ABSTRACT
Lyme borreliosis is caused by tick-transmitted spirochetes of the Borrelia burgdorferi sensu lato group and is the most common vector-borne disease in the United States and Europe. Outer surface protein C (OspC) is a 23-kDa outer surface lipoprotein expressed during spirochete transmission from the tick to the vertebrate host. In a previous study, we found that immunization with a recombinant disulfide-bridged dimeric form of OspC (D-OspC) stimulates increased antibody responses relative to immunization with commonly employed monomeric OspC. Here, we report that mice immunized with dimeric OspC proteins also exhibited enhanced protection against infection with the cognate B. burgdorferi strain. Mice were protected by four immunizations containing as little as 100 ng of dimeric OspC, suggesting that this form of the protein can induce protective immunity within a dose range reasonable for a human or veterinary vaccine. In contrast, monomeric OspC was only partially protective at much higher doses. IgG subclass analysis revealed that D-OspC-immunized animals mainly possessed anti-OspC-IgG1. In contrast, infected animals develop anti-OspC restricted to the IgG3 isotype. A subset of antibodies generated by dimeric OspC immunization did not recognize the monomeric variant, indicating that unique epitopes exist on the dimeric form. Moreover, monoclonal antibodies that recognized only dimeric OspC protected mice from B. burgdorferi challenge, whereas another monoclonal that recognized both immunogens was not protective. These studies suggest that this dimeric OspC presents distinctive epitopes that generate antibodies protective against B. burgdorferi infection and could be a useful vaccine component.
KEYWORDS: OspC, vaccine, ELISA, immunoblot, Lyme disease, Borrelia burgdorferi, Borrelia
INTRODUCTION
Lyme borreliosis (LB) is the most commonly reported arthropod-borne illness in North America and Europe (1), with an estimated annual incidence of 300,000 cases in the United States (2). Caused by spirochetes of the Borrelia burgdorferi sensu lato group (B. burgdorferi sensu stricto, B. afzelii, B. garinii, and related species), these bacteria are transmitted by hard-bodied ticks of the genus Ixodes in wooded areas of moderate climate. They can infect many mammals but generally find reservoir hosts in rodent and bird populations. In the United States, B. burgdorferi sensu stricto is the most common LB-associated strain, while the most common species in Europe are B. afzelii and B. garinii.
LB is a multisystemic bacterial infection and is characterized by localized, disseminated, and late manifestations. Localized disease often presents as erythema migrans, an expanding red rash that develops at the site of the tick bite. Patients may also experience fatigue, headaches, fever, chills, and muscle and joint pain. After a period of several days or weeks, the bacteria disseminate by hematogenous or lymphatic routes to other organs and cause systemic manifestations (3–5). These patients may present with multiple erythema, Lyme neuroborreliosis (LNB), arthritis, and cardiac symptoms. Late manifestations (6, 7) vary but may consist of arthritis, neuroborreliosis, or carditis (7, 8). In North America, a significant proportion of late-manifestation patients develop chronic arthritis, especially in the large joints. The disease phenotype varies somewhat between the different genospecies; for example, B. burgdorferi infection tends to favor arthritic symptoms, whereas neurologic manifestations are more commonly associated with B. garinii infections. Antibiotic therapy is frequently effective, but 10 to 20% of patients, especially those diagnosed late in the disease process, develop posttreatment Lyme disease syndrome and continue to have symptoms of fatigue, joint and muscle pain, and cognitive deficits (9–11).
At present, the prevention of LB is restricted to personal protection measures, including the use of pesticides and personal tick checks. Plans to eradicate Borrelia or tick populations on a large scale are only in conceptual stages and appear to be both impractical and cost-ineffective. LYMErix, a Lyme disease vaccine, was approved for human use in 1998 (12). It was composed of a recombinant form of the B. burgdorferi outer surface protein A (OspA). Although OspA is primarily expressed when the spirochete is in the Ixodes tick, LYMErix was found to be effective at preventing Lyme borreliosis by protecting against the transmission of B. burgdorferi from the tick to the human. LYMErix had some limitations, including a vaccine efficacy of <80%, uncertainty about the length of vaccine-induced immunity (potential need for booster doses), and efficacy only against the predominant North American Borrelia strain (13). In addition, concerns were raised by antivaccine groups regarding vaccine safety, and in response to these concerns and low public demand, the manufacturer voluntarily withdrew LYMErix from the market in 2002. Currently, new strategies employing mixtures of chimeric OspAs from several strains are being tested as vaccine candidates (14).
Another vaccine candidate is the outer surface protein C (OspC). The expression of OspC is induced during tick feeding and transmission of Borrelia and is essential for the initial colonization of mammalian hosts (15–18). In the tick host, OspC binds to the Salp15, a tick salivary protein (19). This interaction appears to facilitate the localization of the spirochetes to the salivary gland from which the spirochetes enter the mammalian host. The OspC-Salp15 interaction further enhances invasion of the host and may also enhance evasion of the host immune system (20). Furthermore, OspC appears to interact with plasminogen, perhaps utilizing this interaction to facilitate hematologic dissemination to tissues distant from the infection site (21, 22). Finally, mutagenesis studies support the existence of a ligand binding domain in OspC that interacts with an unidentified mammalian factor that is critical for survival and dissemination in early infection (23).
OspC is highly immunogenic and is one of the primary proteins against which host immune memory is developed (24, 25). It generates an IgM antibody response early during the course of infection, and the detection of anti-OspC IgM antibodies is useful in the diagnosis of early LB (26, 27). However, the amino acid sequence of OspC is highly diverse, even within strains of the same Borrelia species (28), and at least 25 families of OspC variants have been defined, with each geographic area harboring as many as 10 to 15 different types (29). The membrane-proximal N and C termini of OspC tend to be more conserved, especially a 10-amino-acid epitope near the C terminus. Both humans and mice can produce borreliacidal IgM to this C-terminal epitope (30–32).
OspC has previously been evaluated as a vaccine candidate and was found to be mostly protective against the Borrelia strain from which it originated; however, it failed to provide satisfactory cross-protection against strains with divergent OspC sequences (33–37). To overcome this problem, tetra- or octavalent recombinant OspC proteins have been designed but thus far have not been tested in depth in animal infection models (38, 39).
In previous studies, Probst et al. (40) found that a recombinant OspC dimer has higher binding efficiency for anti-OspC antibodies in patient specimens and elicits an improved antibody response compared to monomeric OspC. OspC contains an intrinsic signal sequence followed by a cysteine residue, the attachment site of the lipid membrane anchor. Removal of the signal sequence allows isolation of a recombinant OspC homodimer that is covalently linked via an N-terminal disulfide bridge. The goal of the current study was to examine whether recombinant dimers of OspC (e.g., dimeric recombinant OspC from B. burgdorferi strain B31 [D-OspCB31]) result in enhanced protection in comparison with the corresponding recombinant monomers (such as monomeric recombinant OspC from B. burgdorferi strain B31 [M-OspCB31]) against challenge with LB Borrelia by either needle inoculation or tick transmission. Our data indicate that this dimeric form of OspC is a more protective immunogen than monomeric OspC and produces protective antibodies to epitopes unique to the dimeric form.
RESULTS
Characterization of recombinant OspCs.
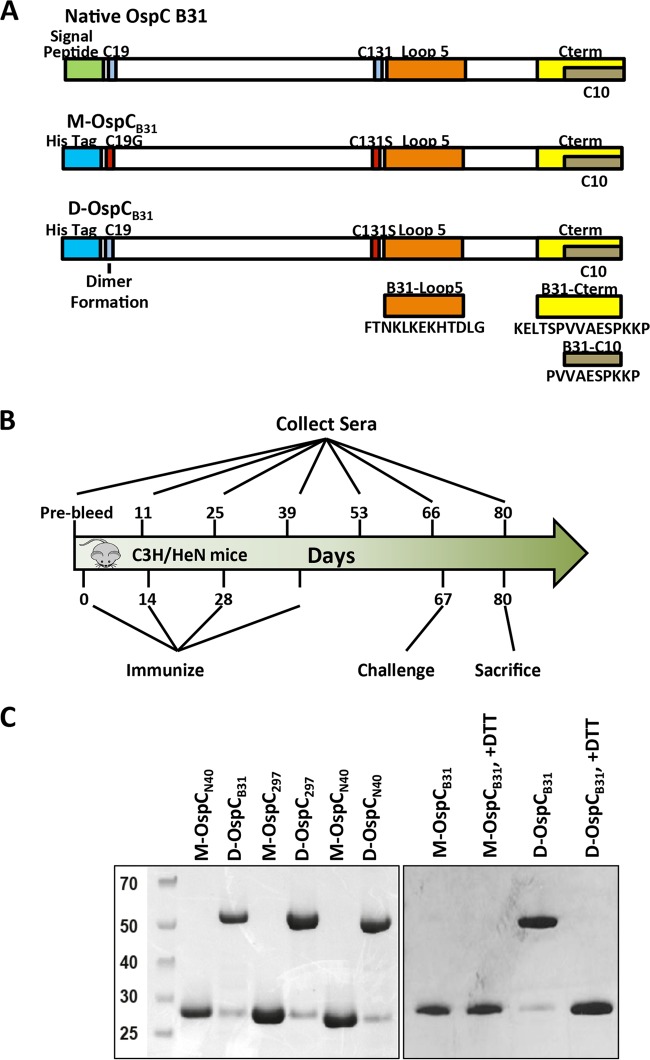
We constructed six different expression vectors to produce OspC variants from several Borrelia burgdorferi strains as monomers or as dimers. In all cases, sequences encoding the lipoprotein leader (amino acids 1 to 18) were omitted from the recombinant sequence, resulting in a soluble cytoplasmically expressed protein product. An N-terminal octahistidine tag was added to aid in protein purification. Three recombinant constructs lacked any cysteine residues and therefore were predicted to form monomers (M-OspCB31, monomeric recombinant OspC from B. burgdorferi strain 297 [M-OspC297], and monomeric recombinant OspC from B. burgdorferi strain N40 [M-OspCN40]). An additional three recombinant forms (D-OspCB31, D-OspC297, and D-OspCN40) contained cysteines at position 19 and were predicted to form dimers, based on prior results (40). Details of the vector construction and protein expression and purification are described in the supplemental material. Mass spectrometry verified the identity and purity of the prepared recombinant OspC variants (data not shown).
In nonreducing SDS-PAGE, M-OspCB31, M-OspC297, and M-OspCN40 showed the same migration behavior as in reducing SDS-PAGE and migrated with apparent molecular masses in the range of 25 to 30 kDa. In contrast, the OspC variants with intact Cys19 migrated more slowly in nonreducing gels and produced 50- to 60-kDa bands (Fig. 1C, left panel). Less than 5% of the total protein of the dimer constructs migrated, with an Mr of 25,000 to 30,000, as calculated from densitometric analysis of nonreducing gels. Individually, their apparent molecular masses were twice those observed under reducing/alkylating conditions, in agreement with the formation of disulfide-bridged dimers. The 50- to 60-kDa bands could be reduced to 25 to 30 kDa by heating the sample with reducing agents prior to electrophoresis (Fig. 1C, right panel).
FIG 1.
Antigens and a representative immunization protocol used in this study. (A) Schematic of the recombinant monomeric and dimeric OspC proteins. OspC from strain B31 is given as an example. The signal peptide (amino acids [aa] 1 to 18) is replaced with an oligohistidine tag. Monomeric OspC also carries a C19G mutation. Both monomeric and dimeric variants carry a C→S mutation of the central cysteine, thereby preventing aggregation. Peptides B31loop5 and B31Cterm are indicated in orange and yellow, respectively. (B) Schematic overview of a representative immunization schedule. Mice were immunized up to 4 times using up to 100 μg of protein per injection with adjuvant (typically alum). Adjuvant only was used as a control. Sera were sampled before the initial immunization, ∼12 days after each immunization, on the day of challenge, and on the day of sacrifice. Challenge was done either by subcutaneous injection of in vitro-cultured B. burgdorferi (needle inoculation) or by applying B. burgdorferi B31-infected I. scapularis ticks onto each individually caged mouse for a period of 14 days. (C) SDS-PAGE of monomeric and dimeric recombinant OspC proteins, showing dimerization mediated by Cys19. Left panel, SDS-PAGE of M-OspCB31, D-OspCB31, M-OspC297, D-OspC297, M-OspCN40, and D-OspCN40 in the absence of dithiothreitol (DTT) reducing agent. Right panel, SDS-PAGE migration of recombinant B31 OspC proteins in the presence and absence of DTT. Each lane contains 1 μg of purified recombinant protein. M, molecular mass (in kilodaltons) ladder; M-OspCB31, monomeric OspC of strain B31; D-OspCB31, dimeric OspC of strain B31; M-OspC297, monomeric OspC of strain 297; D-OspC297, dimeric OspC of strain 297; M-OspCN40, monomeric OspC of strain N40; and D-OspCN40, dimeric OspC of strain N40.
To determine the size distribution of OspC variants in solution, M-OspCB31 and D-OspCB31 were also analyzed by dynamic light scattering in reducing and nonreducing buffer in the presence of various amounts of sodium chloride (Fig. S1). In all cases, a model for globular proteins was applied. The hydrodynamic radius (HR) of M-OspCB31 was calculated as ∼5 nm under all conditions. D-OspCB31 showed a hydrodynamic radius of >10 nm under nonreducing conditions in buffers of low ionic strength (≤150 mmol/liter NaCl). However, the HR of D-OspCB31 was reduced to ∼5 nm under reducing conditions or when the NaCl concentration was ≥500 mmol/liter, indicating that D-OspCB31 is capable of forming multimeric structures not observed for M-OspCB31.
Immunization with dimeric OspC protects mice from B. burgdorferi challenge better than immunization with monomeric OspC.
Since previous experiments indicated that dimerized OspC was more immunogenic than monomeric OspC (40), we undertook a series of immunization and challenge experiments to determine whether dimeric OspC could protect against B. burgdorferi challenge better than monomeric OspC. A total of nine experiments were performed that tested various parameters of protection: required dosage, effect of adjuvants, number of required immunizations, lot-to-lot variability, tick challenge, and cross-strain protection. In most experiments, alum was used as the adjuvant, and 4 immunizations per animal were used. A summary of all the experiments is shown in Table 1.
TABLE 1.
Relative protection of mice against Borrelia infection through immunization with dimeric and monomeric recombinant OspC constructs
| Antigen | Challenge | No. of animals | Dosage (μg) | No. of immunizations | No. infected/total no. | % protection |
|---|---|---|---|---|---|---|
| Summary, experiments with B31 immunization and challenge | ||||||
| Alum only | B31 | 27 | 0 | 4 | 27/27 | 0 |
| Alum + OspC-DB31 | B31 | 63 | 0.1–100 | 4 | 2/60 | 97 |
| Alum + OspC-MB31 | B31 | 45 | 0.1–100 | 4 | 32/39 | 18 |
| MPL only | B31 | 3 | 0 | 3 | 3/3 | 0 |
| MPL + OspC-DB31 | B31 | 6 | 1 | 3 | 0/6 | 100 |
| MPL + OspC-MB31 | B31 | 6 | 1 | 3 | 3/6 | 50 |
| Experiment 1, antigen dosage effects | ||||||
| Alum only | B31 | 6 | 0 | 4 | 6/6 | 0 |
| Alum + OspC-DB31 | B31 | 6 | 100 | 4 | 0/6 | 100 |
| Alum + OspC-DB31 | B31 | 6 | 30 | 4 | 0/6 | 100 |
| Alum + OspC-DB31 | B31 | 6 | 10 | 4 | 0/6 | 100 |
| Alum + OspC-M31 | B31 | 6 | 100 | 4 | 5/6 | 17 |
| Alum + OspC-MB31 | B31 | 6 | 30 | 4 | 5/6 | 17 |
| Alum + OspC-MB31 | B31 | 6 | 10 | 4 | 4/6 | 33 |
| Experiment 2, antigen dosage effects | ||||||
| Alum only | B31 | 6 | 0 | 4 | 6/6 | 0 |
| Alum + OspC-DB31 | B31 | 6 | 10 | 4 | 1/6 | 83 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 4 | 0/6 | 100 |
| Alum + OspC-DB31 | B31 | 6 | 0.1 | 4 | 1/6 | 83 |
| Alum + OspC-MB31 | B31 | 6 | 100 | 4 | 4/6 | 33 |
| Alum + OspC-MB31 | B31 | 3 | 10 | 4 | 2/3 | 33 |
| Alum + OspC-MB31 | B31 | 3 | 1 | 4 | 3/3 | 0 |
| Alum + OspC-MB31 | B31 | 3 | 0.1 | 4 | 3/3 | 0 |
| Experiment 3, no. of immunizations | ||||||
| Alum only | B31 | 3 | 0 | 4 | 3/3 | 0 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 4 | 0/6 | 100 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 3 | 1/6 | 83 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 2 | 2/6 | 67 |
| Alum + OspC-MB31 | B31 | 6 | 1 | 4 | 6/6 | 0 |
| Experiment 4, no. of immunizations | ||||||
| Alum only | B31 | 3 | 0 | 4 | 3/3 | 0 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 4 | 0/6 | 100 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 3 | 1/6 | 83 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 2 | 4/6 | 33 |
| Experiment 5, cross-strain challenge | ||||||
| Alum only | B31 | 3 | 0 | 4 | 3/3 | 0 |
| Alum + OspC-DB31 | B31 | 6 | 1 | 4 | 0/6 | 100 |
| Alum only | N40 | 6 | 0 | 4 | 6/6 | 0 |
| Alum + OspC-DB31 | N40 | 6 | 1 | 4 | 6/6 | 0 |
| Alum only | 297 | 6 | 0 | 4 | 6/6 | 0 |
| Alum + OspC-DB31 | 297 | 6 | 1 | 4 | 6/6 | 0 |
| Experiment 6, different lots of recombinant proteins | ||||||
| Alum only | B31 | 3 | 1 | 4 | 3/3 | 0 |
| Alum + OspC-DB31 (lot 1) | B31 | 3 | 1 | 4 | 0/3 | 100 |
| Alum + OspC-DB31 (lot 2) | B31 | 3 | 1 | 4 | 0/3 | 100 |
| Alum + OspC-DB31 (lot 3) | B31 | 3 | 1 | 4 | 0/3 | 100 |
| Alum + OspC-MB31 (lot 2) | B31 | 3 | 1 | 4 | 3/3 | 0 |
| Alum + OspC-MB31 (lot 3) | B31 | 3 | 1 | 4 | 1/3 | 67 |
| Experiment 7, OspC from different strains | ||||||
| Alum only | 297 | 3 | 0 | 4 | 3/3 | 0 |
| Alum + OspC-D297 | 297 | 6 | 1 | 4 | 0/6 | 100 |
| Alum + OspC-M297 | 297 | 6 | 1 | 4 | 5/6 | 17 |
| Alum only | N40 | 3 | 0 | 4 | 3/3 | 0 |
| Alum + OspC-DN40 | N40 | 6 | 1 | 4 | 0/6 | 100 |
| Alum + OspC-MN40 | N40 | 6 | 1 | 4 | 0/6 | 100 |
| Experiment 8, Cross-strain challenge, MPL adjuvant, and reduced dimer immunization | ||||||
| MPL only | B31 | 3 | 1 | 3 | 3/3 | 0 |
| MPL + OspC-MB31 | B31 | 6 | 1 | 3 | 3/6 | 50 |
| MPL + OspC-DB31 | B31 | 6 | 1 | 3 | 0/6 | 100 |
| MPL + OspC-DB31 | B31 | 6 | 1 | 2 | 2/6 | 67 |
| MPL + OspC-DN40 | B31 | 3 | 1 | 3 | 3/3 | 0 |
| None | 297 | 3 | 1 | 3 | 3/3 | 0 |
| MPL + OspC-DN40 | 297 | 3 | 1 | 3 | 2/3 | 33 |
| MPL only | N40 | 3 | 1 | 3 | 3/3 | 0 |
| MPL + OspC-DN40 | N40 | 3 | 1 | 3 | 0/3 | 100 |
An initial needle inoculation experiment indicated that high doses of M-OspCB31 (10 to 100 μg per immunization) could protect some immunized mice (33%) from B. burgdorferi infection, but protection was not observed when doses lower than 100 μg per immunization were used (Tables 1 and 2, experiments 1 and 2). In contrast, D-OspCB31 protected challenged animals with immunization doses as low as 0.1 μg (Tables 1 and 2, experiments 1 and 2). When large doses of antigen were used (10 μg or more of either recombinant OspC), we observed a nodular reaction at the site of immunization. This reaction was diminished or absent when lower doses of antigen (0.1 μg to 1.0 μg) were used. Since 1 μg per immunization of D-OspCB31 routinely protected all animals, we chose this dose for subsequent immunizations. No variation in protection was seen between different lots of OspC proteins (Table 1, experiment 6). Fisher's exact test was used to examine protection bias between animals immunized with 1-μg doses of D-OspCB31 or M-OspCB31. The result was significant at a P value of <0.01.
TABLE 2.
Protection obtained by immunization with dimeric OspC at clinically relevant dosages
| Group | No. of cultures positive/total no. |
No. of mice positive/total no. | % mice protected | |||||
|---|---|---|---|---|---|---|---|---|
| Skin | Ear | Joint | Bladder | Heart | All sites | |||
| Alum only | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 30/30 | 6/6 | 0 |
| 0.1 μg D-OspCB31 + alum | 1/6 | 1/6 | 0/6 | 1/6 | 1/6 | 4/30 | 1/6 | 83 |
| 1.0 μg D-OspCB31 + alum | 0/6 | 0/6 | 0/6 | 0/6 | 0/6 | 0/30 | 0/6 | 100 |
| 10 μg D-OspCB31 + alum | 1/6 | 0/6 | 1/6 | 1/6 | 1/6 | 4/30 | 1/6 | 83 |
| 0.1 μg M-OspCB31 + alum | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 15/15 | 3/3 | 0 |
| 1.0 μg M-OspCB31 + alum | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 15/15 | 3/3 | 0 |
| 10 μg M-OspCB31 + alum | 2/3 | 2/3 | 2/3 | 2/3 | 2/3 | 10/15 | 2/3 | 33 |
| 100 μg M-OspCB31 + alum | 4/6 | 4/6 | 4/6 | 4/6 | 4/6 | 20/30 | 4/6 | 33 |
We also tested the ability of the recombinant monomeric and dimeric OspCs to protect against tick challenge (Table 3). Immunization with M-OspCB31 failed to protect mice from B. burgdorferi infection via tick bite. However, as with needle inoculation, D-OspCB31 protected all tick-inoculated mice (10/10) from infection.
TABLE 3.
Immunization with dimeric OspC protects mice against tick challenge
| Challenge | Group | No. of mice infected/total no. | No. of tissues infected/total no.a | % mice protected |
|---|---|---|---|---|
| Needle inoculation with B31 5A4 | Alum | 12/12 | 57/60 | 0 |
| M-OspCB31 + alum | 12/12 | 56/60 | 0 | |
| D-OspCB31 + alum | 0/12 | 0/60 | 100 | |
| Bite of B31-infected ticks | Alum | 9/11b | 36/44 | 20 |
| M-OspCB31 + alum | 5/6b | 20/24 | 17 | |
| D-OspCB31 + alum | 0/10b | 0/40 | 100 |
Ear, joint, bladder, and heart tissues were analyzed for the presence of viable Borrelia bacteria in all animals as well as skin from the injection site of needle-inoculated animals.
Mice for which no Borrelia-infected fed ticks could be detected after challenge were disregarded.
OspC amino acid sequences vary considerably, with 63 to 90% sequence identity among different LB species and strains (29, 41). To determine whether the dimerization of OspC from other strains also enhanced protective immunogenicity, we immunized animals with monomeric or dimeric OspC variants derived from B. burgdorferi strains 297 and N40 (recombinant proteins M-OspC297, M-OspCN40, D-OspC297, and D-OspCN40). Similar to D-OspCB31, D-OspC297 provided superior protection against the cognate challenge strain, 297, compared to M-OspC297 (Table 1, experiments 7 and 8). All animals immunized with D-OspC297 were protected from 297 challenge, while most animals (5/6) immunized with M-OspC297 became infected in response to 297 challenge. In the case of OspCN40, immunization with either monomeric or dimeric OspCN40 protected against needle challenge with the B. burgdorferi N40. Thus, in cases where monomeric OspC was unable to protect from B. burgdorferi challenge, dimerization improved protection.
We also tested different immunization protocols to determine the effect of reducing the number of immunizations or using different adjuvants. A reduction in the number of immunizations from four injections to three injections with 1 μg of D-OspCB31 led to protection in 10 of 12 mice (83%). Two injections did not induce reliable protection (6 of 12 animals protected) (Table 1, experiments 3 and 4).
We also tested monophosphoryl lipid A (MPL) as an adjuvant, because it has been reported to be more effective than alum in promoting T-cell differentiation (42). Using the standard dose of antigen (1 μg), we tested protection from B31 challenge with two or three immunizations of D-OspCB31 (Table 1, experiment 8). Three immunizations gave 100% protection from B. burgdorferi challenge. Two immunizations protected less well (67% protection). Analysis of sera from mice receiving two doses of antigen showed relatively low antibody titers compared to those obtained after three immunizations (data not shown).
The ability of dimeric OspCs to provide protection to challenge by nonhomologous strains varied. Using our standard protocol, immunization with DN40 and D297 led to no protection (0/3) against B. burgdorferi B31 challenge and 33% (2/6) protection against B. burgdorferi 297 challenge. Immunization with D-OspCB31 protected poorly against challenge with either B. burgdorferi 297 (1/6) or against B. burgdorferi N40 (0/6) (Table 1, experiments 5 and 8).
Characterization of antibody response to OspC immunization.
In an effort to understand the difference in protective immunogenicity between monomeric and dimeric forms of recombinant OspC, we characterized the antibody responses of immunized animals. In examining total IgG, all OspC variants produced a strong anti-OspC response (see Fig. 2A for a representative example). While there was significant variability between experiments and among individual animals, immunization with dimeric OspCs generally led to a faster development of endpoint titers than with the monomeric homologues. However, the monomers often produced higher endpoint titers. The specificity of the enzyme-linked immunosorbent assay (ELISA) reactivity against OspC was confirmed by immunoblotting (Fig. 2B, lanes 3 and 5). As expected, infected animals (i.e., positive controls and unprotected vaccinated mice) developed an IgG response against VlsE, p39/BmpA, and p18/DbpA, as determined by immunoblotting (Fig. 2B, lanes 2 and 6 for examples) and ELISA (Table S3). In addition, varied reactions against unidentified antigens occurred in individual animals.
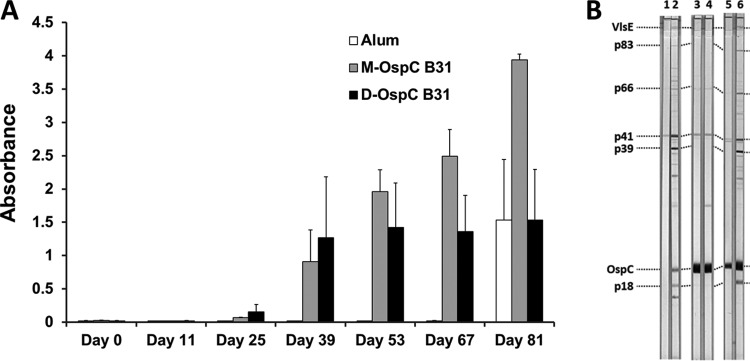
FIG 2.
Mice exhibit a strong differentiated immune response to vaccination with recombinant monomeric and dimeric OspC forms and subsequent Borrelia burgdorferi B31 challenge. (A) ELISA assessment of pan-IgG anti-OspC response to immunization with alum only (white bars), M-OspC31 (gray bars), and D-OspCB31 (black bars). Animals were injected with 1 μg of antigen on days 0, 14, 28, and 42 and were needle inoculated with 104 B. burgdorferi B31 bacteria on day 67; day 81 thus represents a 14-day postchallenge time point. Each group consisted of 6 animals. Sera were tested at a 1:4,000 dilution against dimeric OspC. Mean ± standard error (SE) absorbance is indicated. (B) Immunoblot analysis of murine sera. Sera from immunized and challenged mice were tested for reactivity to various Borrelia antigens using anti-Borrelia Euroline-WB. Immunization was with alum only (lanes 1 and 2), with D-OspCB31 and alum (lanes 3 and 4), and with M-OspCB31 and alum (lanes 5 and 6). Odd lanes (pre) were incubated with sera collected after immunization but prior to Borrelia challenge. Sera incubated in even lanes (post) were collected on day 14 postchallenge. Sera were tested in 1:500 dilutions. Immunization with both forms of OspC resulted in strong prechallenge reactivity with OspC (lanes 3 and 5); nonspecific reactivity to P41 (flagellin) was also observed. After B. burgdorferi challenge, animals immunized with the alum only (strip 2) and M-OspCB31 (strip 6) groups exhibited antibody reactivity to additional B. burgdorferi proteins (VlsE, p39, OspC, and p18) indicative of active infection.
IgG subclass analysis revealed that immunization of mice with either the dimeric or monomeric form of OspCB31 predominantly generated anti-OspC antibodies of subclass IgG1, with minor contributions of IgG2a and IgG2b (Fig. 3 and data not shown). In contrast, infected animals developed a strong IgG3 response to OspC that was absent in uninfected immunized animals (Fig. 3, top panels, and Table S3). In OspC-immunized mice that became infected, antibody titers against OspC increased significantly, while the IgG reactivity remained nearly constant in protected animals (Fig. 2A and 3 top panels, compare prechallenge and postchallenge). A significant difference in antibody reactivity was observed between control animals (alum only) and OspC-immunized mice that were infected upon challenge. OspC-naive mice developed a more pronounced IgG3 response against recombinant OspCs than did infected M-OspCB31-immunized mice (Fig. 3, top panels). Overall, mice immunized with either monomeric or dimeric forms of OspC had predominant IgG1 responses, whereas infected animals exhibited IgG3 responses.
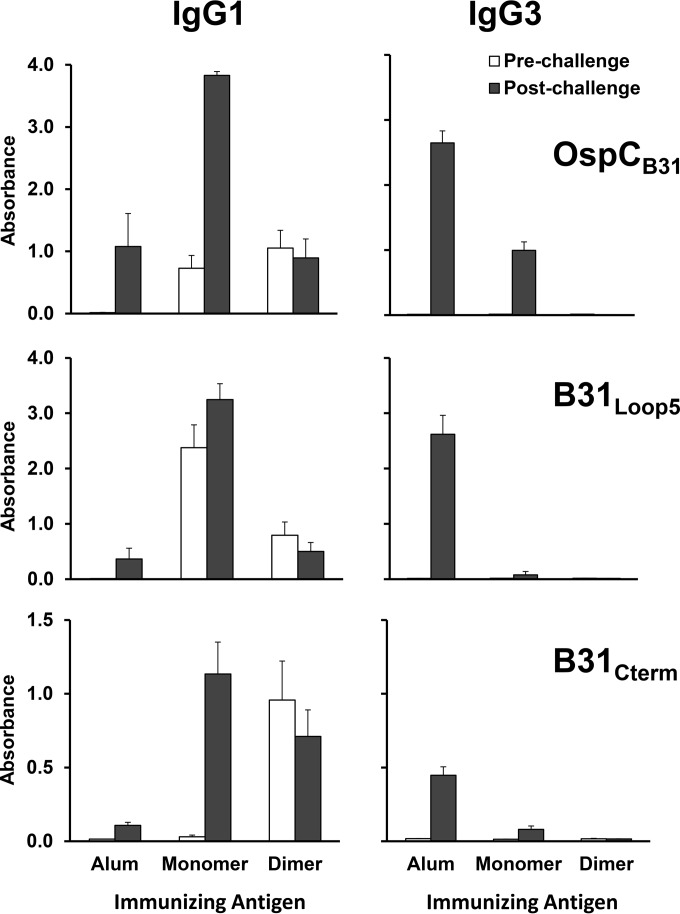
FIG 3.
Animals immunized with OspC can be distinguished from animals with active infection by antibody isotype. IgG1 and IgG3 reactivity after immunization with alum only, M-OspCB31, and D-OspCB31 is shown. All animals were injected with 1 μg at days 0, 14, 28, and 42, needle inoculated with 104 B. burgdorferi B31 bacteria at day 67, and sacrificed at day 81. Each group consisted of 6 animals. Top, IgG1 and IgG3 reactivity against D-OspCB31. Middle, IgG1 and IgG3 reactivity against peptide B31loop5. Bottom, IgG1 and IgG3 reactivity against peptide B31Cterm. Sera were tested at dilutions of 1:4,000 for IgG1 and 1:200 for IgG3. Values represent mean ± SE absorbance.
Liquid-phase inhibition studies were performed to determine the degree of cross-reactivity of anti-OspC antibodies generated through immunization with D-OspCB31 and M-OspCB31. As shown in Fig. S2, preincubation of sera from immunized mice with the antigen used in immunization resulted in nearly complete inhibition of antibody binding to antigen-coated ELISA plates (94% inhibition for D-OspCB31 and 93% for M-OspCB31). In general, cross-absorption (e.g., preincubation of sera from D-OspCB31-immunized animals with M-OspCB31 antigen) resulted in less inhibition. These results indicate that, as expected, antibodies from the immunized animals are absorbed more effectively by the antigen used for immunization. However, sera from dimer-immunized mice preincubated with either the dimeric or monomeric forms of recombinant OspC exhibited nearly equivalent inhibition of binding (83% and 87%) in the M-OspCB31 ELISA (Fig. S2, lower panel, left side), suggesting that D-OspCB31 possesses the epitopes found in M-OspCB31. None of the differences are statistically significant (P > 0.05), so they can be interpreted only as trends.
To determine whether antibodies generated by immunization with an OspC monomer or dimer could recognize OspC from other B. burgdorferi strains, sera from animals immunized with M-OspCB31 and D-OspCB31 were tested by immunoblotting against whole-cell extracts from B. burgdorferi N40 and B. burgdorferi 297. Sera from D-OspCB31-immunized animals recognized a single band of the predicted OspC size in both N40 and 297 whole-cell extracts by immunoblotting (Fig. 4A, left panel). In contrast, sera from M-OspCB31-immunized animals did not yield a detectable reaction with extracts from either N40 or 297 (Fig. 4A, right panel). By ELISA, each serum recognized its cognate OspC ortholog most strongly but also reacted with the other orthologs. Cross-reactivity with heterologous OspCs was varied and reached 10 to 30% of the total IgG reactivity against the immunizing proteins, as determined by ELISA (Fig. 4B).
FIG 4.
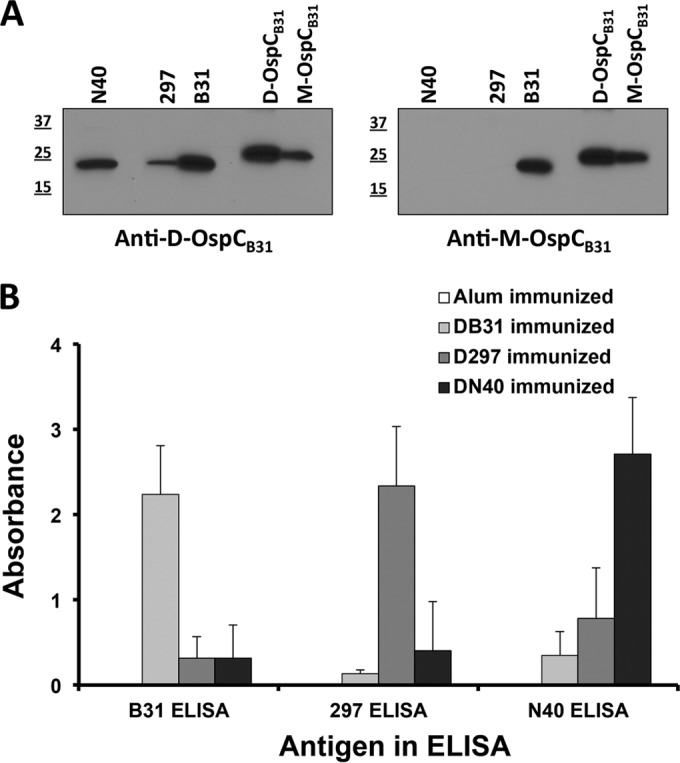
IgG cross-reactivity against recombinant dimeric OspC proteins from different B. burgdorferi strains. (A) Immunoblot analysis of murine sera. Sera from D-OspCB31-immunized (left panel) (pool of sera from two animals) or M-OspCB31-immunized (right panel) (pool of sera from two animals) mice were tested for reactivity to different Borrelia strains. N40, 107 Borrelia burgdorferi N40; 297, 107 Borrelia burgdorferi 297; B31, 107 Borrelia burgdorferi B31, M, molecular mass (in kilodaltons) standards; D-OspCB31, 50 ng recombinant D-OspCB31; M-OspC297, 50 ng recombinant M-OspCB31. (B) Limited IgG cross-reactivity against recombinant dimeric OspC proteins from different B. burgdorferi strains by ELISA. Sera from immunized animals were tested against the indicated antigen: alum only (white, pool of sera from 9 animals), D-OspCB31 (black, pool of sera from 3 animals), D-OspC297 (shaded, pool of sera from 12 animals), and D-OspCN40 (dotted, pool of sera from 12 animals). Bars represent mean ± standard deviation (SD) absorbance.
Reactivity with OspC epitopes.
In an effort to identify protective epitopes, we performed PepScan analysis of sera from five representative animals after immunization with D-OspCB31 (data not shown). All animals possessed high-titer antibodies against the loop 5 peptides FTNKLKEKHTDL and TNKLKEKHTDLG. The loop 5 domain of OspC has been identified as surface exposed and highly antigenic in both humans and mice (43). Three animals also had varied reactivity against the loop 5 overlapping peptides NKLKEKHTDLGK, KLKEKHTDLGKE, LKEKHTDLGKEG, and KEKHTDLGKEGV. Reactivity against other peptides was low and varied in comparison to the reactivity observed for the loop 5 region.
We also tested the sera of all animals by ELISA for reactivity to a synthetic peptide corresponding to the B31loop5 region (FTKNKLKEKHTDLG) and with a second peptide, B31Cterm (KELTSPVVAESPKKP). B31Cterm overlaps the C-terminal 10 amino acids of OspCB31 (C10, PVVAESPKKP), which are well conserved between OspC orthologs and are surface exposed. Antibodies to this region are frequently seen in the sera of early Lyme borreliosis patients and in patients with neuroborreliosis (44, 45). Due to the chemical nature of the peptide linkage in the PepScan, this C-terminal peptide was not well analyzed in the PepScan assay.
Both M-OspCB31 and D-OspCB31 induced high-titer IgG1 against B31loop5, but M-OspCB31-immunized animals exhibited ∼3-fold higher absorbance values (see Fig. 3, middle panels, prechallenge values). In contrast, the absorbance values against B31Cterm were higher in D-OspCB31-immunized mice, although significant variation was found between individual animals (Fig. 3, lower panels, prechallenge values). There was no discernible relationship between prechallenge reactivity with either B31loop5 or B31Cterm and protection against infection. Many D-OspCB31-immunized animals that were protected against infection had low reactivity to either peptide, and conversely, M-OspCB31-immunized animals with high antibody levels to either peptide were frequently not protected from infection (Fig. 5). These data indicate that the protective effect conferred by immunization with D-OspCB31 is not due to the induction of antibodies against these linear epitopes.
FIG 5.
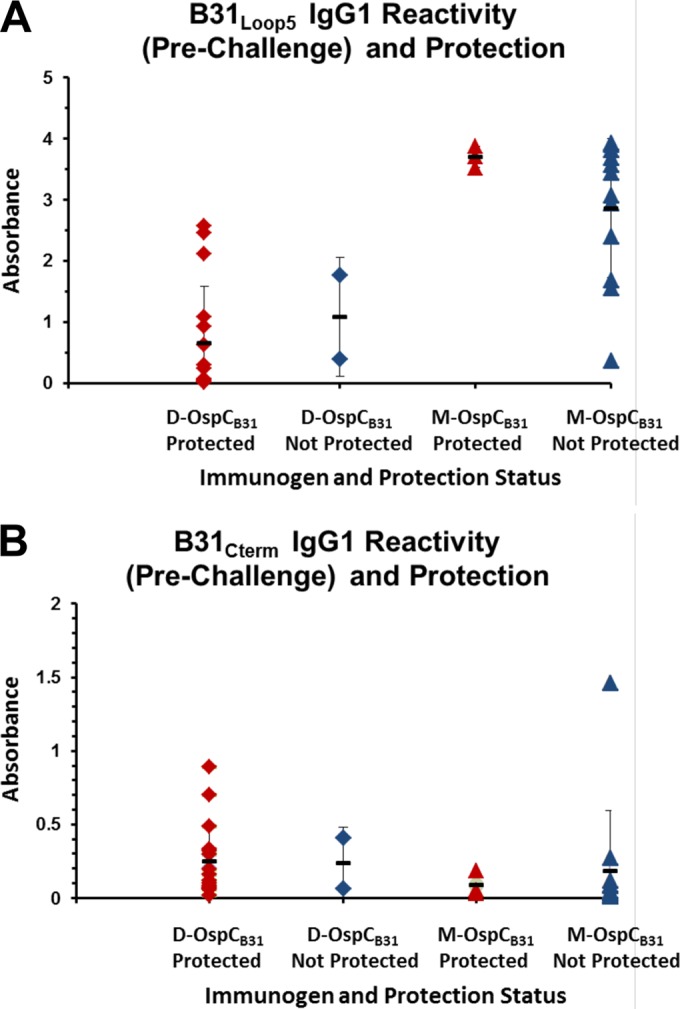
Lack of relationship between serologic reactivity to the OspC peptide B31loop5 or B31Cterm and protection against infection with B. burgdorferi B31. Data points indicate the ELISA absorbance obtained for B31loop5 (A) and B31Cterm (B) in prechallenge sera from individual mice protected against infection (red symbols; no cultures positive) and not protected (blue symbols; one or more cultures positive). Diamonds, animals immunized with D-OspCB31; triangles, animals immunized with M-OspCB31.
To further examine the relationship between the epitopes represented by B31loop5 and B31Cterm and protective immunity, the correlation between ELISA reactivity of sera from immunized mice with these peptides and D-OspCB31 or M-OspCB31 was determined. B31Cterm binding did not correlate well with binding of antibodies to either antigen (R2 = 0.02 to 0.17; data not shown). The ELISA reactivity to B31loop5 of sera from mice immunized with either D-OspCB31 or M-OspCB31 correlated well with reactivity to M-OspCB31 (R2 = 0.7044 to 0.7615) (Fig. S3C and D). When sera from D-OspCB31 mice were examined (Fig. S3B), there was a poor correlation between reactivity to the dimer form of the antigen and the B31loop5 (R2 = 0.117). In contrast, the correlation of reactivities was high when reactivity of sera from M-OspCB31 mice with the dimer form and the B31loop5 were compared (Fig. S3A; R2 = 0.7257). These results again support the concept that D-OspCB31 contains additional epitopes that contribute substantially to the protective antibody response.
Characterization of monoclonal antibodies.
We speculated that we might be able to generate monoclonal antibodies to the unique epitopes in D-OspCB31 and that these antibodies might passively protect mice from B. burgdorferi challenge. Thus, we immunized four mice with D-OspCB31 using the standard protocol. As expected, all mice developed a strong antibody response to D-OspCB31, M-OspCB31, B31loop5, and B31Cterm at the end of the 56-day immunization protocol (data not shown). Two mice were randomly chosen for spleen fusion. Of the resulting 1,572 hybridoma cultures, 36 cultures produced supernatants that reacted strongly with D-OspCB31 and/or M-OspCB31 and expressed antibodies of the IgG1 subclass. Four clones were selected for further study: 1-5A10, 1-8B7, 2-2H8, and 2-8C10. Clones 1-5A10, 1-8B7, and 2-8C10 reacted strongly with D-OspCB31 and only weakly with M-OspCB31 (Table S4). Clone 2-2H8 reacted equally well with D-OspCB31 and M-OspCB31. Similar results were obtained in the immunoblot analyses. After nonreducing SDS-PAGE, clones 1-5A10, 1-8B7, and 2-8C10 reacted with the putative dimeric OspC (50- to 60-kDa form) more strongly than with the smaller 25- to 30-kDa monomeric form of OspC. Conversely, immunoblots after reducing/alkylating SDS-PAGE showed equal reactivity for all 4 clones (data not shown). Only clone 2-2H8 showed reactivity against the B31loop5 peptide (Table S4).
To determine whether clones 1-5A10, 1-8B7, and 2-8C10 recognized the same epitope, we performed a cross-reaction analysis. After incubation with one of the monoclonal antibodies, D-OspCB31-coated ELISA plates were incubated with a second biotinylated monoclonal antibody and binding was detected with streptavidin-horseradish peroxidase (HRP). We found that clones 1-5A10, 1-8B7, and 2-8C10 inhibited binding of each other, indicating that they recognize the same or nearby epitopes (data not shown). Clone 2-2H8 was neither inhibited by, nor inhibited, the binding of the other three clones. Likewise, sera collected after immunization with D-OspCB31 inhibited the binding of 1-5-A10, 1-8-B7, and 2-8-C10 to a greater extent than binding of 2-2H8 was inhibited (data not shown). Similarly, monoclonal 2-2H8 was inhibited to a greater extent by sera from M-OspCB31-immunized mice. These data indicate that the three monoclonal antibodies 1-5A10, 1-8B7, and 2-8C10 may recognize a unique epitope present or revealed in D-OspCB31.
Passive protection with monoclonal antibodies.
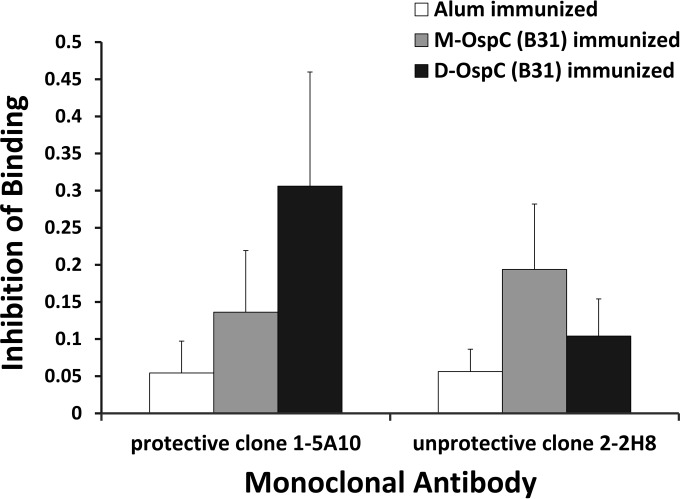
To investigate whether the monoclonal antibodies 1-5-A10, 1-8-B7, and 2-8-C10 that recognize unique D-OspCB31 epitopes could protect against B. burgdorferi challenge, we performed a passive immunization experiment. Mice were injected with each one of the monoclonal antibodies individually, normal mouse serum, serum from D-OspCB31-immunized animals, or serum from M-OspCB31-immunized animals and then challenged with B. burgdorferi B31. We found that monoclonal antibodies 1-5-A10, 1-8-B7, 2-8-C10, and serum from D-OspCB31-immunized mice protected mice from challenge (Table 4; Fig. 6). In contrast, monoclonal antibody 2-2-H8 that recognizes the loop 5 epitope and serum from M-OspCB31-immunized mice were not able to protect mice from infection. ELISA using sera from passively immunized mice demonstrated that all mice still had significant levels of anti-OspC reactivity (data not shown). Taken together, these results demonstrate that unique antibodies that protect against B. burgdorferi challenge can be generated by immunization with dimeric OspC and suggest that D-OspCB31 contains distinctive protective epitopes.
TABLE 4.
Protection against B. burgdorferi challenge by passive immunization with monoclonal antibodies
| Antibody source | No. positive/total no. |
No. of mice positive/total no. | % mice protected | |||||
|---|---|---|---|---|---|---|---|---|
| Skin | Joint | Heart | Bladder | Ear | All sites | |||
| Normal mouse serum | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 15/15 | 3/3 | 0 |
| 1-5A10 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/25 | 0/5 | 100 |
| 1-8B7 | 0/5 | 1/5a | 0/5 | 0/5 | 0/5 | 1/25 | 1/5 | 80 |
| 2-8C10 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/25 | 0/5 | 100 |
| 2-2H8 | 5/5 | 3/3 | 5/5 | 5/5 | 5/5 | 23/23 | 5/5 | 0 |
| Serum pool M-OspCB31 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 15/15 | 3/3 | 0 |
| Serum pool D-OspCB31 | 1/5 | 1/5 | 1/5 | 1/5 | 1/5 | 5/25 | 1/5 | 80 |
Culture did not grow out, but spirochetes were observed in one field on day 7 attached to a fragment of tissue debris.
FIG 6.
Murine sera from protected D-OspCB31-immunized animals inhibited binding of monoclonal antibodies protective against B. burgdorferi B31 challenge. Sera from mice immunized with alum only, D-OspCB31, or M-OspCB31 were used in ELISA to compete for binding with biotinylated monoclonal antibodies 1-5A10 and 2-2H8, and the proportion of inhibition was plotted. The antigen on the ELISA plates was D-OspCB31. Sera were from mice immunized with alum only (white), M-OspCB31 (gray), or D-OspCB31 (black). Clone 1-5A10 was protective against B. burgdorferi challenge. Clone 2-2H8 reacted with the loop 5 epitope and was not protective. Each group consisted of 12 animals. Bars represent the mean ± SD proportion of inhibition.
DISCUSSION
OspC is expressed at high levels during the first few days to weeks of B. burgdorferi infection and is a highly immunogenic lipoprotein (24, 37, 46, 47). These properties indicate that OspC is potentially useful as a vaccine antigen. Indeed, prior reports had indicated that OspC has immunoprotective activity, but the protective activity is allele specific (34, 37, 48). This limitation is important, in that OspC sequences in different B. burgdorferi sensu lato strains are heterogeneous, with a degree of amino acid sequence variability that is second only to that of the antigenic variation protein VlsE (49). Probst et al. (40) previously showed that dimerization of OspC through an N-terminal disulfide bridge greatly enhanced reactivity with serum antibodies from humans with Lyme borreliosis and increased its ability to induce anti-OspC antibody responses in experimentally vaccinated animals.
The current study demonstrates that vaccination with the dimer form of recombinant OspC was much more effective than the monomeric form in inducing protective immunity in a mouse model. We used alum as an adjuvant because of its compatibility with use in humans. High dosages of 10 to 100 μg were utilized initially. Even at these high doses, monomeric OspC was only partially protective against B. burgdorferi B31 infection, whereas the dimeric form was uniformly protective (Table 1). We found that D-OspCB31 at doses as low as 0.1 to 1.0 μg was consistently effective and that three to four immunizations were required for full efficacy. In addition, the nodular reaction observed at the site of immunization was diminished or absent when lower doses of antigen (0.1 μg to 1.0 μg) were used. This reaction has been previously described when using aluminum salts as adjuvants (50) Three immunizations were still needed if monophosphoryl lipid A adjuvant was utilized instead of alum. However, it is possible that the poor protection observed with two immunizations could be due to the amount of time available for antibody response to develop (28 days for the two-immunization protocol versus 42 days for the three-immunization procedure) rather than number of immunizations.
Previous studies (34, 51) found that a single injection of E. coli lysate containing recombinant OspC or a two-immunization protocol of 100 μg recombinant OspC was sufficient for protection against B. burgdorferi challenge. Our studies required three immunizations for full protection but used much lower immunogen doses. In addition, Gilmore et al. (51) found that, as antibody titer waned over a 1-year period, the mice were no longer protected from challenge. Our studies did not test whether a protective anamnestic response was elicited by dimeric OspC. However, since our immunogen and immunization protocol differed significantly from these authors, examination of long-term immunity induced by dimeric OspC is an important next step to explore its vaccine potential.
Similar results were obtained in terms of immunoprotection against the homologous strain when recombinant forms of OspC297 were used, i.e., the disulfide-linked dimeric form was much more effective in protecting against infection than was the monomeric form. For OspCN40, both forms of recombinant protein were effective in protecting mice from B. burgdorferi infection.
Interestingly, while D-OspCB31 generated antibodies that recognized OspC from B. burgdorferi 297 and N40 relatively weakly in ELISA (Fig. 4B), anti-D-OspCB31 (but not anti-M-OspCB31) detected native OspC297 and OspCN40 well on the Western blot (Fig. 4A). However, despite this recognition and the efficacy of low doses of D-OspCB31 against homologous challenge, the B31 antigen did not cross-protect against infection the B. burgdorferi strains 297 and N40. This pattern is consistent with the heterogeneity of OspC and the OspC cross-protection results obtained in prior studies (35, 37). However, D-OspCN40 did provide partial protection from B. burgdorferi 297 challenge and B31 challenge (2 of 6 animals protected in each case; Table 1). This result was unexpected, in that cross-protection was not obtained in prior OspC immunization studies. In fact, a lack of homologous protection using immunization with OspCN40 had been observed in a prior study by Bockenstedt et al. (37). This discrepancy may have resulted from the fact that the initial culture of N40 contained two different clones, which may differ in their immunologic properties; the clone we utilized is called cN40 (52). The unusual cross-protective activity of D-OspCN40 may be due to the presence of immunodominant epitopes that exhibit epitope-spreading properties. This interesting and potentially important observation will require further study to clarify the mechanism.
Ivanova et al. (53) showed that recombinant proteins of five specific OspC types (B, E, F, I, and K) could detect anti-OspC antibody from the serum of mice infected with any one of 15 different strains of B. burgdorferi, each of a different OspC type. The N40 OspC is of the E type, while the B31 OspC is of the A type, and the 297 OspC is of the I type (54). It is interesting to note that the partial cross-protection is observed from one of the broadly reactive OspC types.
Importantly, mice immunized with D-OspCB31 were also protected against tick-transmitted infection with B. burgdorferi B31 (Table 3), indicating that this immunization protocol is effective in preventing infection via the natural route of transmission. Further experiments with this model are ongoing and will explore the use of OspC mixtures to overcome the current limitations regarding cross-protection.
Several parameters were examined in an attempt to determine the mechanisms of heightened protection by the N-terminal dimerized form of OspC. Immunization with the monomeric and dimeric forms of OspC was found to yield similar levels of IgG subtypes, with a predominance of IgG1. In some experiments, higher total IgG or IgG1 levels were obtained with the monomeric antigen, yet the monomer-immunized mice were generally not protected against infection. Thus, the immunoprotective activity of the N-terminal dimerized OspC was not simply due to higher antibody levels or the induction of IgG1 anti-OspC antibodies. Binding inhibition studies indicated that the dimeric form of OspC presented at least one unique epitope that was not bound by antibodies induced by the monomeric form. In contrast to prior immunizations performed without adjuvant (40), sera from animals immunized with either D-OspC or M-OspC and the adjuvant alum have barely detectable levels of IgG3. In contrast, infection of unprotected mice resulted in induction of high levels of anti-OspC IgG3 (Fig. 3, upper panel). We speculate that infection and adjuvant-facilitated immunization result in differential stimulation of T cell activities and thus cytokine profiles, thereby resulting in altered patterns of IgG isotype switching. It is conceivable that infection with B. burgdorferi may drive the maturation of the anti-OspC-producing B lymphocytes weakly and lead to an arrest at the first IgG subclass, IgG3; indeed, OspC is expressed only during the first few weeks of mammalian infection (55, 56), which may limit immune maturation. Interestingly, the Baumgarth laboratory (57, 58) found that infection with B. burgdorferi leads to a strong T-independent B cell response with short-lived germinal centers and production of anti-Borrelial antibodies primarily of the IgM isotype and very few of the IgG1 isotype. These results led them to speculate that B. burgdorferi evades clearance by altering the normal B cell response in the lymph nodes (57, 58). In any case, this differential pattern of isotype reactivity allows infected animals to be distinguished from immunized animals.
Perhaps the most revealing studies were those in which the reactivity and protective activity of monoclonal antibodies were generated by immunization of mice with D-OspCB31. Hybridomas were selected that produced IgG1 antibodies reacting strongly with the immunizing antigen. The resulting monoclonal antibodies had differing reactivity with M-OspCB31. Remarkably, three monoclonal antibodies that reacted strongly with D-OspCB31 but weakly with M-OspCB31 provided complete passive protection against infection of mice with the B31 strain, whereas the fourth monoclonal antibody that reacted strongly with both recombinant OspC proteins did not provide protection. These results support the concept that the antibodies to unique epitopes presented by D-OspCB31 are responsible for this protective activity. We hypothesize that these highly protective epitopes are present at the interface in this recombinant dimer form and mimic naturally occurring epitopes that are present in the OspC dimer on the cell surface of Lyme disease Borrelia.
The protective monoclonal antibodies exhibited a pattern of OspC peptide binding that is consistent with this hypothesis (Fig. 5). The B31loop5 peptide strongly inhibited binding of the nonprotective monoclonal antibody 2-2-H8 to the D-OspCB31 protein, indicating that this monoclonal antibody recognizes an epitope present in this peptide. However, none of the protective monoclonal antibodies exhibited detectable inhibition by this peptide. Therefore, these antibodies likely do not react with loop 5 in the OspC structure. Additionally, the monoclonal antibodies examined did not exhibit inhibition by a peptide corresponding to the C terminus, which has been implicated as a potentially protective antigen. It is of interest, however, that the C-terminal region of OspC is not required for tick transmission or mouse infection (59). The amino acid region(s) of OspC that forms the protective epitope(s) recognized by the passively protecting monoclonal antibodies has yet to be identified. Another future area for research would be to determine whether the protective monoclonal antibodies are OspC allele specific (as expected) or are able to cross-protect against non-B31 strains.
The enhanced protective immunogenicity of N-terminally dimerized forms of OspC provides some optimism that OspC constructs may be useful in vaccines against Lyme borreliosis. The artificial dimerization used here may stabilize a tertiary structure that contains protective epitopes found in the native OspC as expressed on the cell surface of B. burgdorferi. These epitopes may consist of amino acids from both OspC polypeptides (i.e., part of the interface of the dimerized proteins).
Alternatively, these dimer-specific epitopes could result from changes in the secondary structure of the two polypeptide chains arising from the protein-protein interaction; however, such secondary structure changes would be expected to be minor, given that the circular dichroism profiles of the monomer and dimer forms of these recombinant constructs are nearly identical (40).
Interestingly, OspC is believed to exist as a homodimer in vivo, and this interaction results in increased resistance to protease digestion (60). Crystal structures of OspC show that dimerization is mediated by interactions between alpha helices in each subunit (61, 62). In addition, dimerization results in the formation of a putative ligand binding pocket (61, 62) that contains some amino acid residues conserved across OspC phyletic types (63). The ligand binding pocket and one of the conserved residues within the pocket are required for infectivity (23). Possibly, the covalent dimerization stabilizes these important epitopes and thereby facilitates the production of protective antibodies.
Allele specificity still occurs with the disulfide-dimerized OspC constructs, but the results obtained with D-OspCN40 immunization suggest that the enhanced immunoprotective activity obtained may partially overcome this barrier and that specific OspC types may be able to provide some degree of cross-protection. In addition, given the lower quantity of antigen required, immunization with N-terminally dimerized OspC antigens corresponding with multiple different OspC alleles may be a practical approach to future Lyme disease vaccine development.
MATERIALS AND METHODS
Ethics statement.
All procedures involving mice were reviewed for effective experimental design and the humane treatment of animals and approved by the Animal Welfare Committee of The University of Texas Health Science Center at Houston or the Animal Use and Care Committee of the Division of Vector Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), CDC.
Bacterial strains.
The infectious B. burgdorferi strains B31-5A4 (64), N40 (52), and 297 (65) were used for animal studies. The N40 strain was the kind gift of J. D. Radolf, University of Connecticut Health Science Center, Farmington, CT, and the 297 strain was the gift of P. A. Rosa, Rocky Mountain Laboratories, Hamilton, MT. All B. burgdorferi strains were cultured in Barbour-Stoenner-Kelly II (BSK-II) medium at 34°C in 3% CO2, as previously described (64). Cultures used in this study had undergone no more than two passages since thawing or clone isolation, thus minimizing the likelihood of plasmid loss. Infectivity in mice by needle inoculation was verified prior to the use of the strains in immunization studies.
Vector construction and production of recombinant proteins.
Procedures for the construction of expression vectors and for expression and purification of recombinant proteins are described in the supplemental material. The PCR primers used for generating cloning fragments are described in Table S1. A schematic representation of the dimeric and monomeric forms of OspC utilized can be found in Fig. 1A.
Generation of monoclonal antibodies.
Monoclonal antibodies were produced by immunization of mice with dimeric OspC from B. burgdorferi B31 (D-OspCB31) at Eurogentec, using a standard protocol. Mouse sera and supernatants of hybridoma cultures were screened for the presence of antibodies against D-OspCB31, M-OspCB31 (monomeric OspC from B. burgdorferi B31), and the peptides B31loop5 and B31Cterm by ELISA and immunoblotting. Hybridoma clones were grown in RPMI 1640 medium supplemented with 10% (vol/vol) fetal calf serum, 1% (vol/vol) nonessential amino acids, 1 mmol/liter sodium pyruvate, and 50 μmol/liter 2-mercaptoethanol (Thermo Fisher Scientific, Germany) in standard culture flasks at 37°C and 7.5% CO2. Antibodies from selected clones were purified by protein A-Sepharose affinity chromatography (GE Healthcare, Germany), according to the manufacturer's manual, dialyzed against phosphate-buffered saline (PBS), concentrated to 1 mg/ml, and stored at −80°C until use. For some experiments, the antibodies were biotinylated with the EZ-Link Sulfo-NHS-biotinylation kit (Thermo Fisher Scientific), as per the manufacturer's instructions.
Immunization and challenge of mice.
Female C3H/HeN mice (4 to 6 weeks old) were immunized by subcutaneous injection with up to 100 μg of the recombinant OspC proteins with or without adjuvant. Immediately prior to injection, the antigens were emulsified with either Imject alum (Thermo Fisher Scientific) or 50% (vol/vol) monophosphoryl lipid A (MPL; Sigma), according to the manufacturer's instructions. Booster immunizations were administered at 2-week intervals. The amount of antigen and number of booster immunizations varied in different experiments. A typical immunization scheme is depicted in Fig. 1B. At the end of the immunization schedule, the mice were either needle inoculated with in vitro-cultivated B. burgdorferi (104 cells of strain B31, clone 5A4; 105 cells of strain 297; 105 cells of N40) or challenged by tick bite from B. burgdorferi B31-infected nymphal Ixodes scapularis ticks. B. burgdorferi-infected ticks were generated as previously described (66). In the case of tick challenge, fed ticks were collected, and their infection states were analyzed by PCR. Mice were disregarded for further analysis if none of the ticks that fed on that animal were PCR positive for B. burgdorferi (Table S2).
Serum samples were collected from each animal prior to immunization, before each boost, at challenge, and at sacrifice. The mice were sacrificed at 2 weeks postinfection, and selected tissues (ear, inoculation site, heart, joint, and bladder) were harvested and cultured in BSK-II medium. Cultures were monitored by dark-field microscopy for the presence of spirochetes at 7, 14, and 28 days.
For passive immunization experiments, mice received a 200-μg dose of monoclonal antibody or 0.1 ml of previously frozen preimmune or immune mouse serum intraperitoneally 1 day prior to challenge with B. burgdorferi. Five days after inoculation, animals were given an additional 200-μg dose of monoclonal antibody or a 0.05-ml dose of preimmune or immune serum. Immune sera were derived from pooled serum collected prior to challenge from protected/D-OspCB31-immunized mice or from unprotected/M-OspCB31-immunized mice. Two weeks after challenge, the animals were euthanized. Blood was drawn to test for anti-OspC activity, and tissues were cultured as indicated above.
Immunoassays for the detection of murine antibodies.
Ninety-six-well plates (Nunc, Germany) were coated with 100 μl of the recombinant protein at a concentration of 1 μg/ml in PBS for 2 h at 25°C, washed three times with washing buffer (0.05% [wt/vol] Tween 20 in PBS), and then blocked with blocking buffer (0.1% [wt/vol] casein in PBS) for 1 h. The success of antigen immobilization was confirmed by incubation with a murine monoclonal anti-hexahistidine tag antibody (Sigma-Aldrich, Germany) diluted 1:2,000. Experimental serum samples were diluted in sample buffer (1% [wt/vol] casein, 0.05% [wt/vol] Tween 20 in PBS) and incubated for 30 min at room temperature. After washing three times, bound antibodies were detected by incubation with anti-mouse IgG-HRP conjugate (Jackson Research, UK) diluted 1:2,000 in sample buffer, for 30 min, washed as described above, and incubated with tetramethyl benzidine (TMB) substrate (Euroimmun, Germany) for 15 min. All incubation steps were carried out at room temperature. The optical density (OD) at 450 nm was read using an automated spectrophotometer (Tecan, Germany). The reactivity of murine sera was analyzed for IgG subclasses using the same procedure, except for the use of subclass-specific conjugates diluted 1:10,000 (Jackson Research).
For liquid-phase inhibition experiments, 10 μg/ml recombinant protein was added to the diluted sera 30 min prior to their incubation on the microplate. Percent inhibition was calculated as 100 × [1 − (Abs with inhibitor)/(Abs without inhibitor)] (Abs, absorbance).
For the analysis of antibody reactivity to synthetic peptides (OspC B31loop5 [FTNKLKEKHTDLGK] and OspC B31Cterm [KELTSPVVAESPKKP]), N-terminally biotinylated C-terminally carboxylated peptides (Eurogentec, Belgium) were immobilized on streptavidin-coated microplates (Euroimmun) at a concentration of 0.5 μg/ml. Blocking and sample buffers contained 0.5% (wt/vol) bovine serum albumin in PBS; all other parameters were identical to those of the protein-based ELISA. Sera were also analyzed by immunoblotting, as described by Probst et al. (40).
For the analysis of competition between murine serum antibodies and monoclonal antibodies against OspC, biotinylated monoclonal antibodies diluted in sample buffer were incubated for 30 min following the serum incubation step. Immobilized biotin was visualized using streptavidin-HRP conjugate (Sigma-Aldrich, Germany) diluted 1:5,000 in sample buffer, followed by washing and detection as described above (Fig. 6).
PepScan analysis.
PepScan analysis is described in the supplemental material.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nadine Rochow, Rita Komorowski, David Gräser, and Beatrice Witt for their brilliant technical support and Nicole Baumgarth for her insightful comments.
This work was supported in part by a Sponsored Research Agreement from Euroimmun Medizinische Labordiagnostik AG and by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under award R01AI037277.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00306-16.
REFERENCES
- 1.Steere AC, Coburn J, Glickstein L. 2004. The emergence of Lyme disease. J Clin Invest 113:1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead PS. 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.García Moncó JC, Wheeler CM, Benach JL, Furie RA, Lukehart SA, Stanek G, Steere AC. 1993. Reactivity of neuroborreliosis patients (Lyme disease) to cardiolipin and gangliosides. J Neurol Sci 117:206–214. [DOI] [PubMed] [Google Scholar]
- 4.Luft BJ, Steinman CR, Neimark HC, Muralidhar B, Rush T, Finkel MF, Kunkel M, Dattwyler RJ. 1992. Invasion of the central nervous system by Borrelia burgdorferi in acute disseminated infection. JAMA 267:1364–1367. doi: 10.1001/jama.267.10.1364. [DOI] [PubMed] [Google Scholar]
- 5.Shih CM, Telford SR III, Pollack RJ, Spielman A. 1993. Rapid dissemination by the agent of Lyme disease in hosts that permit fulminating infection. Infect Immun 61:2396–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malawista SE. 1989. Pathogenesis of Lyme disease. Rheumatol Int 9:233–235. [DOI] [PubMed] [Google Scholar]
- 7.Chaaya G, Jaller-Char JJ, Ali SK. 2016. Beyond the bull's eye: recognizing Lyme disease. J Fam Pract 65:373–379. [PubMed] [Google Scholar]
- 8.Steere AC. 2001. Lyme disease. N Engl J Med 345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez JL. 2015. Clinical manifestations and treatment of Lyme disease. Clin Lab Med 35:765–778. doi: 10.1016/j.cll.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Ljøstad U, Mygland A. 2013. Chronic Lyme; diagnostic and therapeutic challenges. Acta Neurol Scand 127:38–47. doi: 10.1111/ane.12048. [DOI] [PubMed] [Google Scholar]
- 11.Dersch R, Sommer H, Rauer S, Meerpohl JJ. 2016. Prevalence and spectrum of residual symptoms in Lyme neuroborreliosis after pharmacological treatment: a systematic review. J Neurol 263:17–24. doi: 10.1007/s00415-015-7923-0. [DOI] [PubMed] [Google Scholar]
- 12.Poland GA. 2011. Vaccines against Lyme disease: what happened and what lessons can we learn? Clin Infect Dis 52(Suppl 3):S253–S258. [DOI] [PubMed] [Google Scholar]
- 13.Nigrovic LE, Thompson KM. 2007. The Lyme vaccine: a cautionary tale. Epidemiol Infect 135:1–8. doi: 10.1017/S0950268806007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wressnigg N, Barrett PN, Pollabauer EM, O'Rourke M, Portsmouth D, Schwendinger MG, Crowe BA, Livey I, Dvorak T, Schmitt B, Zeitlinger M, Kollaritsch H, Esen M, Kremsner PG, Jelinek T, Aschoff R, Weisser R, Naudts IF, Aichinger G. 2014. A novel multivalent OspA vaccine against Lyme borreliosis is safe and immunogenic in an adult population previously infected with Borrelia burgdorferi sensu lato. Clin Vaccine Immunol 21:1490–1499. doi: 10.1128/CVI.00406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwan TG, Piesman J. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A 101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilly K, Bestor A, Jewett MW, Rosa P. 2007. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun 75:1517–1519. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchal C, Schramm F, Kern A, Luft BJ, Yang X, Schuijt TJ, Hovius JW, Jaulhac B, Boulanger N. 2011. Antialarmin effect of tick saliva during the transmission of Lyme disease. Infect Immun 79:774–785. doi: 10.1128/IAI.00482-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagal V, Portnoi D, Faure G, Postic D, Baranton G. 2006. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect 8:645–652. doi: 10.1016/j.micinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Önder Ö, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, Brisson D. 2012. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem 287:16860–16868. doi: 10.1074/jbc.M111.290775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, Marconi RT. 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol 76:393–408. doi: 10.1111/j.1365-2958.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung BP, McHugh GL, Leong JM, Steere AC. 1994. Humoral immune response to outer surface protein C of Borrelia burgdorferi in Lyme disease: role of the immunoglobulin M response in the serodiagnosis of early infection. Infect Immun 62:3213–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaz A, Glickstein L, Field JA, McHugh G, Sikand VK, Damle N, Steere AC. 2001. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun 69:7437–7444. doi: 10.1128/IAI.69.12.7437-7444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dressler F, Whalen JA, Reinhardt BN, Steere AC. 1993. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 27.Engstrom SM, Shoop E, Johnson RC. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 33:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brisson D, Dykhuizen DE. 2004. OspC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbour AG, Travinsky B. 2010. Evolution and distribution of the ospC Gene, a transferable serotype determinant of Borrelia burgdorferi. mBio 1(4):e00153-10. doi: 10.1128/mBio.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovrich SD, Jobe DA, Schell RF, Callister SM. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin Diagn Lab Immunol 12:746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jobe DA, Lovrich SD, Schell RF, Callister SM. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin Diagn Lab Immunol 10:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikushima M, Matsui K, Yamada F, Kawahashi S, Nishikawa SK. 2000. Specific immune response to a synthetic peptide derived from outer surface protein C of Borrelia burgdorferi predicts protective borreliacidal antibodies. FEMS Immunol Med Microbiol 29:15–21. doi: 10.1111/j.1574-695X.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 33.Probert WS, LeFebvre RB. 1994. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect Immun 62:1920–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilmore RD Jr, Kappel KJ, Dolan MC, Burkot TR, Johnson BJ. 1996. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun 64:2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Probert WS, Crawford M, Cadiz RB, LeFebvre RB. 1997. Immunization with outer surface protein (Osp) A, but not OspC, provides cross-protection of mice challenged with North American isolates of Borrelia burgdorferi. J Infect Dis 175:400–405. doi: 10.1093/infdis/175.2.400. [DOI] [PubMed] [Google Scholar]
- 36.Barthold SW, Feng S, Bockenstedt LK, Fikrig E, Feen K. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin Infect Dis 25:S9–S17. doi: 10.1086/516166. [DOI] [PubMed] [Google Scholar]
- 37.Bockenstedt LK, Hodzic E, Feng S, Bourrel KW, de Silva A, Montgomery RR, Fikrig E, Radolf JD, Barthold SW. 1997. Borrelia burgdorferi strain-specific OspC-mediated immunity in mice. Infect Immun 65:4661–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Earnhart CG, Marconi RT. 2007. An octavalent Lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Hum Vaccin 3:281–289. doi: 10.4161/hv.4661. [DOI] [PubMed] [Google Scholar]
- 39.Earnhart CG, Buckles EL, Marconi RT. 2007. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 25:466–480. doi: 10.1016/j.vaccine.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 40.Probst C, Ott A, Scheper T, Meyer W, Stocker W, Komorowski L. 2012. N-terminal disulfide-bridging of Borrelia outer surface protein C increases its diagnostic and vaccine potentials. Ticks Tick Borne Dis 3:1–7. doi: 10.1016/j.ttbdis.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Wilske B, Preac-Mursic V, Gobel UB, Graf B, Jauris S, Soutschek E, Schwab E, Zumstein G. 1993. An OspA serotyping system for Borrelia burgdorferi based on reactivity with monoclonal antibodies and OspA sequence analysis. J Clin Microbiol 31:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacLeod MK, McKee AS, David A, Wang J, Mason R, Kappler JW, Marrack P. 2011. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc Natl Acad Sci U S A 108:7914–7919. doi: 10.1073/pnas.1104588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckles EL, Earnhart CG, Marconi RT. 2006. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clin Vaccine Immunol 13:1162–1165. doi: 10.1128/CVI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathiesen MJ, Christiansen M, Hansen K, Holm A, Asbrink E, Theisen M. 1998. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol 36:3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baum E, Randall AZ, Zeller M, Barbour AG. 2013. Inferring epitopes of a polymorphic antigen amidst broadly cross-reactive antibodies using protein microarrays: a study of OspC proteins of Borrelia burgdorferi. PLoS One 8:e67445. doi: 10.1371/journal.pone.0067445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenedy MR, Lenhart TR, Akins DR. 2012. The role of Borrelia burgdorferi outer surface proteins. FEMS Immunol Med Microbiol 66:1–19. doi: 10.1111/j.1574-695X.2012.00980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuchs R, Jauris S, Lottspeich F, Preacmursic V, Wilske B, Soutschek E. 1992. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in E. coli. Mol Microbiol 6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown EL, Kim JH, Reisenbichler ES, Höök M. 2005. Multicomponent Lyme vaccine: three is not a crowd. Vaccine 23:3687–3696. doi: 10.1016/j.vaccine.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Norris SJ. 2014. vls antigenic variation systems of Lyme disease Borrelia: eluding host immunity through both random, segmental gene conversion and framework heterogeneity. Microbiol Spectr 2:MDNA3-0038-2014. doi: 10.1128/microbiolspec.MDNA3-0038-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrack P, McKee AS, Munks MW. 2009. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilmore RD Jr, Bacon RM, Carpio AM, Piesman J, Dolan MC, Mbow ML. 2003. Inability of outer-surface protein C (OspC)-primed mice to elicit a protective anamnestic immune response to a tick-transmitted challenge of Borrelia burgdorferi. J Med Microbiol 52:551–556. doi: 10.1099/jmm.0.05068-0. [DOI] [PubMed] [Google Scholar]
- 52.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol 143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 53.Ivanova L, Christova I, Neves V, Aroso M, Meirelles L, Brisson D, Gomes-Solecki M. 2009. Comprehensive seroprofiling of sixteen B. burgdorferi OspC: implications for Lyme disease diagnostics design. Clin Immunol 132:393–400. doi: 10.1016/j.clim.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crother TR, Champion CI, Whitelegge JP, Aguilera R, Wu XY, Blanco DR, Miller JN, Lovett MA. 2004. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect Immun 72:5063–5072. doi: 10.1128/IAI.72.9.5063-5072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tunev SS, Hastey CJ, Hodzic E, Feng S, Barthold SW, Baumgarth N. 2011. Lymphoadenopathy during Lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog 7:e1002066. doi: 10.1371/journal.ppat.1002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hastey CJ, Elsner RA, Barthold SW, Baumgarth N. 2012. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J Immunol 188:5612–5622. doi: 10.4049/jimmunol.1103735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Earnhart CG, Rhodes DVL, Smith AA, Yang X, Tegels B, Carlyon JA, Pal U, Marconi RT. 2014. Assessment of the potential contribution of the highly conserved C-terminal motif (C10) of Borrelia burgdorferi outer surface protein C in transmission and infectivity. Pathog Dis 70:176–184. doi: 10.1111/2049-632X.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zückert W, Kerentseva T, Lawson C, Barbour A. 2001. Structural conservation of neurotropism-associated VspA within the variable Borrelia Vsp-OspC lipoprotein family. J Biol Chem 276:457–463. doi: 10.1074/jbc.M008449200. [DOI] [PubMed] [Google Scholar]
- 61.Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Hook M, Sacchettini JC. 2001. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem 276:10010–10015. doi: 10.1074/jbc.M010062200. [DOI] [PubMed] [Google Scholar]
- 62.Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. 2001. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. EMBO J 20:971–978. doi: 10.1093/emboj/20.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Earnhart CG, Marconi RT. 2007. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clin Vaccine Immunol 14:628–634. doi: 10.1128/CVI.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Norris SJ, Howell JK, Garza SA, Ferdows MS, Barbour AG. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun 63:2206–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 66.Piesman J. 1993. Standard system for infecting ticks (Acari: Ixodidae) with the Lyme disease spirochete, Borrelia burgdorferi. J Med Entomol 30:199–203. doi: 10.1093/jmedent/30.1.199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.