Energy contained in lactate, glucose, ketones and fatty acids is captured by metabolic processes in the heart to produce mechanical and electrical work (1). The actual contribution of each substrate to energy production and the specific metabolic pathways involved are very sensitive to both physiological conditions and disease. This knowledge of cardiac biochemistry is derived primarily from studies in isolated hearts and from invasive in vivo studies in experimental animals. Animal models of some diseases, notably acute ischemia and reperfusion, provided valuable insights, but in general the relevance of animal studies to human disease is uncertain because it is difficult to meaningfully model heart failure, hypertrophy, cardiomyopathies, hibernating myocardium, and other complex conditions. Methods to quantify biochemical events in the heart are important because it is becoming increasingly apparent that chronic adaptations in metabolism may drive processes with adverse consequences such as impaired energy capture and oxidative stress. Positron tomography (PET) provides some metabolic information in patients. However, in spite of the popularity and the very high sensitivity for detecting a radionuclide, the “fit” between metabolic complexity and the information accessible by PET is actually quite poor. For example, uptake, phosphorylation and trapping of 18FDG is widely accepted as a biomarker of glucose metabolism. Yet the PET signal does not inherently make the simple distinction between metabolism of glucose to acetyl-CoA and subsequent oxidation in the Krebs cycle versus anaerobic glycolysis to pyruvate and lactate. For basic science studies, an alternative to radiotracers is the use of 13C-enriched substrates with detection by NMR spectroscopy. 13C is a stable isotope of carbon that is normally present at about 1% of carbon nuclei. After enriching a compound with 13C to ~99%, intermediary metabolism has been studied for decades using 13C NMR spectroscopy (2). Aside from the convenience of avoiding radiation, 13C NMR provides very high chemical specificity and it is a simple task to distinguish the many metabolic products and relevant pathways after exposure to 13C-labeled substrates. However, the sensitivity for 13C detection in vivo is poor primarily because the concentration of metabolically- relevant metabolites is only a few millimolar at best, and the MR sensitivity of 13C is weak. Consequently only a few investigators have attempted to detect 13C in the human heart (3).
In this issue of Circulation Research, Cunningham and colleagues report the first images of ventricular myocardium in healthy humans acquired by 13C MR imaging (4). This achievement was enabled by a method well-known in the physics community, hyperpolarization, to vastly improve sensitivity for detecting 13C by MR. Dynamic nuclear polarization or DNP is based on mixing 13C-enriched pyruvate with a stable radical, freezing the sample at ~1 Kelvin and exposing the mixture to microwaves (Figure 1A). This results in an increase in 13C polarization by 10,000x or more, hence the term hyperpolarization (HP). Many details of DNP were well-known in the 1970s (5) but it was not until 2003 that Ardenkjaer-Larsen, Golman and colleagues demonstrated that the frozen sample could be rapidly warmed with boiling water while preserving, albeit temporarily, 13C polarization (6). An early application was 13C imaging of the pig heart (7,8). Because of the practical advantages of working with stable isotopes and the potentially high information yield of detecting 13C with MR methods, there has been intense interest in developing 13C imaging, particularly for cancer applications. The first results reported in 2013 were obtained in men with prostate cancer. The HP [1-13C]pyruvate was generated in a clean room constructed next to the scanner and an endorectal coil was used to acquire images (9).
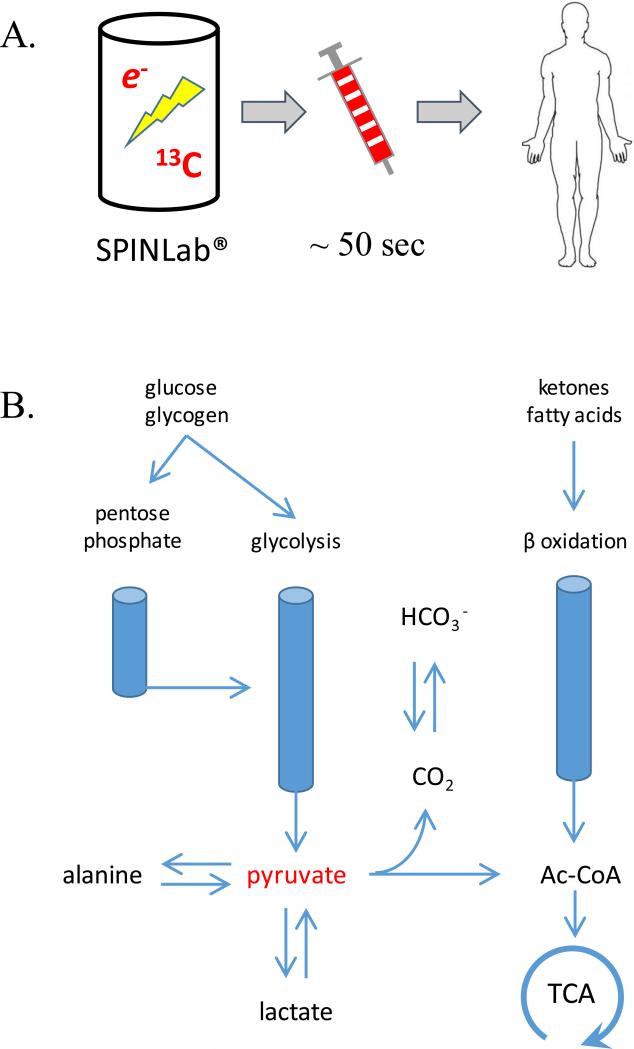
Figure. Schematic of the Hyperpolarization Process (A) and Pyruvate Metabolism in the Heart (B).
[1-13C]pyruvate is mixed with a stable free radical, frozen, and exposed to microwaves to generate hyperpolarized [1-13C]pyruvate. After dissolving with hot water, the solution is rapidly loaded into a syringe for intravenous injection (Panel A). In the heart, [1-13C] pyruvate may undergo transamination to [1-13C]alanine, reduction to [1-13C]lactate or oxidation to 13CO2 and acetyl-CoA. Since the activity of carbonic anhydrase is high in the heart, HP13CO2 is rapidly converted to [13C]bicarbonate.
The current study is somewhat limited by reporting data on only 4 subjects. Nevertheless, the study describes important advances because it confirmed the safety of injecting HP [1-13C]pyruvate in humans. Further, the device used to hyperpolarize pyruvate, the SPINLab®, is self-contained and is designed to be located in a clinical environment. HP[1-13C]pyruvate was imaged in the right and left ventricular cavity, and as would be expected from known biochemical pathways (Figure 1B), [13C]bicarbonate, [1-13C]lactate, [1-13C]alanine and 13CO2 were observed.
Relatively homogenous images of HP [13C]bicarbonate, derived from HP [1-13C]pyruvate, was acquired successfully in the healthy human myocardium. Earlier studies in pigs under general anesthesia were promising and it is reassuring to see that the experiment worked in humans (7,8). The myocardium normally oxidizes primarily fatty acids or ketones, so a transient increase in the concentration of pyruvate might not be sufficient to suppress oxidation of fatty acids and ketones in a conscious, resting person (Figure 1B). The positive results are exciting, but what does it mean to detect HP[13C]bicarbonate? Since pyruvate is metabolized in the heart overwhelmingly via pyruvate dehydrogenase (PDH), the appearance of HP[13C]bicarbonate in principle serves as a reliable imaging biomarker for flux through this enzyme (10). PDH is a large, multi-enzyme complex that resides exclusively in mitochondria, so presumably the appearance of [13C]bicarbonate indicates intact mitochondria. After prolonged ischemia and reperfusion, the absence of a HP[13C]bicarbonate has been attributed to cardiomyocyte injury (7,8). In a model of pacing-induced heart failure, the bicarbonate signal decreased late (11) in the evolution of heart failure. However, in general the absence of a [13C]bicarbonate signal should be interpreted cautiously because high plasma concentrations of fatty acids or ketones – exactly the situation to be anticipated in sick patients – will suppress oxidation of HP[1-13C]pyruvate even with normally-functioning mitochondria (12). An important challenge going forward is to investigate and validate methods to quantify flux in PDH and the other pathways involved with pyruvate metabolism.
HP 13C imaging of the human heart has limitations and disadvantages. The method requires an expensive external device to generate hyperpolarized materials that must be immediately adjacent to the MR scanner. Transfer of the polarized material to the subject must occur within 10s of seconds (Figure 1A). Current 13C coils, acquisition schemes and reconstruction algorithms are almost certainly suboptimal. These limitations are primarily technical and more advanced coils and acquisition schemes will certainly improve image quality. In particular it is important to assure that signals can be reliably compared from different regions of the myocardium. For example, the in figure, there is some variation in HP[13C]bicarbonate signal – does this indicate true differences in metabolism and perfusion of the septum or does it reflect differences in how the coil interacts with tissue in these regions? Once these technical limitations are solved, HP [1-13C]pyruvate will likely provide other insights into metabolism that are simply not available to clinicians today. For example, the [1-13C]lactate signal generated from HP-[1-13C]pyruvate occurs largely through via an exchange reaction in the active site of lactate dehydrogenase so it reflects the size of the existing tissue lactate pool, not newly generated lactate from pyruvate (13). This is important because it offers the potential to image lactate pool sizes in different tissue regions within a few seconds after injection of HP-[1-13C]pyruvate. This could potentially allow rapid imaging of tissue ischemia and, in addition, allow monitoring of generation of newly generated lactate after a pharmacological intervention (14). It must be remembered that the mass of injected [1-13C]pyruvate is significant and potentially could influence cardiac metabolism. Although hyperpolarization methods provide a new window into cardiac metabolism, it will be important to validate, to the extent possible, with well-accepted existing methods to evaluate metabolism such as PET and other techniques.
This study demonstrates two important points. First, currently-available technologies including the SPINLab as well as radiofrequency coils and pulse sequences are at sufficient to begin studies of the myocardium in human subjects. Second, metabolism of [1-13C]pyruvate to downstream metabolites such as [13C]bicarbonate can be detected in normal human myocardium, opening the opportunity for better understanding of metabolism in high-impact myocardial disease. Additional 13C-enriched metabolic probes for interrogating other pathways in the heart such as [1-13C]lactate (see figure) will likely become available (15). Since the method inherently provides detailed chemical information about the 13C-enriched probes, it is possible to co-polarize two compounds, one targeting metabolic processes and the other measuring myocardial perfusion (16). If the technology can be refined, there are three advantages over current methods for a clinician: the absence of ionizing radiation, the capacity to integrate with any other cardiac MR exam, and access to specific information about cardiac metabolism that is not provided by radionuclide methods.
Acknowledgments
Funding Sources: This work was supported by the National Institutes of Health (P41EB015908)
Footnotes
Financial disclosures: none
References
- 1.Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, Gropler RJ, Holzhuetter HG, Kizer JR, Lewandowski ED, Malloy CR, Neubauer S, Peterson LR, Portman MA, Recchia FA, Van Eyk JE, Wang TJ. American Heart Association Council on Basic Cardiovascular Sciences. Assessing Cardiac Metabolism: A Scientific Statement from the American Heart Association. Circ Res. 2016;118:1659–701. doi: 10.1161/RES.0000000000000097. PMID: 27012580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffrey FM, Rajagopal A, Malloy CR, Sherry AD. 13C-NMR: a simple yet comprehensive method for analysis of intermediary metabolism. Trends Biochem Sci. Jan. 1991;16(1):5–10. doi: 10.1016/0968-0004(91)90004-f. PMID: 2053137. [DOI] [PubMed] [Google Scholar]
- 3.Bottomley PA, Hardy CJ, Roemer PB, Mueller OM. Proton-decoupled, Overhauser-enhanced, spatially localized carbon-13 spectroscopy in humans. Magn Reson Med. 1989;12:348–63. doi: 10.1002/mrm.1910120307. PMID: 2560801. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016 Sep 15; doi: 10.1161/CIRCRESAHA.116.309769. PMID: 27635086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abragam A, Goldman M. Principles of Dynamic Nuclear Polarization. Reports on Progress in Physics. 1978;41:395–467. [Google Scholar]
- 6.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10, 000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golman K, Petersson JS, Magnusson P, Johansson E, Akeson P, Chai CM, Hansson G, Månsson S. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn Reson Med. 2008;59:1005–13. doi: 10.1002/mrm.21460. PMID: 18429038. [DOI] [PubMed] [Google Scholar]
- 8.Golman K, Petersson JS. Metabolic imaging and other applications of hyperpolarized 13C1. Acad Radiol. 2006;13:932–42. doi: 10.1016/j.acra.2006.06.001. PMID: 16843845. [DOI] [PubMed] [Google Scholar]
- 9.Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci Transl Med. 2013;5:198ra108. doi: 10.1126/scitranslmed.3006070. PMID: 23946197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007;104:19773–19777. doi: 10.1073/pnas.0706235104. PMID: 18056642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JR, Tyler DJ, Clarke K, Connelly KA, Wright GA, Cunningham CH. Hyperpolarized (13)C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail. 2013;15:130–40. doi: 10.1093/eurjhf/hfs192. PMID: 23258802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno KX, Sabelhaus SM, Merritt ME, Sherry AD, Malloy CR. Competition of Pyruvate with Physiological Substrates for Oxidation by the Heart: Implications for Studies with Hyperpolarized [1-13C]Pyruvate. Am J Physiol Heart Circ Physiol. 2010;298:H1556–64. doi: 10.1152/ajpheart.00656.2009. PMID: 20207817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 14.Khemtong C, Carpenter NR, Lumata LL, Merritt ME, Moreno KX, Kovacs Z, Malloy CR, Sherry AD. Hyperpolarized 13C NMR detects rapid drug-induced changes in cardiac metabolism. Magn Reson Med. 2015;74:312–319. doi: 10.1002/mrm.25419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen AP, Lau JY, Alvares RD, Cunningham CH. Using [1-13C]lactic acid for hyperpolarized 13C MR cardiac studies. Magn Reson Med. 2014 doi: 10.1002/mrm.25354. PMID: 25046652. [DOI] [PubMed] [Google Scholar]
- 16.Lau AZ, Miller JJ, Robson MD, Tyler DJ. Simultaneous assessment of cardiac metabolism and perfusion using copolarized [1-13C]pyruvate and 13C-urea. Magn Reson Med. 2016 Jan 7; doi: 10.1002/mrm.26106. doi: 10.1002/mrm.26106. [Epub ahead of print] PMID: 26743440. [DOI] [PMC free article] [PubMed] [Google Scholar]