ABSTRACT
One potential advantage of live attenuated bacterial vaccines is the ability to stimulate responses to antigens which are only expressed during the course of infection. To determine whether the live attenuated cholera vaccine CVD 103-HgR (Vaxchora) results in antibody responses to the in vivo-induced toxin-coregulated pilus antigen TcpA, we measured IgA and IgG responses to Vibrio cholerae O1 El Tor TcpA in a subset of participants in a recently reported experimental challenge study. Participants were challenged with V. cholerae O1 El Tor Inaba N16961 either 10 days or 90 days after receiving the vaccine or a placebo. Neither vaccination nor experimental infection with V. cholerae alone resulted in a robust TcpA IgG or IgA response, but each did elicit a strong response to cholera toxin. However, compared to placebo recipients, vaccinees had a marked increase in IgG TcpA antibodies following the 90-day challenge, suggesting that vaccination with CVD 103-HgR resulted in priming for a subsequent response to TcpA. No such difference between vaccine and placebo recipients was observed for volunteers challenged 10 days after vaccination, indicating that this was insufficient time for vaccine-induced priming of the TcpA response. The priming of the response to TcpA and potentially other antigens expressed in vivo by attenuated V. cholerae may have relevance to the maintenance of immunity in areas where cholera is endemic.
KEYWORDS: TcpA, challenge, cholera, priming, vaccines
INTRODUCTION
Cholera continues to be a major public health issue for much of the developing world. There are an estimated 2.9 million cases of cholera, resulting in 95,000 deaths, worldwide each year (1). Vibrio cholerae O1 is the primary etiologic agent of cholera, which produces ADP-ribosylating cholera toxin (CT) that causes the intense secretory diarrhea of cholera. In volunteers, ingestion of as little as 5 μg of CT can mimic severe cholera (2).
To deliver CT to the mucosal surface, V. cholerae adheres to the small intestine. The toxin-coregulated pilus (TCP), a type IV pilus, is required for attachment to and colonization in humans and in animal models of cholera (3–6). Similar to CT, the expression of TCP, including its main structural component, TcpA, is dependent on activation of the ToxR regulon during passage of the bacteria through the small intestine (5, 7). Once in the intestine, the B subunit (CtxB) pentamer of CT binds the GM1 ganglioside on epithelial cells, where the A subunit of the toxin is translocated intracellularly (8). The activation of adenylate cyclase by the A subunit ultimately leads to the secretion of chloride and the fluid loss characteristic of cholera (9).
While the vibriocidal antibody response, a T-cell-independent response which largely targets the O antigen of V. cholerae lipopolysaccharide (LPS), is the best-characterized marker of protection against cholera (10–12), there is an interest in understanding whether responses to T-cell-dependent protein antigens could also contribute to protective immunity against cholera. While T-cell-dependent anti-CT antibodies are a sensitive immunologic marker of V. cholerae infection, antitoxin responses alone do not appear to confer long-lasting protection against disease in humans. For example, in Bangladesh, where cholera is endemic, approximately 75% of individuals who develop clinical cholera had a 2-fold or greater rise in serum IgG antibodies against CT within 20 days of infection (10). In addition, following severe cholera, IgG memory B cells to CT can be detected up to 1 year following exposure (13). However, neither baseline levels of anti-CtxB IgG antibodies nor circulating CtxB-specific IgG producing memory B cells are associated with protection from cholera in household contacts of cholera patients (10, 12, 14).
Previous data on the role of CtxB responses in vaccination also support a limited role of this antigen in protection. For example, North American volunteers immunized with three monthly doses of 8 mg of enterally administered CtxB toxoid had equivalent attack rates and similar diarrhea outcomes compared to controls when challenged with live V. cholerae despite having an increase in antitoxin titers (15). In field trials comparing three doses of oral, whole-cell killed cholera vaccine with and without the CtxB toxoid, the whole-cell vaccine with CtxB had a protective efficacy of 62% compared to 53% for the whole-cell-only vaccine after 1 year (16). However, after 3 years, the protective efficacy of the whole-cell vaccine with CtxB dropped to 17% compared to 43% for whole-cell-only vaccine (16).
TCP is also required for full pathogenesis of V. cholerae in humans, but the role of anti-TcpA antibodies in protection remains uncertain. When volunteers ingested a classical O395 V. cholerae O1 strain with a tcpA gene deletion, the strain was unable to colonize the volunteers, no robust vibriocidal antibody responses were detected, and none of the volunteers who were subsequently challenged with wild-type V. cholerae were protected against clinical cholera (4). Nonetheless, when North American volunteers were experimentally infected with a single dose of V. cholerae O395, none demonstrated a serum anti-TCP IgG or IgA response, defined as a 4-fold rise in titer, and yet when four of these volunteers were rechallenged 9 weeks later, all were protected against disease (15, 17).
In contrast, in Bangladesh, where cholera is endemic, mucosal or systemic anti-TcpA responses have been observed in over 90% of cholera patients infected with V. cholerae O1 El Tor, and memory B-cell responses against TcpA antigen can be detected up to 1 year after infection (13, 18). Furthermore, in household contacts of cholera patients, higher baseline levels of circulating anti-TcpA IgA antibodies are associated with protection against V. cholerae infection, while baseline anti-TcpA IgG levels are not associated with the risk of V. cholerae infection (12). Given these observations, it is unclear whether immune responses to TcpA contribute to long-term protection following natural infection with V. cholerae and whether including TcpA as a vaccine component enhances the effectiveness of oral cholera vaccination (18, 19).
CVD 103-HgR is an attenuated classical V. cholerae O1 Inaba 569B strain that has a 94% gene deletion of the cholera toxin A subunit while the nontoxigenic CtxB subunit remains intact (20, 21). When CVD 103-HgR was administered as a single-dose, live oral vaccine, it was well tolerated and immunogenic (22–24). CVD 103-HgR was previously licensed and manufactured as Orochol and was recently relicensed and manufactured as Vaxchora (PaxVax, USA), which was approved by the U.S. Food and Drug Administration in 2016 and recommended by the Advisory Committee on Immunization Practices for the prevention of cholera in travelers to areas where cholera is endemic.
In a recently reported multicenter clinical trial conducted using a human cholera challenge model, CVD 103-HgR elicited protection against moderate and severe diarrhea (the clinical protocol endpoint of interest) with efficacies of 90% and 80% in North American vaccinees challenged with wild-type V. cholerae at 10 or 90 days postvaccination, respectively (24). In this study, a 4-fold or greater increase in vibriocidal antibodies was observed in 90% of vaccinees, and these vibriocidal responses were strongly associated with protection (24). In another immunogenicity study, Vaxchora elicited a rise in IgG anti-cholera toxin antibodies in 57% of recipients 28 days following vaccination (23). While it is inferred that TcpA remains intact since this strain is capable of colonizing the small intestine in humans and in animal models of cholera (22, 25), it has not been previously reported whether vaccination induces a significant immune response to the TcpA antigen (19).
In this study, we utilized remaining serum samples from the previously reported human challenge study (24) to address whether Vaxchora induced TcpA IgG and IgA antibody responses and compared these with responses to a known T-cell-dependent protein antigen, CtxB, for which responses have been established following both vaccination and infection (10, 12, 15, 16, 23).
RESULTS
Participants.
Samples obtained from 84 of the 134 challenged volunteers enrolled in the phase 3 clinical trial conducted by PaxVax were used in this immunogenicity study. The subset of participants selected for this study included all subjects for which there were remaining serum samples from each time point, and those with missing or inadequate sample volume were excluded. These subjects had a mean age of 32 years (standard deviation of 7) comprised of 62% males and 38% females. The proportion of participants with blood type O, a population more susceptible to severe cholera, was enriched to 50% as part of the design of the clinical trial, and this enrichment is reflected in the selected subset as well. Among the subset of participants in the 10-day challenge cohort, 26 received a single dose of CVD 103-HgR while 22 received placebo. From the subset of participants in the 90-day challenge cohort, 20 received the vaccine and 16 received placebo. Age, sex, race, and blood type were recorded for all subjects, and this information is presented in Table 1 for participants only in the subset of samples used in this study. There were no significant differences in demographic characteristics or reported adverse events between placebo and vaccine recipients in either the 10- or 90-day challenge cohort (Table 1).
TABLE 1.
Participant demographics
| Characteristic | Value for: |
|||
|---|---|---|---|---|
| 10-Day challenge |
90-Day challenge |
|||
| Vaccine (n = 26) | Placebo (n = 22) | Vaccine (n = 20) | Placebo (n = 16) | |
| Mean age, yr (SD) | 31.3 (5.9) | 31.6 (7.8) | 34.8 (8.1) | 30.6 (6.4) |
| Sex, no. (%) | ||||
| Male | 17 (65.4) | 11 (50.0) | 14 (70.0) | 10 (62.5) |
| Female | 9 (34.6) | 11 (50.0) | 6 (30.0) | 6 (37.5) |
| Race, no. (%) | ||||
| Black/African-American | 14 (53.9) | 13 (59.1) | 14 (70.0) | 12 (75.0) |
| White | 8 (30.8) | 9 (40.9) | 6 (30.0) | 4 (25.0) |
| Other | 4 (15.3) | 0 | 0 | 0 |
| Blood group, no. (%) | ||||
| O | 11 (42.3) | 12 (54.5) | 13 (65.0) | 6 (37.5) |
| Non-O | 15 (57.7) | 10 (45.5) | 7 (35.0) | 10 (62.5) |
Prechallenge serum anti-CtxB antibody responses.
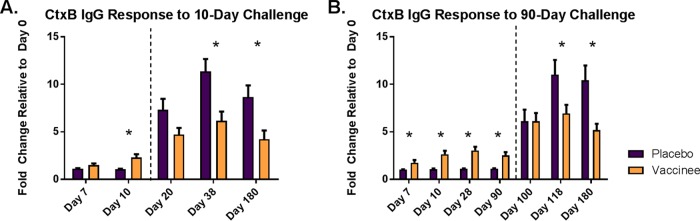
We evaluated anti-CtxB IgG antibody responses in serum from participants who received either placebo or a single dose of V. cholerae CVD 103-HgR and were subsequently challenged with wild-type V. cholerae at either 10 or 90 days postvaccination. The prechallenge, postvaccination responses are shown to the left of the dashed lines in Fig. 1A for the 10-day challenge cohort and Fig. 1B for the 90-day challenge cohort. Following vaccination, significant increases in anti-CtxB IgG levels were seen by day 7 and remained increased through day 90, the time point that reflects the longest postvaccination, prechallenge time interval in the study. As expected, vaccinees had significantly higher CtxB IgG antibody responses than placebo recipients at all prechallenge time points after day 0. Although the aggregate responses to CtxB were significant in the vaccinees, there was considerable variation between participants. While 30 of the 46 (65%) had a ≥50% increase in CtxB IgG antibody level, some participants from both challenge cohorts had no significant response following vaccination.
FIG 1.
CtxB-specific IgG response for vaccine and placebo recipients before and after cholera challenge. Mean fold change of anti-CtxB IgG antibodies for placebo and vaccine recipients challenged at day 10 (A) and day 90 (B). To the left of the dashed line are days before cholera challenge, and to the right are days following challenge. Error bars represent standard errors of the means, and an asterisk denotes differences between placebo and vaccine recipients with a P value of <0.05.
Postchallenge serum anti-CtxB antibody responses.
Following challenge with live V. cholerae 10 or 90 days postvaccination, both vaccine and placebo recipients had increases in anti-CtxB antibody levels on all days tested (Fig. 1). By 28 days following the 10- and 90-day challenges (day 38 and day 118, respectively), the placebo groups had a significantly higher average fold change of 11.3 and 11.0 in anti-CtxB IgG antibody levels compared to 6.2 and 6.9 for the vaccine group (P = 0.006 and P = 0.034, respectively). The higher postchallenge CtxB response observed in the placebo recipients persisted through the final assessment on day 180, where the placebo group for the 10-day challenge cohort had a fold increase of 8.7 compared to 4.2 for vaccinees (P = 0.002), and the placebo group for the 90-day challenge cohort had an increase of 10.4 compared to 5.2 for vaccinees (P = 0.007).
Prechallenge serum anti-TcpA antibody responses.
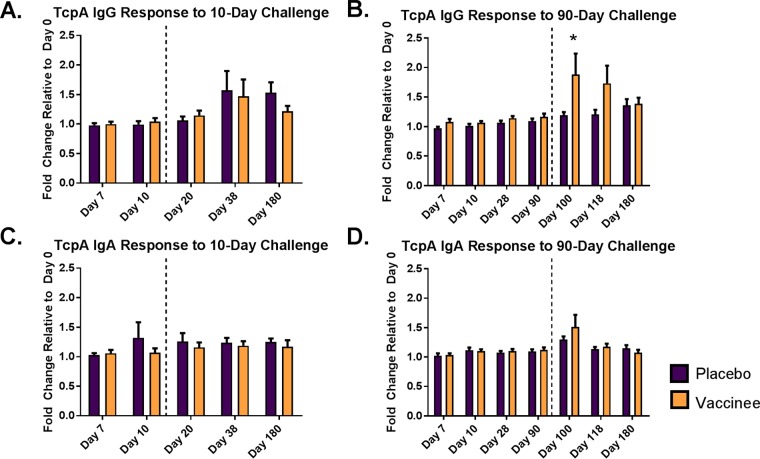
Serum from the same 84 participants was also tested for IgG and IgA anti-TcpA antibodies. Following vaccination alone, there was no significant observed difference between vaccine and placebo recipients in serum TcpA-specific IgG or IgA antibodies for either challenge cohort (Fig. 2). Only 4% of vaccinees in the 10-day challenge group and 20% of vaccinees in the 90-day challenge group (which were evaluated at multiple time points before experimental infection) demonstrated a greater than 50% increase in anti-TcpA IgG antibodies prior to cholera challenge. There were no observed associations between the participant demographics (Table 1) and their TcpA responses following vaccination.
FIG 2.
TcpA-specific IgG and IgA response for vaccine and placebo recipients before and after cholera challenge. Mean fold change of anti-TcpA IgG (A and B) and IgA (C and D) antibodies for placebo and vaccine recipients challenged at day 10 (A and C) and day 90 (B and D). To the left of the dashed line are days before cholera challenge, and to the right are days following challenge. Error bars represent standard errors of the means, and an asterisk denotes differences between placebo and vaccine recipients with a P value of <0.05.
Postchallenge serum anti-TcpA antibody responses.
Following challenge with V. cholerae, both vaccine and placebo recipients in the 10-day challenge group had an increase in TcpA IgG antibodies on day 20 relative to the day of challenge (P = 0.045 for vaccinees and 0.040 for placebo recipients). However, these responses were generally modest, as only 4 of the 26 (15%) vaccinees and 2 of the 22 (9%) placebo recipients had a greater than 50% increase in anti-TcpA antibodies 10 days following challenge. Additionally, there was no difference in the TcpA responses between vaccinees and placebo recipients following the 10-day challenge (Fig. 2A and C).
Similarly, the vaccine and placebo group in the 90-day challenge cohort had increased anti-TcpA IgG antibodies 10 days following challenge (P value of 0.007 and 0.018, respectively). However, vaccinees challenged 90 days postvaccination had a markedly higher-level anti-TcpA IgG response than placebo recipients (P = 0.033) (Fig. 2B). In addition, 10 of the 20 vaccinees (50%) in the 90-day challenge group seroconverted on day 100 compared to only 1 of 16 (6%) placebo recipients (P = 0.009). Comparing vaccinees challenged at 10 versus 90 days postvaccination, the 90-day challenge cohort had significantly higher IgG responses 10 days after challenge (P = 0.004) (Fig. 3). There was no relation between sex, race, or blood group and IgG TcpA response after either challenge day for vaccine or placebo recipients. Furthermore, no significant differences in anti-TcpA IgA responses were seen between vaccinees and placebo recipients for the 90-day challenge cohort (Fig. 2D).
FIG 3.
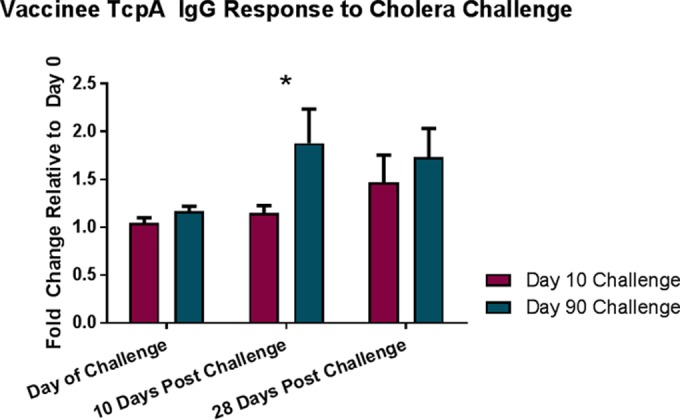
TcpA-specific IgG response for vaccinees following challenge with V. cholerae. Mean fold change relative to day 0 for anti-TcpA IgG antibodies for vaccinees challenged either 10 or 90 days following vaccination. Error bars represent standard errors of the means, and an asterisk denotes differences between challenge cohorts with a P value of <0.05.
Correlation of CtxB and TcpA antibody responses and diarrhea after challenge.
To assess the association between CtxB- and TcpA-specific antibodies and protection against cholera challenge, we evaluated the relationship between antibody response following vaccination and the volume and severity of diarrhea after challenge. As previously described, vaccine recipients were protected against moderate (≥3.0 liters) and severe (≥5.0 liters) diarrhea (MSD) at both day 10 (90% efficacy) and day 90 (80% efficacy) after challenge (24). In our subset of individuals derived from the original study, 2 of 26 (8%) vaccinees challenged after day 10 developed MSD, and 3 of 20 (15%) challenged after day 90 developed MSD. There was no evidence of an association between prechallenge TcpA IgG, TcpA IgA, or CtxB responses and the severity of diarrhea after challenge when correlating diarrhea with fold rise in antibody (Fig. 4). In contrast, there was perhaps a modest association between CtxB IgG responses and diarrhea after challenge by seroconversion. The 14 vaccinees who seroconverted to CtxB before the 90-day challenge had an average of 688 ml of diarrhea compared to 3,623 ml for the 6 vaccinees who did not CtxB seroconvert (P = 0.037). No such difference was seen for TcpA, as the 4 vaccinees who IgG seroconverted to TcpA before the 90-day challenge had an average of 2,665 ml of diarrhea compared to 1,294 ml for the 16 vaccinees who did not TcpA seroconvert (P = 0.420).
FIG 4.
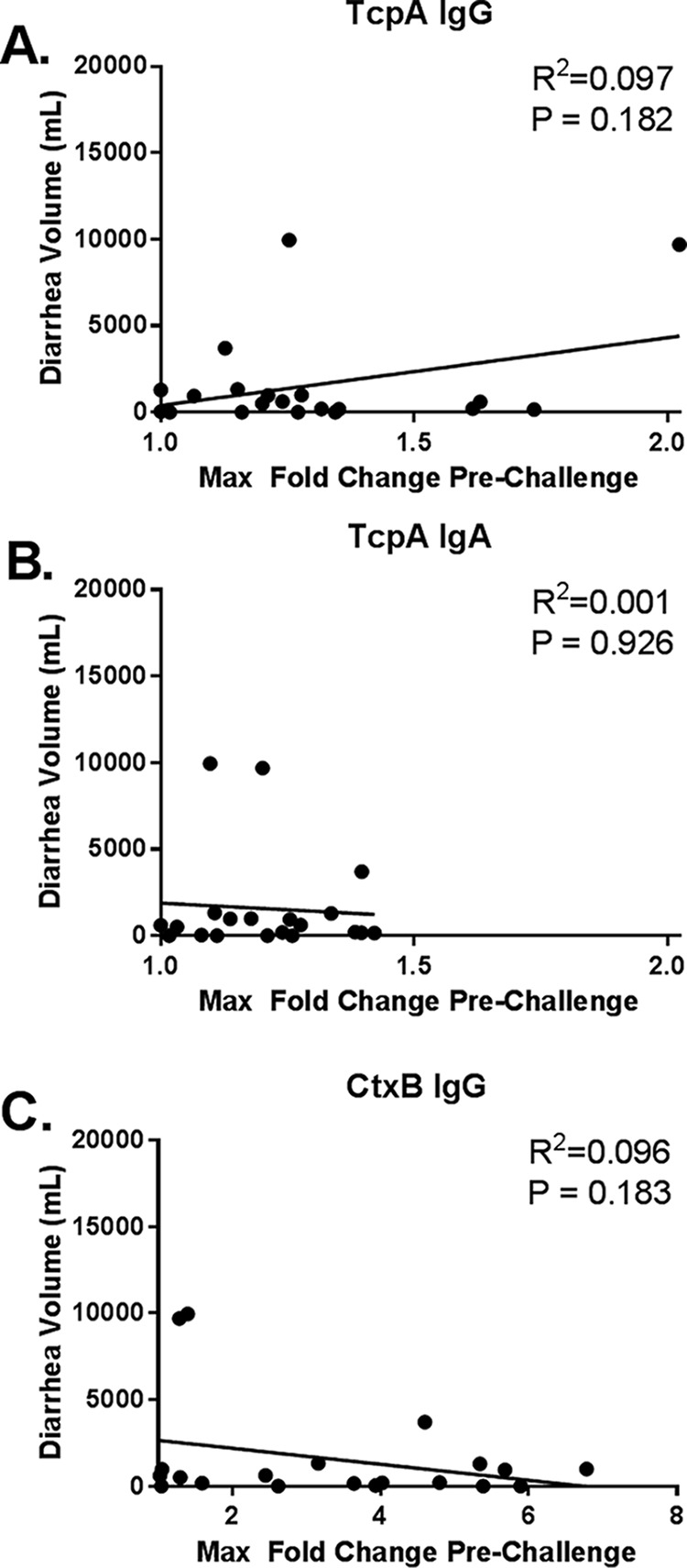
Correlation of maximum fold change before 90-day challenge relative to diarrhea volume following cholera challenge. Shown are the vaccinee maximum (max) fold changes for anti-TcpA IgG (A), anti-TcpA IgA (B), and anti-CtxB IgG (C) antibodies before 90-day challenge relative to cumulative diarrhea volume following 90-day challenge. The line represents a linear regression with R2 and P values for normalized data.
DISCUSSION
In this study, we demonstrate that oral vaccination with a single dose of Vaxchora, containing live attenuated CVD 103-HgR, primes an anamnestic response to TcpA that is induced by challenge with wild-type V. cholerae 90 days after vaccination. This finding underscores a potential advantage of live attenuated bacterial vaccines, which is the ability to stimulate responses to antigens that are only expressed during the course of infection but are not present in killed vaccine. Since TcpA is essential for V. cholerae attachment to and colonization of the human small intestine (3–6), it is possible that the priming of a response to TcpA and potentially other in vivo-expressed antigens following vaccination with CVD 103-HgR could contribute to long-term efficacy of the vaccine in settings where cholera is endemic.
One potential advantage of vaccine-induced memory against T-cell-dependent antigens such as CtxB and TcpA is that these responses may persist longer than responses against T-cell-independent polysaccharide antigens. It has previously been demonstrated that in Bangladesh, IgG memory B-cell responses to the T-cell-dependent antigens CtxB and TcpA are detected in circulation for up to 1 year following natural infection with V. cholerae, while circulating memory B cell responses to LPS appear to wane more quickly (13). However, while it does not appear that CtxB memory B cell responses are associated with protection against cholera (14), it is unknown whether TcpA responses impact protective immunity. In our study, the fact that vaccine-derived priming of the TcpA response was only observed after the 90-day experimental V. cholerae challenge, but not the 10-day challenge, suggests that this priming is dependent on the development of an initial memory B-cell response, as seen in areas where cholera is endemic, to CVD 103-HgR that takes over 10 days to develop.
It is notable that neither a single dose of vaccine alone nor a single experimental infection (in the placebo recipients) was sufficient to induce a robust serum IgG response to TcpA comparable to the response seen in the subjects with vaccination-induced priming followed by experimental infection. The limited TcpA response from only a single antigenic exposure supports previous results demonstrating the lack of TcpA response in experimentally challenged North American volunteers (17). Based on this observation, it is most likely that the high percentage of TcpA responders observed in cholera patients in Bangladesh is due to natural priming occurring from repeated exposure in this area where cholera is endemic, and that relative to CtxB, TcpA is significantly less immunogenic.
Interestingly, the only previous study of anti-TcpA antibody responses to a live, oral cholera vaccine assessed responses to Peru-15, which is derived from a V. cholerae El Tor Ogawa strain lacking the cholera toxin A subunit (26). Seven days after vaccination with Peru-15, only 10% of Bangladeshi vaccinees had demonstrated a ≥2-fold increase in TcpA IgA antibodies (26). Unlike Peru-15, the CVD 103-HgR vaccine is derived from a V. cholerae O1 classical strain which expresses a TcpA antigen that differs by 18% at the amino acid level from the TcpA antigen present in the N16961 El Tor challenge strain (27, 28). In this study, we specifically measured responses using the El Tor TcpA, because this antigen better reflects the currently circulating V. cholerae strains, and strains producing the classical TcpA antigen are no longer a cause of cholera (8). While it is possible that antibody responses to the classical TcpA antigen after vaccination with CVD 103-HgR differ from our results using the El Tor antigen, it is unlikely that we would have observed a strong response to classical TcpA after vaccination alone, given our results that vaccine-based priming was required to induce a robust TcpA response in experimentally infected individuals. This is consistent with the earlier Peru-15 study, which demonstrated vaccination alone did not induce a strong TcpA response even to the homologous TcpA antigen. Importantly, the results of our study demonstrate that vaccinating with a classical V. cholerae biotype still resulted in priming an antibody response to an El Tor antigen given sufficient time following vaccination.
Given the small number of vaccinees that developed a response to TcpA prior to challenge, as well as the limited number of challenged individuals who went on to develop MSD, it is not surprising that we were unable to detect a significant association between prechallenge TcpA responses and the occurrence of diarrhea after challenge. We also did not observe a robust association between CtxB responses and protection against diarrhea. In contrast, previous studies of this cohort demonstrated a very strong association between a 4-fold increase in the vibriocidal antibody response and protection against experimental challenge (24).
In summary, our findings suggest that vaccination with CVD 103-HgR results in priming of subsequent antibody response to the TcpA antigen. While the priming of the response to TcpA and potentially other antigens expressed in vivo during colonization by CVD 103-HgR may have relevance to the maintenance of immunity in areas where cholera is endemic, their potential contribution to protection following immunization will require further evaluation.
MATERIALS AND METHODS
Study subjects and sample collection.
Adult volunteers were enrolled in a randomized, double-blind, placebo-controlled phase 3 clinical trial, which has been described in detail previously (24). In brief, participants were screened for eligibility, which included exclusion based on abnormal stool pattern and current or recent antibiotic use, at three study sites (Baltimore, MD, Cincinnati, OH, and Burlington, VT) before receiving either a single dose of vaccine containing ∼5 × 108 CFU of CVD 103-HgR or normal saline as a placebo (24). Subjects were challenged with 1 × 105 CFU of V. cholerae O1 El Tor Inaba strain N16961 either 10 or 90 days following vaccination. For subjects challenged 10 days postvaccination, serum was collected at days 0, 7, 10, 20, 38, and 180, and for subjects challenged 90 days postvaccination, it was collected at days 0, 7, 10, 28, 90, 100, 118, and 180. For the 10 days following challenge, subjects were closely monitored in an inpatient setting, and all stools were graded according to consistency and for assessment of diarrhea. For diarrhea volumes over 200 ml, the corresponding severity, number of total stools, and number of total days with diarrhea were measured.
Antigens used for immunological assays.
CtxB from V. cholerae was purchased from List Biological Laboratories, Inc. (Campbell, CA). TcpA was prepared as described previously (29). In brief, recombinant histidine-tagged TcpA was generated from V. cholerae O1 El Tor strain C6706, which produces a TcpA protein identical to that of challenge strain N16961. TcpA-His was ligated into pET-15b (Novagen, San Diego, CA) at the N terminus and purified using nickel affinity chromatography. The purified product was concentrated to 1.0 mg/ml. With polyacrylamide gel electrophoresis, protein purity and identity were assessed with Coomassie staining and Western immunoblotting, respectively, which demonstrated a single TcpA-His band at 19.7 kDa.
Detection of antibody responses.
Antibody responses in all subjects were detected by research personnel blinded to vaccine or placebo status by an endpoint enzyme-linked immunosorbent assay (ELISA) as previously described (13). In brief, 96-well polystyrene plates (Nunc, Denmark) were coated directly with 100 ng per well of purified CtxB or V. cholerae TcpA overnight and blocked with 1% bovine serum albumin. Serum was applied at a 1:100 dilution. Following washing, 100 μl of 1:8,000 diluted secondary anti-human IgG antibodies conjugated to horseradish peroxidase (HRP) (Jackson ImmunoResearch, West Grove, PA) was applied to CtxB-coated plates, while 1:4,000 anti-human IgG-HRP or IgA-HRP was applied to TcpA-coated plates. Development was performed with ortho-phenylenediamine (Sigma, St. Louis, MO) in 0.1 M sodium citrate buffer (pH 4.5) with 0.01% hydrogen peroxide and stopped with 100 μl of 1N hydrochloric acid after 5 min. Plates were read spectrophotometrically at 490 nm.
Statistical analysis.
ELISA values for test samples were divided by a standard pool of convalescent-phase sera from patients who had recovered from cholera as a control sample that was included with each assay and normalized by log transformation. Immunological responses at various time points were compared to baseline measures or between cohorts using paired or unpaired t tests, respectively. Seroconversion was defined as a 50% rise in endpoint ELISA values relative to day 0 or the day of challenge. Two-tailed P values of <0.05 were considered statistically significant.
ACKNOWLEDGMENTS
We are grateful for the participation of subjects and staff at each of the clinical sites.
This research was supported by the Massachusetts General Hospital and the following grants: R01 AI103055 (J.B.H.) and R01 AI099243 (J.B.H.). The original clinical challenge study was funded by PaxVax, Inc.
The content of this work is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
J.K.S., M.L., and M.G. were employed by PaxVax, Inc. W.H.C., C.E.L., B.D.K., M.C., and M.M.L. received research funding from PaxVax, Inc. M.M.L. is the patent holder of CVD 103-HgR. J.B.H. served on the data safety and monitoring board for the challenge study and on a scientific advisory committee for PaxVax. All other authors declare no competing interests.
REFERENCES
- 1.Ali M, Nelson AR, Lopez AL, Sack DA. 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev 47:510–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krebs SJ, Taylor RK. 2011. Protection and attachment of Vibrio cholerae mediated by the toxin-coregulated pilus in the infant mouse model. J Bacteriol 193:5260–5270. doi: 10.1128/JB.00378-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrington DA, Hall RH, Losonsky G, Mekalanos JJ, Taylor RK, Levine MM. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med 168:1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A 84:2833–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ritchie JM, Rui H, Bronson RT, Waldor MK. 2010. Back to the future: studying cholera pathogenesis using infant rabbits. mBio 1:e00047-10. doi: 10.1128/mBio.00047-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bina J, Zhu J, Dziejman M, Faruque S, Calderwood S, Mekalanos J. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc Natl Acad Sci U S A 100:2801–2806. doi: 10.1073/pnas.2628026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 10.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. 1985. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis 151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 11.Mosley WH, Ahmad S, Benenson AS, Ahmed A. 1968. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ 38:777–785. [PMC free article] [PubMed] [Google Scholar]
- 12.Harris JB, LaRocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. 2008. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris AM, Bhuiyan MS, Chowdhury F, Khan AI, Hossain A, Kendall EA, Rahman A, LaRocque RC, Wrammert J, Ryan ET, Qadri F, Calderwood SB, Harris JB. 2009. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun 77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SM, Rahman MA, Mohasin M, Riyadh MA, Leung DT, Alam MM, Chowdhury F, Khan AI, Weil AA, Aktar A, Nazim M, LaRocque RC, Ryan ET, Calderwood SB, Qadri F, Harris JB. 2012. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol 19:842–848. doi: 10.1128/CVI.00037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine MM, Nalin DR, Craig JP, Hoover D, Bergquist EJ, Waterman D, Holley HP, Hornick RB, Pierce NP, Libonati JP. 1979. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg 73:3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- 16.Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N. 1990. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 335:270–273. doi: 10.1016/0140-6736(90)90080-O. [DOI] [PubMed] [Google Scholar]
- 17.Hall RH, Losonsky G, Silveira AP, Taylor RK, Mekalanos JJ, Witham ND, Levine MM. 1991. Immunogenicity of Vibrio cholerae O1 toxin-coregulated pili in experimental and clinical cholera. Infect Immun 59:2508–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asaduzzaman M, Ryan ET, John M, Hang L, Khan AI, Faruque AS, Taylor RK, Calderwood SB, Qadri F. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect Immun 72:4448–4454. doi: 10.1128/IAI.72.8.4448-4454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop AL, Camilli A. 2011. Vibrio cholerae: lessons for mucosal vaccine design. Expert Rev Vaccines 10:79–94. doi: 10.1586/erv.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaper JB, Lockman H, Baldini MM, Levine MM. 1984. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature 308:655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- 21.Ketley JM, Michalski J, Galen J, Levine MM, Kaper JB. 1993. Construction of genetically marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett 111:15–21. doi: 10.1111/j.1574-6968.1993.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine MM, Kaper JB, Herrington D, Ketley J, Losonsky G, Tacket CO, Tall B, Cryz S. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet ii:467–470. [DOI] [PubMed] [Google Scholar]
- 23.Chen WH, Greenberg RN, Pasetti MF, Livio S, Lock M, Gurwith M, Levine MM. 2014. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR, prepared from new master and working cell banks. Clin Vaccine Immunol 21:66–73. doi: 10.1128/CVI.00601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, Lyon CE, Pasetti MF, Simon JK, Szabo F, Tennant S, Levine MM. 2016. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with Vibrio cholerae O1 El Tor. Clin Infect Dis 62:1329–1335. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce NF, Cray WC, Kaper JB, Mekalanos JJ. 1988. Determinants of immunogenicity and mechanisms of protection by virulent and mutant Vibrio cholerae O1 in rabbits. Infect Immun 56:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury F, Khan AI, Harris JB, LaRocque RC, Chowdhury MI, Ryan ET, Faruque AS, Calderwood SB, Qadri F. 2008. A comparison of clinical and immunologic features in children and older patients hospitalized with severe cholera in Bangladesh. Pediatr Infect Dis J 27:986–992. doi: 10.1097/INF.0b013e3181783adf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhine JA, Taylor RK. 1994. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol 13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 28.Craig L, Taylor RK, Pique ME, Adair BD, Arvai AS, Singh M, Lloyd SJ, Shin DS, Getzoff ED, Yeager M, Forest KT, Tainer JA. 2003. Type IV pilin structure and assembly: X-ray and EM analyses of Vibrio cholerae toxin-coregulated pilus and Pseudomonas aeruginosa PAK pilin. Mol Cell 11:1139–1150. doi: 10.1016/S1097-2765(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 29.Rollenhagen JE, Kalsy A, Cerda F, John M, Harris JB, Larocque RC, Qadri F, Calderwood SB, Taylor RK, Ryan ET. 2006. Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El Tor challenge in mice. Infect Immun 74:5834–5839. doi: 10.1128/IAI.00438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]