ABSTRACT
Adenylate cyclase toxin (ACT) is an essential virulence factor of Bordetella pertussis, and antibodies to ACT protect against B. pertussis infection in mice. The toxin is therefore a strong candidate antigen for addition to future acellular pertussis vaccines. In order to characterize the functionality of the immunologic response to ACT after infection, we developed an assay for testing the ability of serum samples from subjects infected with B. pertussis to neutralize ACT-induced cytotoxicity in J774 macrophage cells. Baboons develop neutralizing anti-ACT antibodies following infection with B. pertussis, and all sera from baboons with positive anti-ACT IgG enzyme-linked immunosorbent assay (ELISA) results neutralized ACT cytotoxicity. The toxin neutralization assay (TNA) was positive in some baboon sera in which ELISA remained negative. Of serum samples obtained from humans diagnosed with pertussis by PCR, anti-ACT IgG ELISA was positive in 72%, and TNA was positive in 83%. All samples positive for anti-ACT IgG ELISA were positive by TNA, and none of the samples from humans without pertussis neutralized toxin activity. These findings indicate that antibodies to ACT generated following infection with B. pertussis consistently neutralize toxin-induced cytotoxicity and that TNA can be used to improve understanding of the immunologic response to ACT after infection or vaccination.
KEYWORDS: Bordetella pertussis, nonhuman primate, adenylate cyclase, assay development, bacterial toxin, neutralization
INTRODUCTION
In 1976, only 1,010 cases of pertussis were reported to the CDC (1). Since that time, the number of cases has increased, reaching a peak of 48,277 reported cases in 2012 in the United States, despite a high rate of vaccination (2). The reemergence of pertussis has been attributed to a combination of improved detection, vaccine refusal, and failure of the current acellular pertussis vaccine (aP) (3). Formulations of aP licensed in the United States contain between three and five Bordetella pertussis antigens (pertussis toxin, filamentous hemagglutinin, pertactin, fimbriae 2, and fimbriae 3), and the addition of antigens to aP has been suggested as a possible remedy to the limited vaccine efficacy and duration of protection (4).
Adenylate cyclase toxin (ACT) is a strong candidate for addition to future acellular vaccines (5) because it is a protective antigen and essential virulence factor (6). ACT is critical for the infection of adult mice and for causing death in infant mice infected with B. pertussis (7, 8). Passive transfer of serum from mice infected with B. pertussis to naive mice protects from infection with B. pertussis (9). In addition, the duration of B. pertussis infection has been found to be shorter in mice vaccinated with aP containing ACT than in those vaccinated with aP alone (10–12). While infection of humans by B. pertussis results in the generation of anti-ACT antibodies (13, 14), the ability of these antibodies to neutralize ACT function is incompletely characterized (15, 16), and the role of the antibodies in protection is not known.
ACT is a single polypeptide consisting of a catalytic domain that converts ATP to cyclic AMP (cAMP) and a cell-binding domain that associates with CD11b/CD18, the β2-integrin present on phagocytic leukocytes. After binding, ACT translocates its catalytic domain directly across the plasma membrane into the cytoplasm and generates supraphysiologic levels of intracellular cAMP, resulting in cell dysfunction and, under some conditions, death (17–20). For example, 30 ng/ml ACT kills 30% of J774 cells, a macrophage-like cell line, in 2 h.
We have taken advantage of the potent and rapid cytotoxic effects of ACT, developing an assay for measuring serum neutralization of cytotoxicity. With this easy-to-perform, sensitive, and specific assay, we have found that the anti-ACT antibody response to infection is neutralizing. The assay will be a valuable method for evaluating the immunologic response to B. pertussis infection and vaccines in future studies.
RESULTS
The nonhuman primate model of pertussis allows for study at specific intervals postimmunization and frequent sampling of serum after challenge to characterize the host antibody response. Following primary infection of unvaccinated baboons with B. pertussis, serum anti-ACT IgG, measured by enzyme-linked immunosorbent assay (ELISA), was detected in each subject by day 24 to 33 (Fig. 1A). While previously infected baboons were negative for anti-ACT IgG 30 to 60 days after their primary infection, IgG response to ACT was present on days 13 to 21 following secondary infection. Of the samples from previously infected baboons, those from subject b showed a weak positive response (3.77 ± 0.13 SD U/ml on day 21) following secondary infection. Interestingly, no anti-ACT IgG response was detected in serum from baboons that had been vaccinated with a whole-cell pertussis vaccine (wP) or aP and then infected with B. pertussis.
FIG 1.
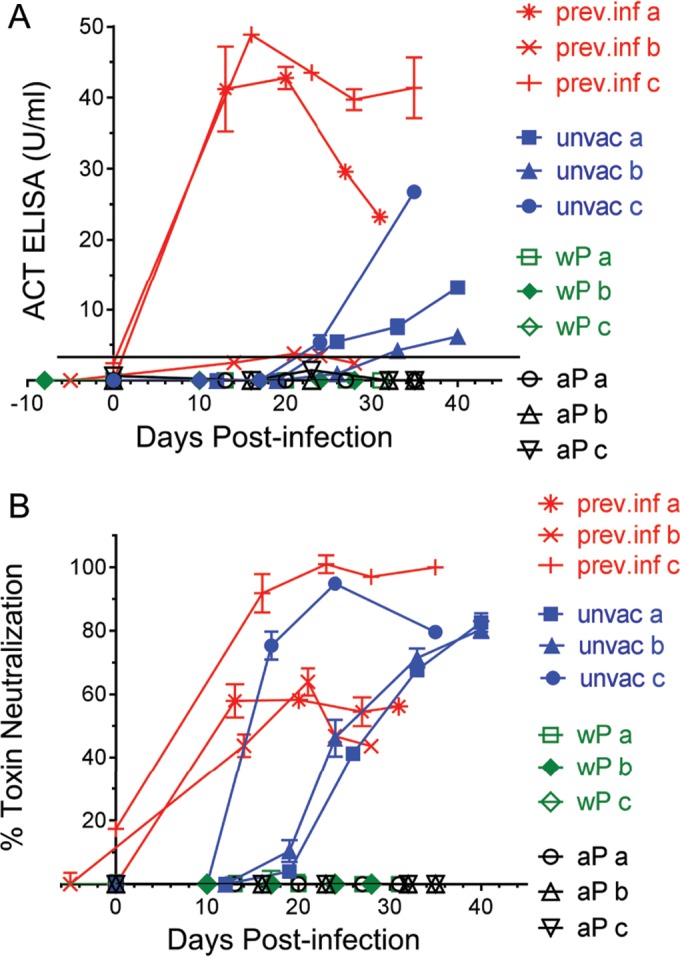
Use of ELISA and toxin neutralization assay to evaluate serial serum samples from baboons inoculated with B. pertussis. Sera from baboons previously infected (prev. inf) and given a secondary challenge with pertussis 1 to 2 months after clearing primary infection, unvaccinated (unvac) and given a primary challenge, wP vaccinated prior to primary challenge, and aP vaccinated prior to primary challenge were tested for anti-ACT response following inoculation, as described in Materials and Methods. Serum samples from 3 different subjects (a, b, and c) were tested within each group. (A) Shown are serum anti-ACT IgG ELISA titers calculated as described in Materials and Methods. Values of ≥3.32 U/ml (represented by a black horizontal line) are considered positive. (B) Shown are results from the TNA using a 1:50 final dilution of serum. Data represent the mean and SD of the results from each sample tested in duplicate (ELISA) or triplicate (TNA).
To further characterize the anti-ACT response, we measured neutralization by serum of ACT-induced cytotoxicity toward J774 cells. As described in Materials and Methods, recombinant purified ACT was combined with serum (at 1:50 final dilution) and added to J774 cells. Cell viability was measured after 2 h, and the results were expressed as percent toxin neutralization.
ACT-neutralizing activity was detected at days 17 to 19 following primary infection of unvaccinated baboons, at least 7 days earlier than anti-ACT IgG was detected using ELISA (Fig. 1). The toxin neutralization assay (TNA) was positive on day 0 in two of the three previously infected baboons, and the magnitude of inhibition increased after secondary challenge, whereas the ELISA was negative for these samples on day 0. TNA remained negative throughout the testing period on serum of baboons vaccinated with wP or aP prior to challenge with B. pertussis.
In summary, all of the baboon sera with anti-ACT IgG response possessed neutralizing activity. The TNA was positive earlier in the time course of infection than was ELISA for both unvaccinated primarily infected baboons and previously infected baboons infected a second time. In animals vaccinated with wP and aP, there was no evidence of an anti-ACT antibody response, by ELISA or TNA, during the 28 to 35 days following challenge.
We used the same assays to study sera from humans recently infected with B. pertussis. All human subjects tested positive for pertussis by PCR and reported symptoms consistent with pertussis. Most subjects were identified during a multicounty outbreak of pertussis in central Virginia in 2015, and all case subjects whom we tested had been vaccinated (Table 1). Of the 18 serum samples tested within 4 to 9 weeks after reported symptom onset, 13 samples were positive by anti-ACT IgG ELISA. When serum samples were tested by TNA, 14/18 samples were positive. Sample V014 was negative by anti-ACT IgG ELISA but was positive by TNA. All serum samples that were positive by anti-ACT IgG ELISA possessed neutralizing activity against ACT.
TABLE 1.
Serum from PCR-positive cases 4 to 9 weeks after symptom onseta
| Sample | Sex/age (yr)b | CT value | Primary vaccine series | aP boost | Date (mo/day/yr) of: |
Time from symptom onset to sample (wk) | ACT ELISA (U/ml) | TNA (%)d | PT ELISA (U/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Last vaccination | Approx symptom onsetc | |||||||||
| V002 | F/14 | 26.3 | 5 aP | + | 03/14/11 | 08/25/14 | 8 | 47.3 | 100 | 300 |
| V003 | M/17 | HCe | 5 aP | + | 08/14/08 | 08/11/14 | 4 | 44.8 | 100 | 640 |
| V006 | F/18 | 28.1 | 5 ap | + | 07/08/08 | 05/15/15 | 6 | 4.8 | 0 | 24.1 |
| V007 | M/16 | 14.0 | 5 aP | + | 07/20/10 | 05/28/15 | 4 | 63.9 | 100 | 464 |
| V008 | F/17 | 27.8 | 5 aP | + | 08/08/11 | 05/28/15 | 5 | 30.5 | 85.4 | 184 |
| V009 | M/12 | 20.6 | 5 aP | 08/06/08 | 05/05/15 | 8 | 93.7 | 100 | 215 | |
| V010 | M/13 | 23.2 | 5 aP | + | 10/14/12 | 06/01/15 | 5 | 156 | 100 | 73.8 |
| V011 | M/22 | 34.5 | 3 wP, 2 aP | + | 04/27/07 | 07/26/15 | 4 | 30.6 | 95.5 | 413 |
| V012 | M/15 | 29.0 | 5 aP | + | 08/30/10 | 05/07/15 | 9 | 45.5 | 85.4 | 803 |
| V013 | F/18 | 19.5 | 5 ap | + | 07/10/08 | 06/17/15 | 9 | 23.6 | 69.8 | 329 |
| V014 | M/13 | 32.9 | 5 aP | + | 08/23/13 | 07/01/15 | 7 | 9.8 | 19.3 | 94.1 |
| V015 | F/15 | 32.7 | 5 aP | + | 08/07/12 | 09/21/15 | 5 | 21.7 | 60.4 | 276 |
| V016 | F/14 | 36.2 | 5 aP | + | 01/18/12 | 09/20/15 | 6 | 54.3 | 97.4 | 170 |
| V017 | F/15 | 17.5 | 5 aP | + | 01/14/11 | 10/23/15 | 6 | 44.4 | 98.0 | 290 |
| V018 | M/15 | 31.9 | 5 aP | + | 06/28/11 | 10/22/15 | 7 | 0.71 | 0 | 1,404 |
| V019 | F/17 | 29.8 | 5 aP | + | 08/18/09 | 11/01/15 | 5 | 30.0 | 81.1 | 563 |
| V020 | M/17 | 27.5 | 5 aP | + | 04/23/10 | 11/14/15 | 4 | 3.28 | 0 | 293 |
| V021 | M/14 | 32.1 | 5 aP | + | 02/13/13 | 10/12/15 | 7 | 0.75 | 0 | 1,040 |
Bolded values are considered positive (see Table 2 for data from normal controls). Data represent the mean of the results from each sample tested in duplicate (ELISA) or triplicate (TNA).
F, female; M, male.
At onset of clinical illness, all subjects reported symptoms consistent with pertussis.
Percent toxin neutralization using a 1:50 final serum dilution, as described in Materials and Methods.
HC, sample was from a household contact of subject V002 with clinical symptoms of pertussis.
In addition to using anti-ACT ELISA and TNA, we tested all sera for anti-pertussis toxin (PT) IgG by ELISA, since this is the only serologic test currently used as a single-sample assay for the diagnosis of pertussis (21, 22). Serum samples V018, V020, and V021 all showed negative TNA and anti-ACT ELISA results while having positive PCR results and positive anti-PT IgG ELISA results, consistent with a diagnosis of pertussis without anti-ACT antibody response, 4 to 7 weeks after symptom onset. Sample V006 was negative by anti-ACT ELISA, TNA, and anti-PT ELISA, findings that likely represent a false-positive PCR result. V010 showed positive anti-ACT ELISA and TNA results but was negative by anti-PT ELISA, consistent with pertussis with negative PT antibody response at 5 weeks. Overall, anti-PT ELISA was positive in 16/18 subjects.
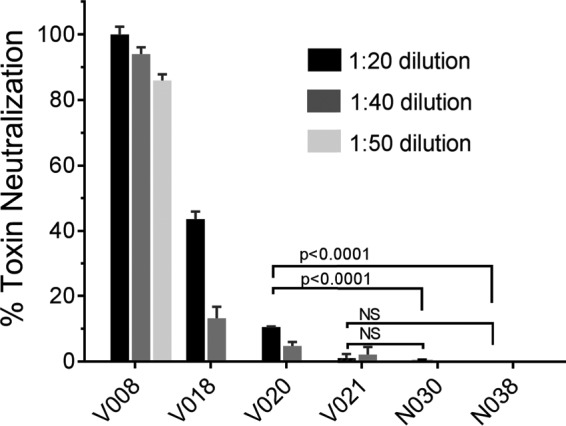
Because V018, V020, and V021 had positive anti-PT ELISA results but were negative by both ELISA and TNA for ACT, we tested these 3 samples at higher concentrations (dilutions of <1:50) to determine if there were lower levels of antibodies that could neutralize the cytotoxicity of ACT. As controls, pertussis-positive sera (V008, Fig. 2) and pertussis-negative sera (N030 and N038 are shown) were also tested at higher concentrations. When a 1:20 dilution of serum was used, instead of 1:50, serum sample V018 neutralized the cytotoxicity of ACT, V020 weakly neutralized ACT, and V021 exhibited no neutralizing activity (Fig. 2). The pertussis-negative control sera had no neutralizing activity, even at these higher serum concentrations (lower dilutions). Thus, dilutions of serum lower than 1:50 (i.e., higher concentrations) can be used to detect low-level ACT-neutralizing antibodies in serum samples without compromising the performance of the neutralization assay.
FIG 2.
Use of more-concentrated serum in the TNA results in positive tests from convalescent-phase samples, while control samples remain negative. Serum from samples V018, V020, and V021 was tested for neutralization of ACT activity using a higher concentration of serum. Serum samples from a pertussis-positive subject (V008 and others not shown) and pertussis-negative control subjects (N030, N038, and others not shown) were also tested. The 1:20 dilution of serum sample V020 is significantly different from 1:20 dilutions of serum from pertussis-negative control samples N030 and N038. The 1:20 dilution of sample V021 is not significantly different (NS) from 1:20 dilutions of N030 or N038. Shown are P values from multiple comparison testing using ANOVA with Tukey's post hoc test to determine statistical significance (P ≤ 0.05). Data represent the mean and SD of the results from each sample tested in triplicate.
Serum samples from 22 control subjects with no symptoms or reported epidemiologic linkage to pertussis were tested by TNA, and none possessed neutralizing activity (Table 2). One of 22 control samples was positive by anti-ACT ELISA, and 1/22 samples was positive by anti-PT ELISA (N042 and N050, respectively, Table 2).
TABLE 2.
Serum samples from control subjectsa
| Sample | Sex/age (yr)b | Primary vaccine series | aP boost | Date (mo/day/yr) of: |
ACT ELISA (U/ml)c | TNA (%)d | PT ELISA (U/ml)e | |
|---|---|---|---|---|---|---|---|---|
| Last vaccination | Serum sample | |||||||
| N030 | M/17 | 5 aP | + | 06/02/10 | 12/22/15 | 3.7 | 0 | 4.1 |
| N032 | M/16 | 5 aP | + | 09/27/10 | 12/22/15 | 3.3 | 0 | 21.1 |
| N033 | M/13 | 5 aP | + | 07/29/13 | 12/22/15 | 4.1 | 0 | 37.3 |
| N036 | F/17 | 5 aP | + | 08/04/09 | 12/22/15 | 9.0 | 0 | 6.4 |
| N037 | F/17 | 5 DTP | + | 08/26/10 | 12/22/15 | 1.6 | 0 | 6.0 |
| N038 | F/20 | 5 DTP | 05/22/99 | 12/22/15 | 2.3 | 0 | 3.0 | |
| N039 | F/16 | 5 aP | + | 05/25/10 | 01/07/16 | 2.5 | 0 | 5.1 |
| N040 | F/25 | 5wP | + | 04/19/94 | 07/23/15 | 14.1 | 0 | 7.1 |
| N041 | F/24 | 5wP | + | 08/20/13 | 07/23/15 | 12.5 | 0 | 19.0 |
| N042 | F/25 | 5wP | + | 02/20/08 | 07/23/15 | 58.8, 10.3f | 0 | 5.7 |
| N043 | F/25 | 5wP | + | 01/10/15 | 03/30/15 | 8.0 | 0 | 75.3 |
| N044 | F/25 | 5wP | + | 04/20/10 | 07/23/15 | 7.7 | 0 | 23.3 |
| N045 | F/28 | 5wP | + | 04/02/14 | 07/23/15 | 9.7 | 0 | 20.1 |
| N046 | F/39 | 5wP | 01/27/85 | 16.9 | 0 | 7.9 | ||
| N047 | F/57 | 5wP | + | 01/2012 | 02/02/12 | 3.0 | 0 | 37.0 |
| N048 | F/26 | 5wP | + | 08/20/13 | 01/28/14 | 15.3 | 0 | 21.9 |
| N049 | F/27 | 5wP | + | 01/22/10 | 01/29/15 | 10.2 | 0 | 59.9 |
| N050 | F/28 | 5wP | + | 02/15/15 | 03/30/15 | 12.8 | 0 | 106 |
| N051 | F/24 | 5wP | + | 04/24/11 | 02/26/14 | 7.4 | 0 | 66.0 |
| N052 | F/25 | 5wP | + | 01/10/15 | 01/29/15 | 9.8 | 0 | 72.9 |
| N053 | M/39 | 5wP | 01/27/85 | 12.9 | 0 | 11.0 | ||
| N054 | M/40 | 5wP | + | 07/24/06 | 01/10/12 | 17.6 | 0 | 3.6 |
Bolded values are considered positive (see titers below). Data represent the mean of the results from each sample tested in duplicate (ELISA) or triplicate (TNA).
M, male; F, female.
ACT titer mean ± SD, 8.85 ± 4.95 U/ml; normal, ≤18.8 U/ml (2 SD).
Percent toxin neutralization using a 1:50 final serum dilution, as described in Materials and Methods.
PT titer mean ± SD, 28.17 ± 29.4 IU/ml; normal, ≤87.0 IU/ml (2 SD).
Titer obtained using catalytic domain as antigen.
ACT is a member of the repeats-in-toxin (RTX) family of bacterial toxins, which includes HlyA of Escherichia coli and other bacterial proteins. Serum testing by anti-ACT IgG ELISA using the ACT holotoxin molecule as an antigen can detect antibodies to other RTX toxins (23, 24), because the C-terminal 1,700-amino-acid hemolysin component of ACT is homologous to other RTX toxins (25, 26). The ELISA used for our studies is based on the detection of ACT holotoxin, and control sample N042 was positive by ELISA. It was, however, negative when retested using the N-terminal catalytic domain of ACT as the ELISA antigen (Table 2), suggesting that the positive result was attributable to reactivity to the RTX domain of ACT.
Serial serum samples were obtained from several subjects with pertussis (Table 3). The TNA showed a positive result as early as 1 week after reported symptoms for V015, when anti-ACT ELISA was negative. Serum sample V002 was positive at 16 months by anti-ACT ELISA and TNA. At 17 months, TNA was weakly positive in serum from subject V003.
TABLE 3.
Serial testing of serum from PCR-positive casesa
| Sample | Sex/age (yr)b | CT value | Primary vaccine series | aP boost | Date (mo/day/yr) of: |
Time from symptom onset to sample | ACT ELISA (U/ml) | TNA (%)d | PT ELISA (U/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Last vaccination | Approx symptom onsetc | |||||||||
| V002A | F/14 | 26.3 | 5 aP | + | 03/14/11 | 08/25/14 | 2 wk | 31.5 | 84.5 | 357 |
| V002B | F/14 | 8 wk | 47.3 | 100 | 300 | |||||
| V002C | F/15 | 16 mo | 30.4 | 97.1 | 60.2 | |||||
| V003A | M/17 | HCe | 5 aP | + | 08/14/08 | 09/15/14 | 4 wk | 44.8 | 100 | 640 |
| V003B | M/17 | 10 wk | 24.8 | 92.7 | 512 | |||||
| V003C | M/18 | 17 mo | 3.59 | 1.3 | 110 | |||||
| V011A | M/22 | 34.5 | 3 wP, 2 aP | + | 04/27/07 | 07/31/15 | 5 days | 4.2 | 0 | 7.4 |
| V011B | M/22 | 4 wk | 30.6 | 95.5 | 413 | |||||
| V011C | M/22 | 4.5 mo | 22.3 | 92.1 | 182 | |||||
| V015A | F/15 | 32.7 | 5 aP | + | 08/07/12 | 09/28/15 | 1 wk | 13.3 | 61.3 | 86.7 |
| V015B | F/15 | 5 wk | 21.7 | 60.4 | 276 | |||||
Bolded values are considered positive (data for normal controls are given in Table 2). Data represent the mean of the results from each sample tested in duplicate (ELISA) or triplicate (TNA).
F, female; M, male.
At onset of clinical illness, all subjects reported symptoms consistent with pertussis.
Percent toxin neutralization using a 1:50 final serum dilution, as described in Materials and Methods.
HC, sample was from a household contact of subject V002 with clinical symptoms of pertussis.
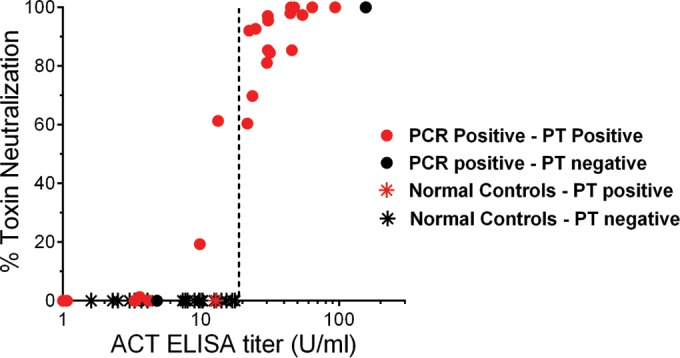
All results of the anti-ACT ELISA, TNA, and anti-PT ELISA are summarized in Fig. 3, which illustrates two important points: first, that serum samples with positive anti-ACT ELISA results uniformly exhibit neutralizing activity, and second, that there is neutralizing activity in some sera that are negative by anti-ACT ELISA. The sample with a positive TNA and negative anti-PT ELISA was acquired 16 months following symptom onset. In the 4- to 9-week period, the sensitivity of TNA for B. pertussis infection was 83.3%, specificity was 100%, and the positive predictive value (PPV) was 100%. The sensitivity of anti-ACT ELISA in the 4- to 9-week period was 72.2%, specificity was 95.5%, and the PPV was 92.9%. The sensitivity of anti-PT ELISA in the 4- to 9-week period was 88.9%, specificity was 95.5%, and the PPV was 94.1%.
FIG 3.
Summary of anti-ACT IgG ELISA, toxin neutralization assay, and anti-PT IgG ELISA results. Shown are the results of the anti-ACT IgG ELISA (x axis) and TNA (y axis) for all human serum samples. Red symbols indicate anti-PT ELISA-positive samples, and black symbols represent anti-PT IgG ELISA-negative samples. Closed circles represent serum from pertussis-diagnosed subjects (PCR positive with symptoms), and stars represent serum samples from control subjects. The dashed line indicates the cutoff for a positive anti-ACT IgG ELISA value. Data represent the mean of the results from each sample tested in duplicate (ELISA) or triplicate (TNA).
DISCUSSION
In this study, all serum samples from humans and baboons with anti-ACT IgG response after infection with B. pertussis neutralized ACT activity. ACT inhibits multiple functions of phagocytic leukocytes in vitro: neutrophil extracellular trap (NET) formation, the oxidative burst, and phagocytosis (15, 27). However, it is unknown whether blocking these functions in vivo promotes clearance of the organism. Because ACT inhibits opsonin-mediated phagocytosis by leukocytes, neutralizing this activity would be expected to promote antibody-mediated bacterial clearance (28). Vaccination of mice with the acellular pertussis vaccine (aP) that includes ACT results in improved clearance of B. pertussis in comparison to vaccination with aP alone (11); this finding may be due to the generation of anti-ACT neutralizing antibodies or other effects of ACT. The implications of generating neutralizing antibodies must be taken in the context of the entire immune response. For example, blocking inhibition of the oxidative burst may result in more reactive oxygen species and NET formation, eliciting greater local inflammation in response to B. pertussis. Further testing of these hypotheses in animal models is required in order to understand the role of neutralizing antibodies in clearance of or protection from pertussis.
Mobberley-Schuman et al. studied serum from patients with pertussis and found that only 1/24 serum samples neutralized ACT function. This low percentage of ACT-neutralizing antisera (compared with our findings) may be attributable to the assay that they used, which measured serum neutralization of the effect of ACT on phagocytosis of live B. pertussis (15). In their experiments, antibodies to ACT may have been adsorbed by ACT on the surface of B. pertussis, whereas active ACT is released (29). In addition, because active secretion and release of ACT from bacteria are required to develop concentrations high enough to inhibit neutrophil function (30), a functionally low concentration of ACT was likely present in the media at the time that bacteria were combined with neutrophils. Furthermore, multiple B. pertussis virulence factors influence phagocytosis (31). Farfel et al. reported that sera from patients with pertussis did not neutralize ACT-induced intracellular cAMP generation (16); however, crude extracts of ACT were used rather than purified ACT, and the primary data were not shown in the paper. Based on the findings of our study, we suggest that neutralizing antibodies would have been identified in most serum samples tested by Mobberley-Schuman et al. (15) and Farfel et al. (16) if the TNA had been used for testing serum.
In comparison to the serum anti-ACT ELISA responses measured by Cherry et al. in unvaccinated study subjects with pertussis (14), serum anti-ACT ELISA responses of subjects with pertussis in the present study are low in magnitude at 4 to 9 weeks after symptom onset. Consistent with the observations in our study, Cherry et al. found that subjects that had been vaccinated with wP or aP prior to infection with B. pertussis responded to infection with an increase in anti-ACT IgG ELISA results that was small in comparison to that of unvaccinated subjects (14). The diminished anti-ACT responses in vaccinated baboons compared to unvaccinated baboons is reminiscent of these findings, suggesting that vaccination with aP or wP prior to infection results in suppression of the anti-ACT response to pertussis. Cherry et al. (14) hypothesized that the immunologic phenomenon of original antigenic sin (OAS) accounted for the reduced antibody response to infection in previously vaccinated hosts. Based on this hypothesis, the response to a secondary exposure to an infecting agent is dominated by memory cells generated by the primary exposure (32, 33). It is unclear how OAS would explain the suppressed anti-ACT response to infection in subjects that received wP, since wP contains ACT. Nonetheless, the observation that the anti-ACT response following infection is reduced by prior vaccination is intriguing and deserves additional investigation.
While the anti-ACT response following infection is reduced in previously vaccinated humans relative to unvaccinated humans, the response is absent in previously vaccinated baboons, and this difference is one of several between human pertussis and the baboon model of pertussis. Interestingly, the anti-ACT IgG ELISA is negative on day 0 in previously infected baboons, 30 to 60 days following their primary infection (Fig. 1). This finding is remarkable because Warfel et al. found that antibody responses to other B. pertussis antigens are detectable in previously infected baboons on day 0 (34), and serial serum samples from humans show anti-ACT IgG ELISA positivity beyond 9 weeks in several cases (Table 3). In addition to serologic differences between baboons and humans, vaccinated and previously infected baboons that are secondarily infected remain asymptomatic (34), whereas human samples in this study were obtained from symptomatic subjects. The human subjects in this study were adolescents and adults, whereas baboons were weanlings. The humans acquired the infection by natural transmission, whereas the baboons were inoculated by intratracheal and intranasal challenge with a large single dose (109 to 1010 CFU) of bacteria. Finally, the B. pertussis strain used to infect baboons is a clinical isolate now used for laboratory experiments (35). Altogether, the baboon model of pertussis is invaluable for a controlled study of B. pertussis infection and vaccination, but one should be aware of the differences between the baboon model of pertussis and human pertussis when interpreting findings.
As demonstrated by this study of human and baboon sera, the same TNA can be used to study sera from different genera. Unlike ELISA, TNA does not require a secondary antibody, so there is no species-specific adjustment of the assay required. The lack of secondary antibody and the design of the TNA eliminate the need for blocking steps, washing steps, and serial dilutions. There is no need for serial dilutions because of the dynamic concentration dependence of ACT-induced cytotoxicity and the concentration of purified ACT used (80 ng/ml), such that any inhibition of ACT results in a substantial reduction in cytotoxicity (Fig. 4). In this study, despite the low anti-ACT IgG ELISA values of infected subjects, the TNA was positive when anti-ACT ELISA was positive and showed a positive result when ELISA was negative in two human cases. The TNA also exhibited positive results at earlier time points after challenge than ELISA in several baboon serum samples. The assay is thus very sensitive.
FIG 4.
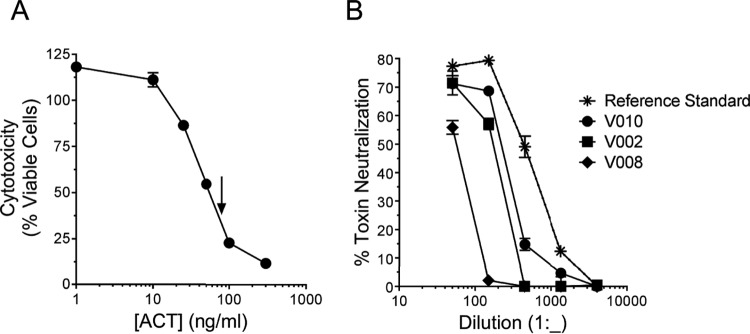
Neutralization of ACT cytotoxicity by serial dilutions of convalescent human serum. (A) The concentration of ACT to be used for the toxin neutralization assay was chosen based on cytotoxicity of ACT toward J774 cells. ACT was added to 30,000 J774 cells grown overnight on a 96-well plate. After 2 h, cytotoxicity (percent viable cells) was measured by a CCK-8 assay. The arrow indicates the concentration of ACT (80 ng/ml) chosen for use in the toxin neutralization assay. Data represent the mean and SD of the results from 3 experiments performed in triplicate. (B) ACT was mixed with serum at the indicated dilution and added to J774 cells as described in Materials and Methods. The final dilution was 1:x, where x is the value on the x axis. Percent toxin neutralization was calculated as described in Materials and Methods. The reference standard for human convalescent anti-pertussis serum was provided by NIBSC (89/530). Data represent the mean and SD of the results from 2 experiments performed in triplicate.
Neutralization requires that an antibody not only bind to ACT but also block its function. Not all anti-ACT monoclonal antibodies block ACT function, as in the case of N042 (Table 2) (36). The positive anti-ACT ELISA result for N042 reflected binding to the RTX component of ACT, the immunodominant component of the toxin in mice (37), which is common to other bacterial toxins. Thus, the finding of neutralization of ACT is more specific than just binding, as is detected by ELISA. In addition, a high concentration (low dilution) of antiserum can be used in the TNA without affecting the test performance, whereas high concentrations of serum in the ELISA can result in a high background signal, requiring greater dilution before use in the assay. While the serum dilution used for most TNA tests was 1:50, the results shown in Fig. 2 indicate that the assay results may be more sensitive without compromising specificity by using a dilution of 1:20. In sum, TNA is a sensitive, specific, and easily performed assay that measures functional activity of the anti-ACT response.
With this novel assay, we have found that B. pertussis infection results in the generation of neutralizing antibodies to ACT in vaccinated humans. This serum neutralization assay will be useful for evaluating the anti-ACT response to infection and vaccines in future studies.
MATERIALS AND METHODS
Bacterial growth and tissue culture.
B. pertussis strain D420 was grown as described previously (38). J774A.1 (J774) murine macrophage cells were cultured in Dulbecco's modified Eagle's medium with 25 mM glucose (Gibco) plus 10% heat-inactivated fetal bovine serum (FBS-HI; Gibco) at 37°C in 5% CO2.
To produce recombinant ACT, Escherichia coli XL1-Blue cells (Stratagene, La Jolla, CA) containing pT7CACT1 plasmid with wild-type cyaA and cyaC were grown as described previously (36, 39). Recombinant ACT was extracted in 8 M urea, purified, and stored as described previously (40).
Vaccination, infection, and evaluation of baboons.
All animal procedures were performed in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International in accordance with protocols approved by the CBER Animal Care and Use Committee and principles outlined in the Guide for the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Resources, National Research Council (46). Baboons obtained from the Oklahoma Baboon Research Resource at the University of Oklahoma Health Sciences Center were inoculated with human doses of a diphtheria-tetanus-acellular pertussis vaccine (DTaP) or a diphtheria–tetanus–whole-cell pertussis vaccine (DTwP) administered intramuscularly (i.m.) at 2, 4, and 6 months of age, as described previously (34). For DTaP, three animals were vaccinated with the U.S.-licensed vaccine Daptacel (Sanofi Pasteur Ltd., Toronto, Canada), which contains diphtheria toxoid, tetanus toxoid, and five pertussis antigens (detoxified pertussis toxin, filamentous hemagglutinin, pertactin, fimbriae 2, and fimbriae 3). For DTwP, three animals were vaccinated with triple antigen (Serum Institute of India Ltd., Pune, India), which meets WHO recommendations for potency, as documented previously (41). Unvaccinated animals were matched for age. Baboons that had previously been infected with B. pertussis were tested by twice-weekly cultures of nasopharyngeal aspirates and found to be clear of infection for 1 to 2 months prior to subsequent infection (34). Direct challenge studies were performed as described previously (38, 42). The inoculum for each direct challenge was between 109 and 1010 CFU, as determined by measurement of optical density and confirmed by serial dilution and plating to determine the number of CFU per milliliter of inoculum. Peripheral blood was collected for serum separation, as previously described (38).
Evaluation of human subjects.
All human subject research was performed according to a protocol reviewed and approved by the Institutional Review Board for Health Sciences Research at the University of Virginia (UVA). Subjects were identified on the basis of a positive B. pertussis diagnostic PCR result from the UVA Health System clinical microbiology laboratory. The PCR test was developed at UVA using commercially available reagents in compliance with laboratory standards and detects the IS481 element of bordetellae (43, 44). Primers, TaqMan fluorescent probes, and master mix were manufactured by BioGX, and analysis was performed on the BD Max instrument. Determination of positive PCR tests was based on the following criteria. A sample was considered positive for pertussis if the pertussis-specific total fluorescence was ≥220 fluorescence units and there was amplification within 40 cycles. A sample processing control (SPC), which does not react with IS481, was incorporated into each specimen and carried through each step of the procedure. The SPC was considered positive if total fluorescence was ≥220 fluorescence units and the threshold cycle (CT) was ≤30.5. A sample was considered negative if the SPC was positive and there was no amplification of pertussis-specific DNA within 40 cycles.
Once identified as positive by PCR, subjects were contacted by phone, as per study protocol, and informed consent was obtained from the subject or his/her parent or guardian. Clinical history was collected, and subjects were asked if they would donate a sample of blood for these studies. All subjects with positive PCR tests (Table 1) reported symptoms consistent with pertussis. Thus, they fulfilled the CDC criteria for a positive pertussis case. Control serum samples were obtained from subjects who reported no known exposure to pertussis, no recent respiratory symptoms, and no history of pertussis (Table 2). Immunization records of PCR-positive and control subjects were obtained using the Virginia Immunization Information System, which provides a record of vaccines administered in the state of Virginia (Tables 1 and 2).
Enzyme-linked immunosorbent assay.
ELISA was used to quantify titers of anti-ACT IgG and anti-PT IgG antibodies in serum samples. For anti-ACT IgG and anti-PT IgG assays, 96-well flat-bottom ImmunoPlate MaxiSorp plates (Thermo Scientific) were coated with 100 μl of ACT at 1 μg/ml or 100 μl PT at 1 μg/ml in 50 mM carbonate-bicarbonate coating buffer (pH 9.6) overnight at room temperature (RT). Plates were washed 3 times with phosphate-buffered saline (PBS) (pH 7.4) plus 0.05% Tween 20 (PBST) and blocked with 100 μl of Blocker Blotto in Tris-buffered saline (TBS; Thermo Scientific) for 1 h at room temperature. Wells were washed, and 3-fold dilutions (starting at 1:100) of reference serum standard (National Institute for Biological Standards and Control [NIBSC] code 89/530 for ACT or 06/140 for PT) and serum samples to be tested were made in PBST plus 10% Blocker Blotto (PBSTB), added to the ELISA plate, and incubated for 1 h, with mixing at room temperature (RT). Negative-control wells were treated similarly but did not contain subject or reference serum. Plates were washed, 100 μl of 1:10,000 goat anti-monkey IgG(H+L)-horseradish peroxidase (HRP) (AbD Serotec, for baboon studies) or goat anti-human IgG(H+L) F(ab′)2-HRP (Novex, Life Technologies for human studies) was added, and plates were incubated for 1 h, with mixing at RT. After washing, 100 μl of SureBlue 3,3′,5,5′-tetramethylbenzidine (TMB) microwell peroxidase substrate was added and plates were incubated in the dark, with mixing at RT. The reaction was stopped by the addition of 100 μl of 1 N HCl, and absorbance at 450 nm was read in a μQuant plate reader (Biotech Instruments). The ACT ELISA results were calculated for each serum sample against reference serum 89/530, which has been assigned an anti-ACT IgG titer of 100 U/ml, using linear regression with ≥3 dilutions in the linear range (14). The PT ELISA results were calculated using reference standard 06/140, which has an anti-PT IgG content of 335 IU/ml (45).
The cutoff values for a positive result were calculated as described previously (14). For serum samples from humans, positive was defined as the mean ± 2 SD of ELISA titers of pertussis-negative control subject samples (positive cutoff for anti-ACT IgG ELISA, ≥18.8 U/ml; positive cutoff for anti-PT IgG ELISA, ≥87.0 IU/ml). For baboon serum samples taken at serial time points (Fig. 1A), a positive value for anti-ACT IgG was defined as the mean ± 2 SD of ELISA titers of initial samples (day 0) from naive, aP-treated, and wP-treated baboons (positive cutoff, ≥3.32 U/ml).
Toxin neutralization assay.
The toxin neutralization assay (TNA) is based on the cytotoxicity of ACT toward J774 macrophage-like cells. The J774 cells (30,000 cells in 90 μl) were seeded in each well of a 96-well plate and allowed to attach overnight at 37°C with 5% CO2. ACT was incubated with serum samples for 10 min, and the samples were mixed at 4°C and added to the cells at a final ACT concentration of 80 ng/ml. Cells were incubated at 37°C for 2 h, and the number of viable cells was determined using the CCK-8 assay (Dojindo Laboratories), which measures the reduction of WST-8, a water-soluble tetrazolium salt, by dehydrogenases in live cells. The percentage of viable cells was determined for ACT alone and for ACT with serum compared to control cells by the following equation: [(experimental − blank)/(control cells − blank)] × 100. The results are reported as percent toxin neutralization, determined as follows: [(% viable cells treated with ACT and serum) − (% viable cells treated with ACT alone)]/[(control cells {set as 100%}) − (% viable cells treated with ACT alone)] × 100. A blank is a well on the 96-well plate containing medium and CCK-8 reagent but no J774 cells. Control cells are J774 cells in the 96-well plate that are not treated with ACT or serum.
The concentration of ACT used in the TNA was chosen based on the highly reproducible concentration-response curve shown in Fig. 4A; with a concentration of ACT of 80 ng/ml, subtle inhibition of toxin activity results in a substantial change in cytotoxicity detected by the CCK-8 assay.
The final dilution of serum used for the TNA was 1:50, unless otherwise specified, and this dilution was chosen based upon characteristics of the assay. Testing of human serum samples and the pooled human reference standard (NIBSC 89/530, which exhibited positive anti-ACT ELISA results ranging from 30.5 to 156 U/ml) showed that inhibition of toxin activity by serum is concentration dependent (Fig. 4B). Due to a high signal-to-noise ratio and no increase in nonspecific signal in wells containing concentrated serum samples from control subjects, a concentrated serum specimen could be examined by TNA, and we selected a dilution of 1:50. In ELISA, serum more concentrated (lower dilution) than 1:100 resulted in signal in the control subject wells. Because none of the 22 serum samples from control subjects neutralized ACT, a cutoff value for positivity of >0% toxin neutralization was used.
Statistics.
Multiple comparisons were made using analysis of variance (ANOVA) with Tukey's post hoc test. A P value of ≤0.05 was considered significant. Statistical analysis was performed by using GraphPad Prism software and Microsoft Excel.
REFERENCES
- 1.Clark TA. 2014. Changing pertussis epidemiology: everything old is new again. J Infect Dis 209:978–981. doi: 10.1093/infdis/jiu001. [DOI] [PubMed] [Google Scholar]
- 2.Warfel JM, Edwards KM. 2015. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol 35:48–54. doi: 10.1016/j.coi.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. 2013. Pertussis: challenges today and for the future. PLoS Pathog 9:e1003418. doi: 10.1371/journal.ppat.1003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meade BD, Plotkin SA, Locht C. 2014. Possible options for new pertussis vaccines. J Infect Dis 209(Suppl 1):S24–S27. doi: 10.1093/infdis/jit531. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin SA. 2014. Pertussis: pertussis control strategies and the options for improving current vaccines. Expert Rev Vaccines 13:1071–1072. doi: 10.1586/14760584.2014.944166. [DOI] [PubMed] [Google Scholar]
- 6.Sebo P, Osicka R, Masin J. 2014. Adenylate cyclase toxin-hemolysin relevance for pertussis vaccines. Expert Rev Vaccines 13:1215–1227. doi: 10.1586/14760584.2014.944900. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin MS, Weiss AA. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun 58:3445–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss AA, Hewlett EL, Myers GA, Falkow S. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis 150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 9.Guiso N, Rocancourt M, Szatanik M, Alonso JM. 1989. Bordetella adenylate cyclase is a virulence associated factor and an immunoprotective antigen. Microb Pathog 7:373–380. doi: 10.1016/0882-4010(89)90040-5. [DOI] [PubMed] [Google Scholar]
- 10.Orr B, Douce G, Baillie S, Parton R, Coote J. 2007. Adjuvant effects of adenylate cyclase toxin of Bordetella pertussis after intranasal immunisation of mice. Vaccine 25:64–71. doi: 10.1016/j.vaccine.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Cheung GY, Xing D, Prior S, Corbel MJ, Parton R, Coote JG. 2006. Effect of different forms of adenylate cyclase toxin of Bordetella pertussis on protection afforded by an acellular pertussis vaccine in a murine model. Infect Immun 74:6797–6805. doi: 10.1128/IAI.01104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hormozi K, Parton R, Coote J. 1999. Adjuvant and protective properties of native and recombinant Bordetella pertussis adenylate cyclase toxin preparations in mice. FEMS Immunol Med Microbiol 23:273–282. doi: 10.1111/j.1574-695X.1999.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 13.Arciniega JL, Hewlett EL, Johnson FD, Deforest A, Wassilak SG, Onorato IM, Manclark CR, Burns DL. 1991. Human serologic response to envelope-associated proteins and adenylate cyclase toxin of Bordetella pertussis. J Infect Dis 163:135–142. doi: 10.1093/infdis/163.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Cherry JD, Xing DX, Newland P, Patel K, Heininger U, Corbel MJ. 2004. Determination of serum antibody to Bordetella pertussis adenylate cyclase toxin in vaccinated and unvaccinated children and in children and adults with pertussis. Clin Infect Dis 38:502–507. doi: 10.1086/381204. [DOI] [PubMed] [Google Scholar]
- 15.Mobberley-Schuman PS, Connelly B, Weiss AA. 2003. Phagocytosis of Bordetella pertussis incubated with convalescent serum. J Infect Dis 187:1646–1653. doi: 10.1086/374741. [DOI] [PubMed] [Google Scholar]
- 16.Farfel Z, Konen S, Wiertz E, Klapmuts R, Addy PA, Hanski E. 1990. Antibodies to Bordetella pertussis adenylate cyclase are produced in man during pertussis infection and after vaccination. J Med Microbiol 32:173–177. doi: 10.1099/00222615-32-3-173. [DOI] [PubMed] [Google Scholar]
- 17.Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, Ladant D, Leclerc C. 2001. The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the alpha(M)beta(2) integrin (CD11b/CD18). J Exp Med 193:1035–1044. doi: 10.1084/jem.193.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vojtova J, Kamanova J, Sebo P. 2006. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr Opin Microbiol 9:69–75. doi: 10.1016/j.mib.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Gordon VM, Young WW Jr, Lechler SM, Gray MC, Leppla SH, Hewlett EL. 1989. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J Biol Chem 264:14792–14796. [PubMed] [Google Scholar]
- 20.Hewlett EL, Donato GM, Gray MC. 2006. Macrophage cytotoxicity produced by adenylate cyclase toxin from Bordetella pertussis: more than just making cyclic AMP! Mol Microbiol 59:447–459. [DOI] [PubMed] [Google Scholar]
- 21.Marchant CD, Loughlin AM, Lett SM, Todd CW, Wetterlow LH, Bicchieri R, Higham S, Etkind P, Silva E, Siber GR. 1994. Pertussis in Massachusetts, 1981–1991: incidence, serologic diagnosis, and vaccine effectiveness. J Infect Dis 169:1297–1305. doi: 10.1093/infdis/169.6.1297. [DOI] [PubMed] [Google Scholar]
- 22.Guiso N, Berbers G, Fry NK, He Q, Riffelmann M, Wirsing von Konig CH. 2011. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur J Clin Microbiol Infect Dis 30:307–312. doi: 10.1007/s10096-010-1104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe M, Connelly B, Weiss AA. 2006. Characterization of serological responses to pertussis. Clin Vaccine Immunol 13:341–348. doi: 10.1128/CVI.13.3.341-348.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimprel E, Begue P, Anjak I, Njamkepo E, Francois P, Guiso N. 1996. Long-term human serum antibody responses after immunization with whole-cell pertussis vaccine in France. Clin Diagn Lab Immunol 3:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanski E. 1989. Invasive adenylate cyclase toxin of Bordetella pertussis. Trends Biochem Sci 14:459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- 26.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. 1988. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol 2:19–30. doi: 10.1111/j.1365-2958.1988.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 27.Eby JC, Gray MC, Hewlett EL. 2014. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun 82:5256–5269. doi: 10.1128/IAI.02487-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weingart CL, Mobberley-Schuman PS, Hewlett EL, Gray MC, Weiss AA. 2000. Neutralizing antibodies to adenylate cyclase toxin promote phagocytosis of Bordetella pertussis by human neutrophils. Infect Immun 68:7152–7155. doi: 10.1128/IAI.68.12.7152-7155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray MC, Donato GM, Jones FR, Kim T, Hewlett EL. 2004. Newly secreted adenylate cyclase toxin is responsible for intoxication of target cells by Bordetella pertussis. Mol Microbiol 53:1709–1719. doi: 10.1111/j.1365-2958.2004.04227.x. [DOI] [PubMed] [Google Scholar]
- 30.Eby JC, Gray MC, Warfel JM, Paddock CD, Jones TF, Day SR, Bowden J, Poulter MD, Donato GM, Merkel TJ, Hewlett EL. 2013. Quantification of the adenylate cyclase toxin of Bordetella pertussis in vitro and during respiratory infection. Infect Immun 81:1390–1398. doi: 10.1128/IAI.00110-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fazekas de St. Groth S, Webster RG. 1966. Disquisitions on original antigenic sin. I. Evidence in man. J Exp Med 124:331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzenberg LA, Tokuhisa T, Herzenberg LA. 1980. Carrier-priming leads to hapten-specific suppression. Nature 285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 34.Warfel JM, Zimmerman LI, Merkel TJ. 2014. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad Sci U S A 111:787–792. doi: 10.1073/pnas.1314688110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boinett CJ, Harris SR, Langridge GC, Trainor EA, Merkel TJ, Parkhill J. 2015. Complete genome sequence of Bordetella pertussis D420. Genome Announc 3(3):e00657-15. doi: 10.1128/genomeA.00657-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Gray MC, Guo L, Sebo P, Hewlett EL. 1999. Epitope mapping of monoclonal antibodies against Bordetella pertussis adenylate cyclase toxin. Infect Immun 67:2090–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, Maynard JA, Hewlett EL, Maynard JA. 2015. The Bordetella adenylate cyclase repeat-in-toxin (RTX) domain is immunodominant and elicits neutralizing antibodies. J Biol Chem 290:3576–3591. doi: 10.1074/jbc.M114.585281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warfel JM, Beren J, Kelly VK, Lee G, Merkel TJ. 2012. Nonhuman primate model of pertussis. Infect Immun 80:1530–1536. doi: 10.1128/IAI.06310-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Betsou F, Sebo P, Guiso N. 1993. CyaC-mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun 61:3583–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray MC, Lee SJ, Gray LS, Zaretzky FR, Otero AS, Szabo G, Hewlett EL. 2001. Translocation-specific conformation of adenylate cyclase toxin from Bordetella pertussis inhibits toxin-mediated hemolysis. J Bacteriol 183:5904–5910. doi: 10.1128/JB.183.20.5904-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warfel JM, Zimmerman LI, Merkel TJ. 2015. Comparison of three whole-cell pertussis vaccines in the baboon model of pertussis. Clin Vaccine Immunol 23:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warfel JM, Beren J, Merkel TJ. 2012. Airborne transmission of Bordetella pertussis. J Infect Dis 206:902–906. doi: 10.1093/infdis/jis443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McPheat WL, McNally T. 1987. Isolation of a repeated DNA sequence from Bordetella pertussis. J Gen Microbiol 133:323–330. [DOI] [PubMed] [Google Scholar]
- 44.McLafferty MA, Harcus DR, Hewlett EL. 1988. Nucleotide sequence and characterization of a repetitive DNA element from the genome of Bordetella pertussis with characteristics of an insertion sequence. J Gen Microbiol 134:2297–2306. [DOI] [PubMed] [Google Scholar]
- 45.Xing D, Wirsing von Konig CH, Newland P, Riffelmann M, Meade BD, Corbel M, Gaines-Das R. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin Vaccine Immunol 16:303–311. doi: 10.1128/CVI.00372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]