-CoV, severe acute respiratory syndrome coronavirus.
aa, amino acid.
ITR, inverted terminal repeat.
i.m., intramuscularly; i.p., intraperitoneally; s.c., subcutaneously; i.n., intranasally; CCID50, 50% cell culture infective dose; MID50, 50% median infective dose.
MULTIVALENT AND MULTIPATHOGEN VACCINES FOR HUMAN APPLICATIONS
A wide variety of viruses have been investigated as single-pathogen vector vaccines; remarkably fewer have been used for multivalent and multipathogen applications. Viruses have to meet several requirements to be considered suitable multipathogen or multivalent vectors. The viral vector has to be capable of taking up large fragments of immunogenic genes, together with regulatory elements (e.g., promoter, polymerase, terminator, etc.), or immunomodulators like cytokines, to enhance humoral and cellular immune responses (14–16). These need to be expressed efficiently and stably, sometimes from different loci within the genome and preferably without the persistence of the recombinant virus in the host or its integration into the host genome. Other factors that have to be considered when choosing a vector platform are the lack of toxicity in the host, affordable large-scale production, or issues with preexisting vector immunity that may lead to a reduced immune response to the vector (15, 16). Table 2 compares the viral vectors discussed in this article with respect to their individual vector characteristics and suitability as multivalent/multipathogen viral vector candidates.
TABLE 2.
Comparison of the viral vectors discussed in this minireview based on specific vector characteristics
| Vector | Virus group | No integration into the host genome | Replication in the cytoplasm | Insertion capacitya | Induction of cellular and humoral immunity | Preexisting vector immunity has proven problematic |
|---|---|---|---|---|---|---|
| Adenovirus | dsDNA | + | − | ++ | + | + |
| Coxsackievirus group B | ssRNA | + | + | + | + | + |
| Measles virus | ssRNA | + | + | ++ | + | − |
| Modified vaccinia Ankara | dsDNA | + | + | +++ | + | − |
| Parainfluenza virus 3 | ssRNA | + | + | ++ | + | − |
| Varicella-zoster virus | dsDNA | + | − | +++ | + | − |
+++, up to 10 to 30 kb; ++, up to 4 to 8 kb.
Measles virus.
Among viral vector platforms, measles virus (MV) is a promising candidate. Measles virus, an exclusively human pathogen, is an enveloped virus in the family of Paramyxoviridae with a single-stranded, negative-sense RNA genome. Several MV strains (e.g., the Moraten, Schwarz, or Edmonston measles virus vaccine strain) have been safely used as vaccines for many years; they exhibit strong immunogenic properties leading to lifelong protection. MV replication occurs strictly in the cytoplasm of infected cells, which contributes to a consistent safety profile because no viral DNA is integrated into the host's genome (17). The ability to achieve the stable insertion of more than 5,000 nucleotides into the MV genome (unlike other RNA viruses), together with the efficient expression of transgenes and low production costs, makes MV a valuable potential vaccine delivery system (18, 19). In many multipathogen vaccine candidates based on measles virus vectors, the virus itself is used as an immunogen.
One example is a MV vector (Moraten Berna measles vaccine strain sequence) human papillomavirus (HPV) vaccine candidate (rMVb2-HPV-L1), generated by Cantarella and colleagues (20), which proved to induce strong humoral immune responses against MV and HPV in transgenic interferon alpha receptor-deficient (IFNAR−/−) CD46 mice. Reverse genetics technology enabled the rescue of MV (an RNA virus) from cloned plasmid DNA, containing MV antigenomes, in cell culture using the human helper cell line 293-3-46. The structural L1 protein sequence from HPV16, found to be immunogenic in previous studies, was inserted between the M and P sequence of the MV, forming virus-like particles after expression (20–22). The stability of the transgene expression, an important factor for a successful vaccine candidate, was tested over 10 passages in MRC-5 cells (human fetal lung fibroblasts) and showed no reduction. Furthermore, recombinant rMVb2-HPV-L1 did not exhibit a reduced growth kinetic compared to that of the “empty” MV. The immunogenic activity of rMVb2-HPV-L1 was evaluated in a murine immunization study with MV-susceptible mice (IFNAR−/− CD46; devoid of the interferon type I receptor plus expression of human CD46). Mice were injected intraperitoneally with rMVb2-HPV-L1 or the parental MV at day 0 and 4 weeks later with 105 PFU. All of the mice that were immunized with rMVb2-HPV-L1 mounted L1-specific humoral immune responses, which is comparable to the humoral immune response elicited in mice after three intramuscular injections with the standard HPV vaccine (Cervarix; GlaxoSmithKline Biologicals). Cervarix is a licensed virus-like particle vaccine against disease associated with HPV16 and HPV18, where virus-like particles are obtained with a baculovirus expression system (23). The serum of immunized mice was also examined for anti-measles virus antibodies. No difference in the immune response to MV was observed with the recombinant virus compared to that of the parental MV. As MV has the capacity for larger inserts (5 to 6 kb), this vector may be exploited for expressing immunogens of other HPV types (e.g., HPV18). Additionally, the authors suggest the insertion of the E6 or E7 proteins, which may allow the vaccine candidate to be used for immunotherapy (20).
In another approach by Brandler et al. (26), a recombinant measles vaccine expressing chikungunya virus-like particles was generated using a helper cell line rescue approach (24). Chikungunya virus (CHIKV) is an alphavirus with a positive RNA genome and is transmitted by mosquitoes especially in Southeast Asia, Africa, and the Indian subcontinent. In recent years, there has been a tendency for the virus to spread to more temperate regions. The measles virus Schwarz strain was used for the insertion (between the phosphoprotein and matrix gene of MV) of the C, E3, E2, 6K, and E1 structural protein sequences of CHIKV La Reunion strain 06-49, which accounts for most epidemics worldwide (25). Transgenic CD46-IFNAR mice, susceptible to measles infection, were injected intraperitoneally with two consecutive doses (ranging from 103 to 105 50% tissue culture infective dose [TCID50]) 1 month apart. The control group received empty MV Schwarz strain. All mice vaccinated with MV-CHIKV showed the generation of specific antibodies for the MV vector and CHIKV as well as specific cellular immune responses (interferon gamma [IFN-γ] enzyme-linked immunosorbent spot [ELISPOT] assay on splenocytes), which were boosted after the second immunization. Furthermore, a plaque reduction neutralization test showed that CHIKV-neutralizing antibodies were induced. A challenge study, 1 month after the last injection, with 100 PFU of CHIKV 06-49 (equal to 33 times the 50% lethal concentration [LD50] by intraperitoneal injection) was performed. All of the mice that were immunized with 104 or 105 TCID50 of MV-CHIKV were completely protected from CHIKV even when there was preexisting immunity to MV. Of the mice that were immunized with 103 TCID50 of MV-CHIKV, 83% survived the lethal challenge, and all of the control mice injected with the MV Schwarz strain developed disease and died. In addition, antibodies elicited by the MV-CHIKV vaccine candidate showed neutralizing activity against other clinical isolates (La Reunion 2006, India 2011, Congo 2011, and Thailand 2009) in plaque reduction neutralization tests. Furthermore, passively transferred immune serum from MV-CHIKV-vaccinated mice protected five out of six mice against a lethal challenge with CHIKV (100 PFU of CHIKV 06-49) (26). The MV-CHIKV vaccine candidate was further evaluated in a randomized, double-blind, placebo-controlled phase 1, dose-escalating study including an active comparator (Priorix; GlaxoSmithKline Pharma GmbH, Vienna, Austria; live virus vaccine against measles, mumps, and rubella). The measles strain in the Priorix vaccine is homologous to the MV used to design the MV-CHIKV vaccine candidate. Healthy adults received a low dose (1.5 × 104 TCID50 per 0.05 ml), medium dose (7.5 × 104 TCID50 per 0.25 ml), or high dose (3.0 × 105 TCID50 per 1 ml) of MV-CHIKV suspended in HEPES buffer with ammonium sulfate, Priorix, or placebo (sterile saline) on day 0 and 28 (the placebo was administered on day 90) or on day 0 and 90 (the placebo was administered on day 28). The geometric mean titers of neutralizing antibodies in the blood of participants were lower in the low dose and Priorix vaccine groups than in the medium and high dose groups. Nevertheless, all groups showed 100% seroconversion after booster immunization. Further, the impact of preexisting anti-measles immunity on the MV-CHIKV vaccine candidate was investigated and was found to have no impact on the performance of the vaccine candidate. The authors conclude that a phase 2 clinical trial is warranted to evaluate this promising vaccine candidate further (27).
Adenovirus.
Adenoviruses (AdVs) have been widely studied as vectors for gene therapy and vaccines targeting various diseases, such as malaria or hepatitis C. Several characteristics make them attractive as vaccine vectors, including manufacturability and the ability to elicit broad immune responses. Adenoviruses are double-stranded DNA (dsDNA) viruses that replicate in the nuclei of vertebrates. They are easily manipulated into taking up foreign DNA (up to 8 kb) and can be cultivated in several cell types (dividing and nondividing cells as well as dendritic cells) to produce high virus titers and high levels of protein expression (19, 28). Expression levels can even be enhanced by using heterologous promoters. Another advantage of AdV is their ability to induce strong T-cell responses, including cytotoxic T cells. However, preexisting vector immunity—as is present in a large proportion of individuals—inhibits efficient expression of transgenes and inactivates the viral vector. Potential alternatives are nonhuman AdV vectors or engineered vectors. Adenoviral vectors are available as replication deficient and competent for mammalian cells (29–31).
The generation of multivalent Ebola virus (EBOV) vaccines employing an AdV platform was pioneered by Wang and colleagues (32). They created a bivalent complex adenovirus-based vaccine (cAdVax) vector, utilizing complex adenovirus technology and carrying the GP of Ebola virus Sudan (Boniface strain, SEBOV) and Zaire (Zaire-95 strain, ZEBOV). The cAdVax vectors are replication-defective adenovirus vector platforms with deleted E1, E3, and E4 genes, enabling the vector to accommodate large amounts of foreign DNA (33–35). BALB/c and C57BL/6 mice were immunized intraperitoneally with 1 × 108 PFU of the bivalent vaccine candidate at 0, 16, and 24 weeks. The cAdVaxES/Z vaccine candidate was able to induce SEBOV and EBOV Ebola-specific antibodies to both strain responses as well as cell-mediated immune responses. To investigate if the cAdVaxES/Z vaccine candidate protects mice from a lethal challenge with a mouse-adapted Ebola virus strain (ZEBOV-derived), BALB/c and C57BL/6 mice were immunized by subcutaneous injection with 1 × 108 PFU of the bivalent vaccine candidate on day 0 and 35 followed by a challenge with 1,000 PFU of mouse-adapted Ebola virus (intraperitoneally). All vaccinated animals were 100% protected (32).
On the basis of this work, Swenson and colleagues developed a multistrain filovirus vaccine utilizing complex adenovirus technology and an adenovirus vector (cAdVax) (36). Filoviridae, primarily Ebola virus and Marburg virus, cause severe disease in humans and nonhuman primates. The high divergence between these species and the lack of cross-protection make vaccine development a difficult task (37). This pan-filovirus vaccine approach comprised antigens of Zaire Ebola virus (ZEBOV), Sudan Ebola virus (SEBOV), and the Ci67, Ravn, and Musoke strains of the Marburg virus (MARV). Four different cAdVax vectors were designed, the EBO2 vector containing two copies of the nucleoprotein (NP) of ZEBOV, the EBO7 vector expressing glycoproteins (GPs) of EBOV and SEBOV, the M8 vector expressing the Ci67 and Ravn GPs, and the M11 vector expressing the Musoke GP and NP genes. All four vectors were portioned equally (1 × 1010 PFU) into one combination vaccine, which was then injected intramuscularly into cynomolgus macaques on day 0 and with a booster vaccination after 63 days. The vaccinated animals were challenged with 1,000 times the lethal dose of MARV followed by EBOV or vice versa. All vaccinated primates were 100% protected against ZEBOV and SEBOV as well as the three Marburg virus species (Ci67, Ravn, and Musoke) (36).
Concordant with the above studies, Pratt and colleagues reported a multivalent Ebola virus vaccine candidate (EBO7) based on the cAdVax system, which expressed glycoproteins of SEBOV (Boniface strain) and ZEBOV (Kikwit strain) (38). Furthermore, the M8-recombinant-containing (36) Marburg virus Ci67 and Ravn GP, which was designed in the above study, was included. Immunization of cynomolgus macaques with an equal mixture of M8 and EBO7 (1 × 1010 PFU administered intramuscularly on day 0 and boosted on day 65 or 120) led to similar levels of antibodies against ZEBOV and SEBOV. The simultaneous administration of both vaccine candidates (M8 and EBO7) did not interfere with the levels of antibodies generated against ZEBOV or SEBOV. To test whether the bivalent vaccine candidate protects the vaccinated animals from Ebola virus disease, the macaques were challenged (intramuscularly) with 500 PFU of ZEBOV, 800 PFU of SEBOV, or 800 PFU SEBOV followed by 1,100 PFU ZEBOV. All vaccinated macaques survived the lethal challenge without developing signs of disease. The authors further investigated whether the EBO7 vaccine candidate confers protection against aerosol challenge with 900 to 1,000 PFU of aerosolized SEBOV and 100 to 500 PFU of aerosolized SEBOV after vaccination with 1 × 1010 PFU of EBO7 (day 0 and 71). The vaccine protected the vaccinated macaques after aerosol challenge with either virus in an otherwise lethal dose, even with preexisting vector immunity to the adenoviral vector (38). There is no data on the M8 recombinant, which was administered simultaneously with the EBO7. It would be interesting to investigate whether coadministration protects macaques from a challenge with the respective Marburg virus species. Based on the results reported by Swenson and colleagues (36), M8-vaccinated macaques survived a challenge with 1,000 times the lethal dose of MARV; it seems likely that the authors are suggesting that similar protection against MARV may be achieved, but this needs to be investigated in the future. The administration of multiple viral vectors simultaneously has to be evaluated carefully. Viral vectors harbor the potential risk of unintended recombination events (e.g., with other viral vector vaccines or naturally occurring viruses) in the host, which may lead to hybrid species with unknown characteristics. The use of replication-defective vectors reduces this risk but may not eliminate it completely.
Varicella-zoster virus.
Varicella-zoster virus (VZV) is endemic worldwide, with infection rates as high as 90% before adolescence. The first vaccines against VZV were developed as early as 1984. Currently used vaccines (ATC codes J07BK01 and J07BK02; http://www.whocc.no/atc_ddd_index/?code = J07BK&showdescription=no) consisting of live attenuated VZV have been monitored for years and have demonstrated a high safety profile with only minor side effects in healthy people (39, 40). Besides this, the host range of VZV is restricted to humans, obstructing uncontrolled environmental spread. The safety and the opportunity to insert and maintain large DNA inserts, combined with the ability to induce strong cellular and humoral immunity, sparked interest in using it as a recombinant vaccine candidate for generating multivalent/multipathogen vaccines (40, 41).
Matsuura and colleagues reported a successful application of VZV as a vaccine vector by introducing the two major surface proteins of the mumps virus (MuV) (hemagglutinin-neuraminidase [HN] and fusion protein) into the VZV vOka strain using a bacterial artificial chromosome (BAC) system (42). The integral membrane protein HN is responsible for receptor binding on host cells and MuV neuraminidase and hemagglutinin activity, whereas the F protein's main activity lies in viral penetration and hemolysis. The recombinant vOka-HN-F exhibited reduced growth kinetics with atypical cytopathogenic effects and syncytium formation leading to cell detachment in MRC-5 cells (human fetal lung fibroblast cells). These effects were overcome by introducing a S195Y mutation (serine-to-tyrosine substitution) to prevent membrane fusion (vOka-HN-F-S195Y). Guinea pigs were immunized four times (subcutaneously) with 2 × 106 to 5 × 106 MRC-5 cells infected with either vaccine candidate in 2-week intervals. Both vaccine candidates induced neutralizing antibodies against the VZV vector, and the animals immunized with VZV vOka-HN-F mounted slightly higher titers. Further, neutralizing antibodies against MuV were induced (higher in the group vaccinated with vOka-HN-F-S195Y). Only slight differences in the immune responses of both recombinant constructs were observed (42). These data suggest that VZV is a strong candidate for future multivalent or multipathogen vaccine development. It may be advised to use this viral vector cautiously, as it is replication competent in the human host.
Human parainfluenza virus 3.
Human parainfluenza virus 3 (PIV3) is a member of a group of four parainfluenza viruses in the family Paramyxoviridae.
Parainfluenza viruses are nonsegmented negative-strand RNA viruses with a genome size of roughly 15,000 nucleotides, which can be easily manipulated or attenuated by reverse genetics (see also MV). The viral replication takes place in the cytoplasm of the host with no need for integration into the genome. Recombination events are rare in PIV, which contributes to the stability of inserted transgenes (43, 44).
As PIV3 is the second leading cause of hospitalization for viral respiratory tract disease, the development of a PIV3 vaccine is encouraged, particularly in combination with an already established vaccine to facilitate implementation into routine vaccine schedules.
The parenterally administered standard MV vaccine harbors the risk of being neutralized during the first months of life by serum antibodies passively transferred from mother to baby. A new MV vaccine candidate bypassing this would be a valuable asset in infant vaccination schedules. Therefore, a combined vaccination strategy, including PIV and MV, has been suggested. The viral backbone was generated from wild-type PIV3 through recovery from plasmid-borne cDNA using recombinant DNA technology (45). Further, attenuated versions of wild-type PIV3 have been developed (e.g., PIV3cp45) and have shown promising results as vaccine candidates (46–48). In this study, the hemagglutinin (HA) protein of the MV (Edmonston strain) was inserted into PIV3 or attenuated PIV3 between the N and P genes, P and M genes, or HN and L genes. These vaccine candidates induced antibodies against MV and PIV3 in golden Syrian hamsters, especially when inserted into the N-P or P-M junction. No significant difference in neutralizing antibodies was observed when using attenuated PIV3 compared to that when using wild-type virus. A challenge experiment with wild-type PIV3 that took place 28 days after intranasal immunization with 106 PFU of a vaccine candidate conferred significant protection to viral replication in the respiratory tract. Previous studies in monkeys suggest that PIV3 is able to replicate efficiently even in the presence of passively acquired PIV3 antibodies, leading to the conclusion that even with preexisting maternally MV antibodies the intranasally administered MV-PIV3-HA vaccine may be protective.
The authors report that their recombinant PIV3-MV vaccine candidate elicits, on average, five times more serum antibodies than the licensed live attenuated measles virus vaccine that is administered by intramuscular injection. It is also suggested to employ antigenic serotypes of PIV3 (e.g., PIV1 or PIV2) for prime boost vaccinations to reduce the risk of vector immunity and efficient transgene expression (49).
Coxsackievirus group B.
Coxsackieviruses are positive-sense single-stranded RNA (ssRNA) viruses. Six serotypes of group B coxsackievirus (CVB1 to CVB6) have been described, of which CVB3 has been particularly identified as a potential vaccine vector. All CVBs incorporate four capsid proteins and seven nonstructural proteins and include two proteases. Although the small genome has a somewhat limited capacity to stably integrate foreign genetic material, CVBs are interesting viral vector candidates because strong immune responses (cellular and humoral) are generated following an infection (50). Coxsackieviruses are known to cause gastrointestinal distress, myocarditis, or dilated cardiomyopathy in humans. Together with CVB, only human adenovirus type 2 (AdV2) has been regularly linked to heart disease (51–54). To date, no vaccines against either virus are commercially available to protect from human heart disease. Hofling and colleagues (55) investigated a chimeric CVB3 vaccine candidate expressing the antigenic L1 loop of AdV2 hexon protein (the L1 loop of AdV2 has produced promising results in a rabbit model before [56]) from a locus between the capsid protein P-1D and the protease P-2A in the CVB3 genome. The inserted sequence was expressed over 10 passages in HeLa cells; however, a virus species corresponding to the parental strain was detected in passages 8 and 10, indicating that the recombinant virus is unstable. Reduced virus titers in comparison to the parental CVB3 strain were observed, suggesting that the insertion leads to an attenuation of the viral vector. To investigate the potential of the CVB3-Ad2L1 vaccine candidate to generate a humoral immune response, murine immunogenicity studies were performed. BALB/c mice were injected (intraperitoneally) with 5 × 105 TCID50 of CVB3-Ad2L1 once, twice, or three times in 2-week intervals. The results showed that the CVB3-Ad2L1 vaccine candidate was able to induce anti-CVB3 and anti-Ad2 hexon L1 loop-neutralizing and -binding antibodies (titers increased with the number of booster injections). Interestingly, preexisting anti-CVB antibodies boosted the immune response further and led to even higher levels of anti-Ad2 antibodies in mice after receiving three injections of the multipathogen vaccine candidate. An evaluation of the CVB-Ad2L1 immune response over time to determine the duration of protection will be necessary. It will also be of interest to determine whether cross-protection against other CVB serotypes can be achieved with this candidate vaccine (55). Although this recombinant virus induced both neutralizing and binding antibodies against the insert and vector in a mouse model, it is questionable if its use would be feasible in human applications due to the issues encountered with the insert instability. Moreover, even though the mouse studies showed no virus inhibition by preexisting anti-CVB3 antibodies, it needs to be evaluated if this proves true for other coxsackievirus serotypes.
Poxvirus—modified vaccinia Ankara.
The family Poxviridae is divided into two subfamilies, Chordopoxvirinae and Entomopoxvirinae. Within the subfamily of Chordopoxvirinae, the genus Orthopoxvirus, which includes variola virus, the causative agent for smallpox, is interesting for human vaccine research. The most widely used candidate for new vaccine design is the vaccinia virus (VacV). This virus exhibits unique features that qualify it for use as an effective expression system and ideal recombinant vector. The large genome size of VacV (∼190 kbp) allows the insertion of large amounts of foreign DNA (∼25 to 30 kb) by homologous recombination, direct cloning, or bacterial artificial chromosome technology. This characteristic enables the design of vaccines against multiple pathogens within a single expression system. Vaccinia virus can also be applied via different routes (injection, oral) and induce long-lasting immunity. Many, so far unsuccessful, efforts to develop vaccines for complex diseases, such as tuberculosis or malaria, that require a presentation of more than one antigen can potentially be overcome with a poxviral antigen presentation platform. The life cycle of VacV is fully accomplished in the cellular cytoplasm and does not require the integration of viral genetic material into the genome of the host. These attributes and the absence of a latent stage in the viral infection cycle are further advantages for vaccine design. The extensive investigation of VacV as a promising candidate for vaccine design has led to a number of modified VacV strains with higher safety profiles, more efficient expression systems, and higher immunogenicity in the host. Among other poxviruses (e.g., fowlpox, canarypox, or New York vaccinia virus), a most promising candidate for vaccine design is the attenuated vaccinia strain modified vaccinia Ankara (MVA). Relative to its parental strain (chorioallantoic vaccinia Ankara), MVA has lost 15% (∼30,000 bp) of its genetic information at six major deletion sites during the attenuation process (57–60). These deletion sites have been shown to function as insertion sites for foreign genes into the MVA genome. Foreign genes can also be inserted upstream from endogenous poxviral promoters (in situ).
Fear of the next influenza pandemic has driven efforts toward the development of novel vaccines. As highly pathogenic avian influenza type H5N1 and heterologous influenza strains seem to be on the rise, Prabakaran and colleagues (61) developed a universal H5N1 vaccine candidate with broad coverage for pandemic preparedness. The HA genes of the A/Vietnam/1203/04, A/Indonesia/CDC669/06, and A/Anhui/01/05 (H5N1) strains were selected based on the neutralizing epitopes in HA covering most variants in the H5N1 clades. All three were inserted into deletion site 3 of MVAtor (Emergent BioSolutions, Gaithersburg, MD, USA), each under the control of a separate promoter (PsynI, PsynII, H5), resulting in the trivalent MVAtor-tri-HA. As a control, rMVAtor carrying only the A/Vietnam/1203/04 gene was constructed. Mice (BALB/c) immunized intramuscularly with a two-step protocol (day 0 and day 28) of 100 μl of 8 × 107 TCID50 of MVAtor-tri-HA exhibited significant hemagglutination inhibition titers for the homologous viruses and the heterologous H5N1 clades, whereas the monovalent candidate induced only poor hemagglutination inhibition titers. A challenge experiment (ten 50% minimal lethal doses [MLD50] intranasally) with a homologous clade 1 (RG-A/Vietnam/1203/04) and heterologous clade 7 (RG-A/chicken/Shanxi/2/06) H5N1 virus showed that the MVtor-tri-HA vaccine candidate conferred complete protection from weight loss and death in the immunized mice. In contrast, a previous experiment had shown that the monovalent counterpart conferred only 66% protection against the homologous H5N1 strain. Cross-clade immunity against 20 heterologous H5N1 clades was confirmed after a serological surveillance study in guinea pigs that were vaccinated with the trivalent vaccine candidate. The authors concluded that the robust and broadly neutralizing activity of their MVAtor-tri-HA vaccine candidate may also protect from yet unknown H5N1 strains. The poxviral vector seems to be an excellent delivery vehicle, as it has been proven safe and efficient in many monovalent recombinant vaccine candidates (61).
PROSPECTS, ADVANTAGES, AND CHALLENGES OF MULTIVALENT AND MULTIPATHOGEN VIRAL VECTOR VACCINES
With the number of vaccines growing and prevention (rather than treatment) being the most effective means of controlling virus infection, combination vaccines are becoming more important. Protection against several diseases with fewer injections while maintaining the efficacy and safety of single-component vaccines helps not only to reduce costs for health services and patients but also to simplify vaccine schedules (62). Despite all progress, some infectious diseases still claim millions of lives every year. For many of them, including malaria, leishmania, HIV, or tuberculosis, vaccine development has produced only suboptimal protection thus far. Viral vectors can potentially overcome this with their unique way of antigen presentation and capacity to express various transgenes at once. In countries with ineffective health services, combined vaccines would be easier to administer than their individual counterparts. Immunization coverage could be achieved more easily, with fewer visits to medical centers, resulting in lower mortality, lower treatment costs, and lower levels of residual morbidity postinfection. Among new vaccine products, polyvalent and multipathogen viral vector vaccines hold great promise. Advantages of these recombinant vaccines include their ability to deliver multiple immunogens into the cells of the vaccinee, where they guarantee efficient expression. Conveniently, many of the viral vector shuttles present proteins to the immune system in the same way as that which occurs in a natural infection cycle and therefore ensure a potent induction of cellular and humoral immune responses (63). This route of antigen presentation holds the promise of long-lasting protection without numerous booster vaccinations. Many of the currently used vectors, such as modified vaccinia Ankara and measles virus vaccine, have been in use for years. They have accumulated significant safety and efficacy data through clinical and laboratory research through their use in prior vaccination applications. Progress in genetic engineering, recombinant DNA technology, and improved expression systems (promoter, terminator, enhancer, etc.) have advanced the field of recombinant vaccine design.
In using multipathogen or multivalent vaccines, special attention must be paid to the interaction of the various components with each other; potential interactions include antagonistic or synergistic effects or antigenic competition and/or epitope suppression, resulting in an inappropriate immune response (64). A common perception about vaccines containing more than one antigen is the overburdening of the immune system. Studies have shown that the immune system is responsive to more than 10 million antigens. With vaccines containing only a few specific antigens, an overload of a functional immune system is clearly extremely unlikely (65). Another challenge that needs to be addressed is the manufacturing and testing of new formulations. Each component of the vaccine must be assessed individually and in combination with standardized tests (stability, sterility, potency, efficacy, etc.) to ensure the consistency of the product (66). In addition, as more complex vaccines emerge, regulatory agencies will need to introduce new policies that provide guidelines for researchers, manufacturers, and practitioners regarding testing, licensing, documentation, information, and marketing of vaccines.
The enormous potential of viral vector vaccines drives the continuous development of novel expression vectors. Rhabdovirus- or influenza virus-based platforms are some of the latest to be suggested with the potential to express various antigens (67–70). The insertion of various transgenes into a vector is of course likely to lead to an impairment of virus replication. Revisiting traditional methods of viral gene expression, for example, by using conditional expression systems, such as Tet-on/Tet-off, may open the door for a new generation of improved viral vectors (71, 72).
Viral vectors have also been used in applications other than immunization against infectious disease, for example, as prophylactic or therapeutic cancer vaccines. Most recently, a study by Qiu and colleagues described a cytomegalovirus vector expressing modified tumor antigens. The vaccine candidate elicited tumor-specific T-cell responses, protecting mice from melanoma (73). Another promising study used a live attenuated poliovirus type 1 to vaccinate against glioblastoma multiforme (74). A valid concern in the use of viral vectors as vaccines is the possibility of the vaccinee developing vector immunity, resulting in reduced immunogenicity of the vaccine. Using a combination of different vectors for prime and booster vaccinations has been found to overcome this issue. Several examples in the literature describe the latter strategy, e.g., a recombinant adenovirus followed by an MVA booster regimen or a DNA prime followed by an adenoviral booster (75–78).
As discussed by Kreijtz and colleagues (79) in their excellent review on poxviral vectors, once regulatory challenges have been overcome, the implementation of the first human recombinant vaccine candidate into an existing vaccine schedule will lead to a major improvement in public health—conceivably sooner rather than later with the fast tracking of recombinant vaccines against Ebola virus disease (80–82). This would pave the way for taking multipathogen and multivalent vaccine candidates from the bench into clinical settings.
ACKNOWLEDGMENTS
We thank Paul Klapper for help with the manuscript.
We declare no conflicts of interest.
All authors contributed equally to this minireview.
REFERENCES
- 1.Moszynski P. 2013. Polio is re-emerging in areas previously considered polio free. BMJ 347:f6061. doi: 10.1136/bmj.f6061. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. 2007. Understanding emerging and re-emerging infectious diseases. Biological sciences curriculum study. NIH Curriculum Supplement Series. National Institutes of Health, Bethesda, MD. [Google Scholar]
- 3.Mire CE, Geisbert JB, Versteeg KM, Mamaeva N, Agans KN, Geisbert TW, Connor JH. 2015. A single-vector, single-injection trivalent filovirus vaccine: proof of concept study in outbred guinea pigs. J Infect Dis 212:S384–S388. doi: 10.1093/infdis/jiv126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas AD, Williams AR, Illingworth JJ, Kamuyu G, Biswas S, Goodman AL, Wyllie DH, Crosnier C, Miura K, Wright GJ, Long CA, Osier FH, Marsh K, Turner AV, Hill AVS, Draper SJ. 2011. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2:601. doi: 10.1038/ncomms1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satti I, Meyer J, Harris SA, Thomas Z-RM, Griffiths K, Antrobus RD, Rowland R, Ramon RL, Smith M, Sheehan S, Bettinson H, McShane H. 2014. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: a phase 1, double-blind, randomised controlled trial. Lancet Infect Dis 14:939–946. doi: 10.1016/S1473-3099(14)70845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeyanathan M, Thanthrige-Don N, Afkhami S, Lai R, Damjanovic D, Zganiacz A, Feng X, Yao XD, Rosenthal KL, Medina MF, Gauldie J, Ertl HC, Xing Z. 2015. Novel chimpanzee adenovirus-vectored respiratory mucosal tuberculosis vaccine: overcoming local anti-human adenovirus immunity for potent TB protection. Mucosal Immun 8:1373–1387. doi: 10.1038/mi.2015.29. [DOI] [PubMed] [Google Scholar]
- 7.Lillie PJ, Berthoud TK, Powell TJ, Lambe T, Mullarkey C, Spencer AJ, Hamill M, Peng Y, Blais ME, Duncan CJ, Sheehy SH, Havelock T, Faust SN, Williams RL, Gilbert A, Oxford J, Dong T, Hill AV, Gilbert SC. 2012. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis 55:19–25. doi: 10.1093/cid/cis327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackowiak M, Maki J, Motes-Kreimeyer L, Harbin T, Van Kampen K. 1999. Vaccination of wildlife against rabies: successful use of a vectored vaccine obtained by recombinant technology. Adv Vet Med 41:571–583. doi: 10.1016/S0065-3519(99)80043-3. [DOI] [PubMed] [Google Scholar]
- 9.Pastoret P-P, Brochier B. 1996. The development and use of a vaccinia-rabies recombinant oral vaccine for the control of wildlife rabies; a link between Jenner and Pasteur. Epidemiol Infect 116:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Santis O, Audran R, Pothin E, Warpelin-Decrausaz L, Vallotton L, Wuerzner G, Cochet C, Estoppey D, Steiner-Monard V, Lonchampt S, Thierry A-C, Mayor C, Bailer RT, Mbaya OT, Zhou Y, Ploquin A, Sullivan NJ, Graham BS, Roman F, De Ryck I, Ballou WR, Kieny MP, Moorthy V, Spertini F, Genton B. 2016. Safety and immunogenicity of a chimpanzee adenovirus-vectored Ebola vaccine in healthy adults: a randomised, double-blind, placebo-controlled, dose-finding, phase 1/2a study. Lancet Infect Dis 16:311–320. doi: 10.1016/S1473-3099(15)00486-7. [DOI] [PubMed] [Google Scholar]
- 11.Ewer K, Rampling T, Venkatraman N, Bowyer G, Wright D, Lambe T, Imoukhuede EB, Payne R, Fehling SK, Strecker T, Biedenkopf N, Krähling V, Tully CM, Edwards NJ, Bentley EM, Samuel D, Labbé G, Jin J, Gibani M, Minhinnick A, Wilkie M, Poulton I, Lella N, Roberts R, Hartnell F, Bliss C, Sierra-Davidson K, Powlson J, Berrie E, Tedder R, Roman F, De Ryck I, Nicosia A, Sullivan NJ, Stanley DA, Mbaya OT, Ledgerwood JE, Schwartz RM, Siani L, Colloca S, Folgori A, Di Marco S, Cortese R, Wright E, Becker S, Graham BS, Koup RA, Levine MM, Volkmann A, et al. . 2016. A monovalent chimpanzee adenovirus Ebola vaccine boosted with MVA. N Engl J Med 374:1635–1646. doi: 10.1056/NEJMoa1411627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley DA, Honko AN, Asiedu C, Trefry JC, Lau-Kilby AW, Johnson JC, Hensley L, Ammendola V, Abbate A, Grazioli F, Foulds KE, Cheng C, Wang L, Donaldson MM, Colloca S, Folgori A, Roederer M, Nabel GJ, Mascola J, Nicosia A, Cortese R, Koup RA, Sullivan NJ. 2014. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 20:1126–1129. [DOI] [PubMed] [Google Scholar]
- 13.Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, Traore A, Coulibaly F, Kodio M, Onwuchekwa U, Sztein MB, Wahid R, Campbell JD, Kieny M-P, Moorthy V, Imoukhuede EB, Rampling T, Roman F, De Ryck I, Bellamy AR, Dally L, Mbaya OT, Ploquin A, Zhou Y, Stanley DA, Bailer R, Koup RA, Roederer M, Ledgerwood J, Hill AVS, Ballou WR, Sullivan N, Graham B, Levine MM. 2016. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 16:31–42. doi: 10.1016/S1473-3099(15)00362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abaitua F, Rodríguez JR, Garzón A, Rodríguez D, Esteban M. 2006. Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma. Virus Res 116:11–20. doi: 10.1016/j.virusres.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AVS. 2011. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol 23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y, Chang J. 2013. Viral vectors for vaccine applications. Clin Exp Vaccine Res 2:97–105. doi: 10.7774/cevr.2013.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangy F, Naim HY. 2005. Live attenuated measles vaccine as a potential multivalent pediatric vaccination vector. Viral Immunol 18:317–326. doi: 10.1089/vim.2005.18.317. [DOI] [PubMed] [Google Scholar]
- 18.Zuniga A, Wang Z, Liniger M, Hangartner L, Pavlovic J, Wild P, Viret JF, Glueck R, Martin A, Naim HY. 2007. Attenuated measles virus as a vaccine vector. Vaccine 25:2974–2983. doi: 10.1016/j.vaccine.2007.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loessner H, Schwantes A, Hamdorf M, Komor U, Leschner S, Weiss S. 2012. Employing live microbes for vaccine delivery, p 87–124. In von Gabain A, Klade C (ed), Development of novel vaccines. Skills, knowledge and translational technologies; Springer, New York, NY. [Google Scholar]
- 20.Cantarella G, Liniger M, Zuniga A, Schiller JT, Billeter M, Naim HY, Glueck R. 2009. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine 27:3385–3390. doi: 10.1016/j.vaccine.2009.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A 89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A 92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szarewski A. 2010. HPV vaccine: Cervarix. Expert Opin Biol Ther 10:477–487. doi: 10.1517/14712591003601944. [DOI] [PubMed] [Google Scholar]
- 24.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, Christiansen G, Billeter MA. 1995. Rescue of measles viruses from cloned DNA. EMBO J 14:5773–5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver SC, Osorio JE, Livengood JA, Chen R, Stinchcomb DT. 2012. Chikungunya virus and prospects for a vaccine. Expert Rev Vaccines 11:1087–1101. doi: 10.1586/erv.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandler S, Ruffié C, Combredet C, Brault J-B, Najburg V, Prevost M-C, Habel A, Tauber E, Desprès P, Tangy F. 2013. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine 31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 27.Ramsauer K, Schwameis M, Firbas C, Müllner M, Putnak RJ, Thomas SJ, Desprès P, Tauber E, Jilma B, Tangy F. 2015. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis 15:519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 28.Bangari DS, Mittal SK. 2006. Development of nonhuman adenoviruses as vaccine vectors. Vaccine 24:849–862. doi: 10.1016/j.vaccine.2005.08.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wold WSM, Toth K. 2013. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr Gene Ther 13:421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R-F, Lee C-Y. 2014. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int Rev Immunol 33:45–53. doi: 10.3109/08830185.2013.823420. [DOI] [PubMed] [Google Scholar]
- 31.Johnson JA, Barouch DH, Baden LR. 2013. Nonreplicating vectors in HIV vaccines. Curr Opin HIV AIDS 8:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Raja NU, Trubey CM, Juompan LY, Luo M, Woraratanadharm J, Deitz SB, Yu H, Swain BM, Moore KM, Pratt WD, Hart MK, Dong JY. 2006. Development of a cAdVax-based bivalent Ebola virus vaccine that induces immune responses against both the Sudan and Zaire species of Ebola virus. J Virol 80:2738–2746. doi: 10.1128/JVI.80.6.2738-2746.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubinchik S, Norris JS, Dong J-Y. 2002. Construction, purification and characterization of adenovirus vectors expressing apoptosis-inducing transgenes. Methods Enzymol 346:529–547. doi: 10.1016/S0076-6879(02)46075-2. [DOI] [PubMed] [Google Scholar]
- 34.Rubinchik S, Wang D, Yu H, Fan F, Luo M, Norris JS, Dong JY. 2001. A complex adenovirus vector that delivers FASL-GFP with combined prostate-specific and tetracycline-regulated expression. Mol Ther 4:416–426. doi: 10.1006/mthe.2001.0478. [DOI] [PubMed] [Google Scholar]
- 35.Rubinchik S, Woraratanadharm J, Schepp J, Dong J. 2002. Improving the transcriptional regulation of genes delivered by adenovirus vectors, p 167–199. In Machida CA. (ed), Viral vectors for gene therapy. Humana Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 36.Swenson DL, Wang D, Luo M, Warfield KL, Woraratanadharm J, Holman DH, Dong JY, Pratt WD. 2008. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin Vaccine Immunol 15:460–467. doi: 10.1128/CVI.00431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SM, Feldmann H, Ströher U, Geisbert JB, Fernando L, Grolla A, Klenk H-D, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 38.Pratt WD, Wang D, Nichols DK, Luo M, Woraratanadharm J, Dye JM, Holman DH, Dong JY. 2010. Protection of nonhuman primates against two species of Ebola virus infection with a single complex adenovirus vector. Clin Vaccine Immunol 17:572–581. doi: 10.1128/CVI.00467-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrillo-Santisteve P, Lopalco PL. 2014. Varicella vaccination: a laboured take-off. Clin Microbiol Infect 20(Suppl):S86–S91. [DOI] [PubMed] [Google Scholar]
- 40.Gray WL. 2013. Recombinant varicella-zoster virus vaccines as platforms for expression of foreign antigens. Adv Virol 2013:219439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perciani CT, Willer DO, Haq K, Iwajomo OH, Chan JK, Pilon R, Antony JM, Sandstrom P, Macdonald KS. 2014. Mechanisms of protection observed with varicella zoster virus as a vaccine vector in the SIV macaque model. AIDS Res Hum Retroviruses 30(Suppl):A44–A45. [Google Scholar]
- 42.Matsuura M, Somboonthum P, Murakami K, Ota M, Shoji M, Kawabata K, Mizuguchi H, Gomi Y, Yamanishi K, Mori Y. 2013. Novel polyvalent live vaccine against varicella-zoster and mumps virus infections. Microbiol Immunol 57:704–714. doi: 10.1111/1348-0421.12087. [DOI] [PubMed] [Google Scholar]
- 43.Durbin AP, Karron RA. 2003. Progress in the development of respiratory syncytial virus and parainfluenza virus vaccines. Clin Infect Dis 37:1668–1677. doi: 10.1086/379775. [DOI] [PubMed] [Google Scholar]
- 44.Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. 2006. Nonsegmented negative-strand viruses as vaccine vectors. J Virol 80:10293–10306. doi: 10.1128/JVI.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durbin AP, Hall SL, Siew JW, Whitehead SS, Collins PL, Murphy BR. 1997. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology 235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- 46.Durbin AP, Cho CJ, Elkins WR, Wyatt LS, Moss B, Murphy BR. 1999. Comparison of the immunogenicity and efficacy of a replication-defective vaccinia virus expressing antigens of human parainfluenza virus type 3 (HPIV3) with those of a live attenuated HPIV3 vaccine candidate in rhesus monkeys passively immunized with PIV3. J Infect Dis 179:1345–1351. doi: 10.1086/314769. [DOI] [PubMed] [Google Scholar]
- 47.Durbin AP, Wyatt LS, Siew J, Moss B, Murphy BR. 1998. The immunogenicity and efficacy of intranasally or parenterally administered replication-deficient vaccinia-parainfluenza virus type 3 recombinants in rhesus monkeys. Vaccine 16:1324–1330. doi: 10.1016/S0264-410X(98)00010-3. [DOI] [PubMed] [Google Scholar]
- 48.Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, Newman F, Cannon JC, Thompson J, Tsai T, Paschalis M, Wu S-L, Mitcho Y, Hackell J, Murphy BR, Tatem JM. 2003. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr Infect Dis J 22:394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 49.Durbin AP, Skiadopoulos MH, Auliffe JMMC, Riggs JM, Surman SR, Collins PL, Murphy BR. 2000. Human parainfluenza virus type 3 (PIV3) expressing the hemagglutinin protein of measles virus provides a potential method for immunization against measles virus and PIV3 in early infancy. J Virol 74:6821–6831. doi: 10.1128/JVI.74.15.6821-6831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D-S, Nam J-H. 2011. Application of attenuated coxsackievirus B3 as a viral vector system for vaccines and gene therapy. Hum Vaccin 7:410–416. [DOI] [PubMed] [Google Scholar]
- 51.Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang YH, Bodurtha J, Gelb B, Ni J. 1994. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation 90:330–339. [DOI] [PubMed] [Google Scholar]
- 52.Tracy S, Chapman NM, Tu Z. 1992. Coxsackievirus B3 from an infectious cDNA copy of the genome is cardiovirulent in mice. Arch Virol 122:399–409. doi: 10.1007/BF01317202. [DOI] [PubMed] [Google Scholar]
- 53.Chapman NM, Tu Z, Tracy S, Gauntt CJ. 1994. An infectious cDNA copy of the genome of a non-cardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent coxsackieviruses. Arch Virol 135:115–130. doi: 10.1007/BF01309769. [DOI] [PubMed] [Google Scholar]
- 54.Lee C, Maull E, Chapman N, Tracy S, Wood J, Gauntt C. 1997. Generation of an infectious cDNA of a highly cardiovirulent coxsackievirus B3(CVB3m) and comparison to other infectious CVB3 cDNAs. Virus Res 50:225–235. doi: 10.1016/S0168-1702(97)00059-2. [DOI] [PubMed] [Google Scholar]
- 55.Hofling K, Tracy S, Chapman N, Kim K-S, Smith Leser J. 2000. Expression of an antigenic adenovirus epitope in a group B coxsackievirus. J Virol 74:4570–4578. doi: 10.1128/JVI.74.10.4570-4578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toogood CI, Crompton J, Hay RT. 1992. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol 73:1429–1435. [DOI] [PubMed] [Google Scholar]
- 57.Altenburger W, Süter C-P, Altenburger J. 1989. Partial deletion of the human host range gene in the attenuated vaccinia virus MVA. Arch Virol 105:15–27. [DOI] [PubMed] [Google Scholar]
- 58.Meyer H, Sutter G, Mayr A. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72:1031–1038. [DOI] [PubMed] [Google Scholar]
- 59.Hochstein-Mintzel V, Huber HC, Stickl H. 1972. Virulenz und immunogenitäteines modifizierten vaccinia-virus (Stamm MVA). immunitätsforsch. Exp Klin Immunol 144:140–145. [PubMed] [Google Scholar]
- 60.Mayr A, Hochstein-Mintzel V, Stickl H. 1975. Abstammung, Eigenschaften und Verwendung des attenuierten vaccinia-stammes MVA. Infection 3:6–14. doi: 10.1007/BF01641272. [DOI] [Google Scholar]
- 61.Prabakaran M, Leyrer S, He F, Auer S, Kumar SR, Kindsmueller K, Mytle N, Schneider J, Lockhart S, Kwang J. 2014. Progress toward a universal H5N1 vaccine: a recombinant modified vaccinia virus Ankara-expressing trivalent hemagglutinin vaccine. PLoS One 9:e107316. doi: 10.1371/journal.pone.0107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halsey NA. 2001. Safety of combination vaccines: perception versus reality. Pediatr Infect Dis J 20:S40–S44. doi: 10.1097/00006454-200111001-00007. [DOI] [PubMed] [Google Scholar]
- 63.Souza APD, Haut L, Reyes-Sandoval A, Pinto AR. 2005. Recombinant viruses as vaccines against viral diseases. Braz J Med Biol Res 38:509–522. doi: 10.1590/S0100-879X2005000400004. [DOI] [PubMed] [Google Scholar]
- 64.Sanyal G, Shi L. 2009. A review of multiple approaches towards an improved hepatitis B vaccine. Expert Opin Ther Pat 19:59–72. doi: 10.1517/13543770802587226. [DOI] [PubMed] [Google Scholar]
- 65.Pickering PJ, Siegelman MH. 1999. Lymphoid tissues and organs, p 479–532. In Paul WE. (ed), Fundamental immunology, 4th ed Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 66.Falk LA, Arciniega J, McVittie L. 2001. Manufacturing issues with combining different antigens: a regulatory perspective. Clin Infect Dis 33(Suppl):S351–S355. doi: 10.1086/322579. [DOI] [PubMed] [Google Scholar]
- 67.Yang P, Li T, Liu N, Gu H, Han L, Zhang P, Li Z, Wang Z, Zhang S, Wang X. 2015. Recombinant influenza virus carrying human adenovirus epitopes elicits protective immunity in mice. Antiviral Res 121:145–151. doi: 10.1016/j.antiviral.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 68.Kurup D, Wirblich C, Feldmann H, Marzi A, Schnell MJ. 2015. Rhabdoviral-based vaccine platforms against henipaviruses. J Virol 89:144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papaneri A, Bernbaum J, Blaney JE, Jahrling PB, Schnell M, Johnson RF. 2015. Controlled viral glycoprotein expression as a safety feature in a bivalent rabies-Ebola vaccine. Virus Res 197:54–58. doi: 10.1016/j.virusres.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee Y-N, Hwang HS, Kim M-C, Lee Y-T, Lee JS, Moore ML, Kang S-M. 2015. Recombinant influenza virus expressing a fusion protein neutralizing epitope of respiratory syncytial virus (RSV) confers protection without vaccine-enhanced RSV disease. Antiviral Res 115:1–8. doi: 10.1016/j.antiviral.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H, Wang D, Xia R, Mao Q, Xia H. 2015. A novel adenoviral vector carrying an all-in-one Tet-On system with an autoregulatory loop for tight, inducible transgene expression. BMC Biotechnol 15:4. doi: 10.1186/s12896-015-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berenjian S, Akusjärvi G. 2006. Binary AdEasy vector systems designed for Tet-ON or Tet-OFF regulated control of transgene expression. Virus Res 115:16–23. doi: 10.1016/j.virusres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Qiu Z, Huang H, Grenier JM, Perez OA, Smilowitz HM, Adler B, Khanna KM. 2015. Cytomegalovirus-based vaccine expressing a modified tumor antigen induces potent tumor-specific CD8+ T-cell response and protects mice from melanoma. Cancer Immunol Res 3:536–546. doi: 10.1158/2326-6066.CIR-14-0044. [DOI] [PubMed] [Google Scholar]
- 74.Goetz C, Gromeier M. 2010. Preparing an oncolytic poliovirus recombinant for clinical application against glioblastoma multiforme. Cytokine Growth Factor Rev 21:197–203. doi: 10.1016/j.cytogfr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AVS. 2010. Prime-boost immunization with adenoviral and modified vaccinia virus ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect Immun 78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ewer KJ, Hara GAO, Duncan CJA, Collins KA, Sheehy SH, Reyes-Sandoval A, Goodman AL, Edwards NJ, Elias SC, Halstead FD, Longley RJ, Rowland R, Poulton ID, Draper SJ, Blagborough AM, Berrie E, Moyle S, Williams N, Siani L, Folgori A, Colloca S, Sinden RE, Lawrie AM, Cortese R, Gilbert SC, Nicosia A, Hill AVS. 2013. Protective CD8+ T-cell immunity to human malaria induced by chimpanzee adenovirus-MVA immunisation. Nat Commun 4:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, de Barra E, Havelock T, Bowyer G, Poulton ID, de Cassan S, Longley R, Illingworth JJ, Douglas AD, Mange PB, Collins KA, Roberts R, Gerry S, Berrie E, Moyle S, Colloca S, Cortese R, Sinden RE, Gilbert SC, Bejon P, Lawrie AM, Nicosia A, Faust SN, Hill AVS. 2015. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis 211:1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, Ganeshan H, Belmonte M, Farooq F, Abot E, Banania JG, Huang J, Newcomer R, Rein L, Litilit D, Richie NO, Wood C, Murphy J, Sauerwein R, Hermsen CC, McCoy AJ, Kamau E, Cummings J, Komisar J, Sutamihardja A, Shi M, Epstein JE, Maiolatesi S, Tosh D, King R, Carucci D, Dutta S, Soisson L, Diggs C. 2013. DNA prime/adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One 8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreijtz JHCM, Gilbert SC, Sutter G. 2013. Poxvirus vectors. Vaccine 31:4217–4219. doi: 10.1016/j.vaccine.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 80.Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, Eickmann M, Finckh A, Goncalves AR, Hooper JW, Kaya G, Krähling V, Kwilas S, Lemaître B, Matthey A, Silvera P, Becker S, Fast PE, Moorthy V, Kieny MP, Kaiser L, Siegrist CA, VSV-Ebola Consortium . 2015. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 15:1156–1166. doi: 10.1016/S1473-3099(15)00154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. 2015. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 349:739–742. doi: 10.1126/science.aab3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, Enwere G, Grais R, Gunther S, Hossmann S, Kondé MK, Kone S, Kuisma E, Levine MM, Mandal S, Norheim G, Riveros X, Soumah A, Trelle S, Vicari AS, Watson CH, Kéïta S, Kieny MP, Røttingen J-A. 2015. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, Hevey M, Juompan LY, Trubey CM, Raja NU, Deitz SB, Woraratanadharm J, Luo M, Yu H, Swain BM, Moore KM, Dong JY. 2006. Complex adenovirus-vectored vaccine protects guinea pigs from three strains of Marburg virus challenges. Virology 353:324–332. doi: 10.1016/j.virol.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 84.Singh M, Cattaneo R, Billeter MA. 1999. A recombinant measles virus expressing hepatitis B virus surface antigen induces humoral immune responses in genetically modified mice. J Virol 73:4823–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P, Brahic M, Tangy F. 2004. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol 78:146–157. doi: 10.1128/JVI.78.1.146-157.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stebbings R, Février M, Li B, Lorin C, Koutsoukos M, Mee E, Rose N, Hall J, Page M, Almond N, Voss G, Tangy F. 2012. Immunogenicity of a recombinant measles-HIV-1 clade B candidate vaccine. PLoS One 7:e50397. doi: 10.1371/journal.pone.0050397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liniger M, Zuniga A, Morin TNA, Combardiere B, Marty R, Wiegand M, Ilter O, Knuchel M, Naim HY. 2009. Recombinant measles viruses expressing single or multiple antigens of human immunodeficiency virus (HIV-1) induce cellular and humoral immune responses. Vaccine 27:3299–3305. doi: 10.1016/j.vaccine.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stebbings R, Li B, Lorin C, Koutsoukos M, Février M, Mee ET, Page M, Almond N, Tangy F, Voss G. 2013. Immunogenicity of a recombinant measles HIV-1 subtype C vaccine. Vaccine 31:6079–6086. doi: 10.1016/j.vaccine.2013.09.072. [DOI] [PubMed] [Google Scholar]
- 89.Desprès P, Combredet C, Frenkiel M-P, Lorin C, Brahic M, Tangy F. 2005. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis 191:207–214. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 90.Escriou N, Callendret B, Lorin V, Combredet C, Marianneau P, Février M, Tangy F. 2014. Protection from SARS coronavirus conferred by live measles vaccine expressing the spike glycoprotein. Virology 452–453:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liniger M, Zuniga A, Tamin A, Azzouz-Morin TN, Knuchel M, Marty RR, Wiegand M, Weibel S, Kelvin D, Rota PA, Naim HY. 2008. Induction of neutralising antibodies and cellular immune responses against SARS coronavirus by recombinant measles viruses. Vaccine 26:2164–2174. doi: 10.1016/j.vaccine.2008.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brandler S, Ruffie C, Najburg V, Frenkiel M-P, Bedouelle H, Desprès P, Tangy F. 2010. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine 28:6730–6739. doi: 10.1016/j.vaccine.2010.07.073. [DOI] [PubMed] [Google Scholar]