Abstract
BACKGROUND
Long-term treatment with supplemental oxygen has unknown efficacy in patients with stable chronic obstructive pulmonary disease (COPD) and resting or exercise-induced moderate desaturation.
METHODS
We originally designed the trial to test whether long-term treatment with supplemental oxygen would result in a longer time to death than no use of supplemental oxygen among patients who had stable COPD with moderate resting desaturation (oxyhemoglobin saturation as measured by pulse oximetry [SpO2], 89 to 93%). After 7 months and the randomization of 34 patients, the trial was redesigned to also include patients who had stable COPD with moderate exercise-induced desaturation (during the 6-minute walk test, SpO2 ≥80% for ≥5 minutes and <90% for ≥10 seconds) and to incorporate the time to the first hospitalization for any cause into the new composite primary outcome. Patients were randomly assigned, in a 1:1 ratio, to receive long-term supplemental oxygen (supplemental-oxygen group) or no long-term supplemental oxygen (no-supplemental-oxygen group). In the supplemental-oxygen group, patients with resting desaturation were prescribed 24-hour oxygen, and those with desaturation only during exercise were prescribed oxygen during exercise and sleep. The trial-group assignment was not masked.
RESULTS
A total of 738 patients at 42 centers were followed for 1 to 6 years. In a time-to-event analysis, we found no significant difference between the supplemental-oxygen group and the no-supplemental-oxygen group in the time to death or first hospitalization (hazard ratio, 0.94; 95% confidence interval [CI], 0.79 to 1.12; P=0.52), nor in the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91 to 1.13), COPD exacerbations (rate ratio, 1.08; 95% CI, 0.98 to 1.19), and COPD-related hospitalizations (rate ratio, 0.99; 95% CI, 0.83 to 1.17). We found no consistent between-group differences in measures of quality of life, lung function, and the distance walked in 6 minutes.
CONCLUSIONS
In patients with stable COPD and resting or exercise-induced moderate desaturation, the prescription of long-term supplemental oxygen did not result in a longer time to death or first hospitalization than no long-term supplemental oxygen, nor did it provide sustained benefit with regard to any of the other measured outcomes. (Funded by the National Heart, Lung, and Blood Institute and the Centers for Medicare and Medicaid Services; LOTT ClinicalTrials.gov number, NCT00692198.)
Two trials that were conducted in the 1970s showed that long-term treatment with supplemental oxygen reduced mortality among patients with chronic obstructive pulmonary disease (COPD) and severe resting hypoxemia.1,2 These results led to the recommendation that supplemental oxygen be administered to patients with an oxyhemoglobin saturation, as measured by pulse oximetry (SpO2), of less than 89%.3,4 In the 1990s, two trials evaluated long-term treatment with supplemental oxygen in patients with COPD who had mild-to-moderate daytime hypoxemia; neither trial showed a mortality benefit, but both were underpowered to assess mortality.5,6 The effects of oxygen treatment on hospitalization,7–9 exercise performance, and quality of life are unclear.10
Medicare reimbursements for oxygen-related costs for patients with COPD exceeded $2 billion in 2011.11 If long-term treatment with supplemental oxygen reduces the incidence of COPD-related hospitalizations, increased use could be cost-effective. Reliable estimates of the number of prescriptions for supplemental oxygen that are written for the indication of exercise-induced desaturation are unavailable. Data suggest that many patients with advanced emphysema who are prescribed oxygen may not have severe resting hypoxemia.12
The Long-Term Oxygen Treatment Trial (LOTT) was originally designed to test whether the use of supplemental oxygen would result in a longer time to death than no use of supplemental oxygen among patients with COPD and moderate resting desaturation (SpO2, 89 to 93%). After 7 months and the randomization of 34 patients, the trial design was judged to be infeasible owing to lower-than-projected mortality and the phenotypic overlap between patients with moderate resting desaturation and those with exercise-induced desaturation. Accordingly, the investigators redesigned the trial to include patients with exercise-induced desaturation and to incorporate the secondary outcome of hospitalization for any cause into the new composite primary outcome. Patients who underwent randomization under the original design continued in the redesigned trial.
The amended trial tested whether the use of supplemental oxygen resulted in a longer time to death or first hospitalization for any cause (composite primary outcome) than no use of supplemental oxygen among patients with moderate resting desaturation or moderate exercise-induced desaturation. The original and amended trial protocols are available with the full text of this article at NEJM.org. Herein we report the primary and secondary outcomes and 11 of the 14 other outcomes listed in the trial protocol (see the Supplementary Appendix, available at NEJM.org, for the reasons that 3 outcomes are not reported).
METHODS
DESIGN
We conducted this parallel-group, randomized clinical trial of long-term supplemental oxygen versus no long-term supplemental oxygen in patients with COPD and moderate resting or exercise-induced desaturation. Randomization was performed in a 1:1 ratio, and the trial-group assignment was not masked. The primary outcome in the time-to-event analysis, measured from randomization, was the composite of death or first hospitalization. The protocol specified that the consistency of treatment effects would be tested in subgroups of patients that were defined according to prespecified baseline characteristics. The protocol and amendments were approved by the data and safety monitoring board for the trial and by the institutional review board at each center. No materials were donated to this trial.
PATIENTS
A total of 14 regional clinical centers and their associated sites (a total of 47 centers) screened patients who had stable COPD and moderate resting desaturation (SpO2, 89 to 93%) or moderate exercise-induced desaturation (during the 6-minute walk test, SpO2 ≥80% for ≥5 minutes and <90% for ≥10 seconds). All the patients signed a contract in which they agreed not to smoke while using oxygen, and they provided written informed consent. Table S1 in the Supplementary Appendix lists all the selection criteria.
INTERVENTIONS
Patients in the supplemental-oxygen group were prescribed 24-hour oxygen if their resting SpO2 was 89 to 93% and oxygen only during sleep and exercise if they had desaturation only during exercise. All the patients in the supplemental-oxygen group were prescribed stationary and portable oxygen systems and 2 liters of oxygen per minute during sleep. Patients in the supplemental-oxygen group who had been prescribed 24-hour oxygen were prescribed 2 liters of oxygen per minute at rest. The ambulatory dose of oxygen was individually prescribed and reassessed annually: 2 liters of oxygen per minute or adjusted higher to maintain an SpO2 of 90% or more for at least 2 minutes while the patient was walking. The protocol specified that patients in the supplemental-oxygen group continue the use of supplemental oxygen regardless of increase in the SpO2 level and that patients in the no-supplemental-oxygen group avoid the use of supplemental oxygen unless severe resting desaturation (SpO2 ≤88%) or severe exercise-induced desaturation (SpO2 <80% for ≥1 minute) developed. If either of these conditions developed, oxygen was prescribed and the oxygen requirement was reassessed after 30 days.
Each patient in the supplemental-oxygen group spoke with an adherence educator regularly to discuss barriers to adherence to the assigned regimen and to report average daily use. Each patient in the group that received no long-term supplemental oxygen (no-supplemental-oxygen group) spoke with an adherence educator 1 week after randomization to discuss living without supplemental oxygen. Every 4 months, all the patients were asked about supplemental-oxygen use; those who reported some oxygen use were asked to estimate the average daily use. Patients in the supplemental-oxygen group who used stationary oxygen concentrators also kept logs of meter readings.
OUTCOMES
In addition to the composite primary outcome and its components, outcomes included the incidence of COPD exacerbation, adherence to the supplemental-oxygen regimen, development of severe resting desaturation (as assessed by means of pulse oximetry), development of severe exercise-induced desaturation (as assessed by means of pulse oximetry), the distance walked in 6 minutes, and scores on the Quality of Well-Being Scale (mean daily scores range from 0 to 1, with higher scores indicating better quality of life; minimum clinically important difference, 0.03)13,14 and the St. George’s Respiratory Questionnaire (total scores range from 0 to 100, with higher scores indicating worse health-related quality of life; minimum clinically important difference, 4).15,16 A total of 33 centers elected to obtain spirometric measurements after randomization and to administer the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36; the summary scores for the physical and mental components each range from 0 to 100, with higher scores indicating better function; minimum clinically important difference, 5),17 the Hospital Anxiety and Depression Scale (scores on each measure [anxiety or depression] range from 0 to 21, with higher scores indicating greater anxiety or depression; minimum clinically important difference, 1.5),18,19 and the Pittsburgh Sleep Quality Index (total scores range from 0 to 21, with higher scores indicating worse sleep quality).20 The protocol lists three additional outcomes (nutritional status, risk of cardiovascular disease, and neurocognitive function) that are not reported here.
Patients attended visits yearly after randomization, were interviewed by telephone twice yearly, and completed mailed questionnaires at 4 months and 16 months (Table S2 in the Supplementary Appendix). Details regarding the ascertainment of the primary composite outcome and procedures for measuring resting and exercise-induced desaturation are provided in the Supplementary Appendix.
STATISTICAL ANALYSIS
Calculation of the final required sample was based on a time–to–composite event survival model with the use of the log-rank test statistic. Assuming 90% power to detect a hazard ratio for death or first hospitalization of 0.60 in the supplemental-oxygen group versus the no-supplemental-oxygen group, a two-sided type I error rate of 0.05, an 11.7% overall crossover rate from the no-supplemental-oxygen group to the supplemental-oxygen group, and a 3.1% overall crossover rate from the supplemental-oxygen group to the no-supplemental-oxygen group, we calculated a sample size of 737 patients. The hazard ratio of 0.60 corresponds to the smallest difference in mortality that the investigators judged to be clinically worthwhile (a 40% lower rate in the supplemental-oxygen group than in the no-supplemental-oxygen group), on the basis of the number of patients needed to treat. Because supplemental oxygen is expensive and its use is burdensome, the hazard ratio of 0.60 was also judged to be appropriate for the composite primary outcome of death or first hospitalization in the time-to-event analysis.
Under the original trial design, we assumed that the crossover rate from the no-supplemental-oxygen group to the supplemental-oxygen group would be 21% and the crossover rate from the supplemental-oxygen group to the no-supplemental-oxygen group would be 50%, on the basis of investigator consensus. In March 2012, the data and safety monitoring board approved the use of the observed crossover rates of 11.7% (from the no-supplemental-oxygen group to the supplemental-oxygen group) and 3.1% (from the supplemental-oxygen group to the no-supplemental-oxygen group) to refine the sample-size calculation. Additional details about the sample-size calculation are provided in the Supplementary Appendix.
Data were analyzed according to the treatment group to which the patients were randomly assigned (intention-to-treat approach) except as otherwise noted. A Cox proportional-hazards model21 with one binary covariate for treatment group was used to estimate the between-group hazard ratio for the primary composite outcome in the time-to-event analysis; the log-rank test was used for the P value. This method was also used for each of the secondary outcomes in the time-to-event analysis.
The consistency of the hazard ratio for the primary outcome across prespecified subgroups was assessed by a series of Cox proportional-hazard models with covariates that included the treatment-group indicator, indicators for the levels of the subgroup factor, and treatment-by-subgroup interaction terms. The P values for consistency of hazard ratios across subgroups were determined by Wald chi-square tests. Per the trial protocol, all reported P values are nominal and two-sided and were not corrected for multiple, prespecified comparisons. A P value of less than 0.05 was considered to indicate statistical significance for the composite primary outcome, and a P value of less than 0.01 was considered to indicate statistical significance for a treatment-by-subgroup interaction effect on the primary outcome. Bonferroni corrections were used to determine the P values that were required for statistical significance of the trial-group differences on the secondary and other outcomes and for statistical significance of the multiple treatment-by-subgroup interaction effects on the primary outcome that were assessed.22 Additional details about the statistical analysis are provided in the protocol and the Supplementary Appendix.
RESULTS
TRIAL POPULATION
From January 2009 through August 2014, a total of 738 patients at 42 centers underwent randomization in the trial: 368 patients were randomly assigned to the supplemental-oxygen group and 370 to the no-supplemental-oxygen group (Fig. S1 and Table S3 in the Supplementary Appendix). In the supplemental-oxygen group, 220 patients were prescribed 24-hour oxygen and 148 were prescribed oxygen during exercise and sleep only. Of the 738 patients who underwent randomization, 133 (18%) had resting desaturation only, 319 (43%) had exercise-induced desaturation only, and 286 (39%) had both types of desaturation. The trial groups were similar at baseline except that the patients in the supplemental-oxygen group had a lower BODE index (a scoring system incorporating information on the body-mass index, airflow obstruction, dyspnea, and 6-minute walk distance; higher scores indicate a greater risk of death)23 than those in the no-supplemental-oxygen group (Table 1, and Table S4 in the Supplementary Appendix).
Table 1.
Characteristics of the Patients at Enrollment.*
| Characteristic | No Supplemental Oxygen (N = 370) | Supplemental Oxygen (N = 368) |
|---|---|---|
| Age — yr | 69.3±7.4 | 68.3±7.5 |
| Male sex — no. (%) | 276 (75) | 266 (72) |
| Race — no. (%)† | ||
| Black | 34 (9) | 46 (12) |
| White | 328 (89) | 311 (85) |
| Other | 11 (3) | 17 (5) |
| Medicare coverage — no. (%) | 273 (74) | 268 (73) |
| Current tobacco-cigarette smoker — no. (%) | 92 (25) | 110 (30) |
| Quality of Well-Being Scale mean daily score‡ | 0.56±0.13 | 0.56±0.13 |
| St. George’s Respiratory Questionnaire total score§ | 50.2±17.1 | 49.8±18.7 |
| Oxygen-desaturation type qualifying the patient for enrollment — no. (%) | ||
| Resting only | 60 (16) | 73 (20) |
| Exercise only | 171 (46) | 148 (40) |
| Resting and exercise | 139 (38) | 147 (40) |
| SpO2 at rest while breathing ambient air — % | ||
| All patients | 93.5±1.9 | 93.3±2.1 |
| Resting only | 92.3±0.8 | 92.4±0.9 |
| Exercise only | 95.2±1.2 | 95.4±1.4 |
| Resting and exercise | 91.9±1.2 | 91.7±1.1 |
| Nadir SpO2 during 6-min walk while breathing ambient air — no./total no. (%)¶ | ||
| <86% | 85/290 (29) | 86/292 (29) |
| 86–88% | 103/290 (36) | 105/292 (36) |
| >88% | 102/290 (35) | 101/292 (35) |
Plus-minus values are means ±SD. There were no significant differences at baseline between the group of patients assigned to receive long-term supplemental oxygen (supplemental-oxygen group) and the group of those assigned to receive no long-term supplemental oxygen (no-supplemental-oxygen group), except that the patients in the supplemental-oxygen group had a lower BODE index (a scoring system incorporating information on body-mass index, airflow obstruction, dyspnea, and 6-minute walk distance; higher scores indicate a greater risk of death)23 than those in the no-supplemental-oxygen group (P = 0.007); details of the BODE index values and other characteristics at baseline are provided in Table S4 in the Supplementary Appendix. SpO2 denotes oxyhemoglobin saturation as measured by means of pulse oximetry.
Race was self-reported. Patients were permitted to select more than one race group.
The Quality of Well-Being Scale is a 77-item quality-of-life questionnaire completed by the patient. A score of 0 indicates death. The mean daily score ranges from 0 to 1, with higher scores indicating better quality of life. The minimum clinically important difference is 0.03.13,14
The St. George’s Respiratory Questionnaire is a 51-item questionnaire on the health-related quality of life with regard to respiratory symptoms that is completed by the patient. The total score ranges from 0 to 100, with lower scores indicating better health-related qualify of life. The minimum clinically important difference is 4.15,16
The nadir SpO2 is the 10th lowest SpO2 observed during the 6-minute walk. A total of 10 patients (6 patients in the supplemental-oxygen group and 4 in the no-supplemental-oxygen group) either did not attempt or began but did not complete the 6-minute walk. Reasons included being in a wheelchair, amputation of foot or leg, sciatic pain, starting the walk and stopping because of back pain, or other reason; these 10 participants met the resting hypoxemia criterion. The nadir SpO2 could not be calculated for 146 patients (70 patients in the supplemental-oxygen group and 76 in the no-supplemental-oxygen group) owing to loss of their oximetry data file or a technical issue with their oximetry data file obtained at enrollment.
Patients were followed for 1 to 6 years; the last visits occurred during the period from May through August 2015 (median follow-up, 18.4 months). Vital status as of August 31, 2015, was ascertained in all patients. A total of 97% of the patients had at least 1 year of follow-up for hospitalization. Most patients in the supplemental-oxygen group used 2 liters of oxygen per minute during exercise throughout follow-up (Table S5 in the Supplementary Appendix).
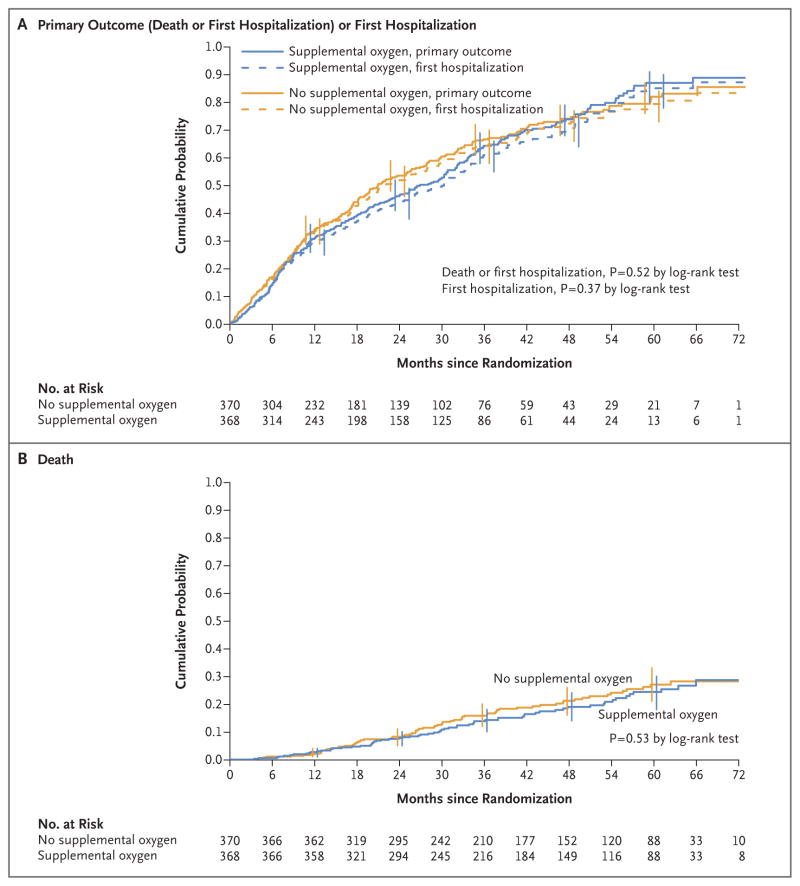
PRIMARY OUTCOME
In a time-to-event analysis, we found no significant difference between the trial groups in the composite outcome of death or first hospitalization for any cause or in either component (Fig. 1 and Table 2). No significant difference was noted in the subgroups defined according to oxygen prescription, desaturation profile, race, sex, smoking status, nadir SpO2 during exercise (the 10th lowest SpO2 observed during the 6-minute walk), forced expiratory volume in 1 second, BODE index, SF-36 physical-component score, body-mass index, or history of anemia (Table S6 in the Supplementary Appendix).
Figure 1. Kaplan–Meier Analyses of the Primary Outcome of Death or First Hospitalization for Any Cause and for the Component Events in the Intention-to-Treat Population.
Panel A shows the results of a time-to-event analysis of the primary outcome, which was a composite of death or first hospitalization for any cause; the median follow-up was 18.4 months. Data for 120 patients who were assigned to receive long-term supplemental oxygen (supplemental-oxygen group) and 120 assigned to receive no long-term supplemental oxygen (no-supplemental-oxygen group) who neither died nor had a hospitalization were censored at the date of the last interview. Error bars indicate 95% confidence intervals (assessed every 12 months). For the time-to-event analysis of the first hospitalization for any cause, the median follow-up was 18.4 months. Data for 139 patients in the supplemental-oxygen group and 133 in the no-supplemental-oxygen group were censored as of their date of death (if there was no hospitalization before death) or as of the date of their last interview (if they were alive and had no hospitalization). Panel B shows the results of a time-to-event analysis of death; the median follow-up was 41.5 months. Data for 302 patients in the supplemental-oxygen group and 297 in the no-supplemental-oxygen group who were alive on August 31, 2015, were censored as of that date. The hazard ratios and 95% confidence limits were derived from Cox regression models, with supplemental oxygen versus no supplemental oxygen as the single model variable. P values were derived from log-rank tests. For the components of the composite primary outcome (death and first hospitalization), a P value of less than 0.025 (0.05 divided by 2) was considered to indicate statistical significance, with the use of a Bonferroni adjustment for multiple comparisons.22
Table 2.
Primary Composite Outcome of Death or First Hospitalization for Any Cause and Composite Events in the Intention-to-Treat Population.*
| Outcome | No Supplemental Oxygen (N = 370) | Supplemental Oxygen (N = 368) | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcome | ||||
|
| ||||
| Death or first hospitalization for any cause | 0.94 (0.79–1.12) | 0.52 | ||
| No. of events | 250 | 248 | ||
| Composite rate per 100 person-yr | 36.4 | 34.2 | ||
|
| ||||
| Primary-outcome component events | ||||
|
| ||||
| Death | 0.90 (0.64–1.25) | 0.53 | ||
| No. of deaths | 73 | 66 | ||
| Rate per 100 person-yr | 5.7 | 5.2 | ||
|
| ||||
| First hospitalization for any cause | 0.92 (0.77–1.10) | 0.37 | ||
| No. of first hospitalizations | 237 | 229 | ||
| Rate per 100 person-yr | 34.5 | 31.6 | ||
The primary outcome was death or first hospitalization for any cause, whichever came first, in patients randomly assigned to receive supplemental oxygen as compared with those assigned to receive no supplemental oxygen. For the composite-event analysis, data from 120 patients in the supplemental-oxygen group and 120 in the no-supplemental-oxygen group who neither died nor had a hospitalization were censored as of their last interview. For the analysis of death, data for 302 patients in the supplemental-oxygen group and 297 in the no-supplemental-oxygen group who were alive on August 31, 2015, were censored as of that date. For the analysis of the first hospitalization, data for 139 patients in the supplemental-oxygen group and 133 in the no-supplemental-oxygen group were censored as of their date of death (if there was no hospitalization before death) or as of the date of their last interview (if they were alive and had no hospitalization). For the components of the composite primary outcome (death and first hospitalization), a P value of less than 0.025 (0.05 divided by 2) was considered to indicate statistical significance, with the use of a Bonferroni adjustment for multiplicity of comparisons.22 P values were calculated by the log-rank test. CI denotes confidence interval.
Patients in the supplemental-oxygen group who reported having had a COPD exacerbation 1 to 3 months before enrollment had a longer time to death or first hospitalization than similar patients in the no-supplemental-oxygen group (hazard ratio, 0.58; 95% confidence interval [CI], 0.39 to 0.88; P = 0.007 for interaction), as did patients who were 71 years of age or older at enrollment (hazard ratio, 0.75; 95% CI, 0.57 to 0.99; P = 0.03 for interaction) and those who had a lower quality of life (Quality of Well-Being Scale score, <0.55) at enrollment (hazard ratio, 0.77; 95% CI, 0.60 to 0.99; P = 0.03 for interaction). However, none of these subgroup-by-treatment interaction effects were significant when the analysis was adjusted for multiple comparisons. In the as-treated analysis, no difference was found between patients who used oxygen for at least 16 hours per day and all others. (Details are provided in Tables S6 and S7 in the Supplementary Appendix.)
ADHERENCE TO REGIMEN
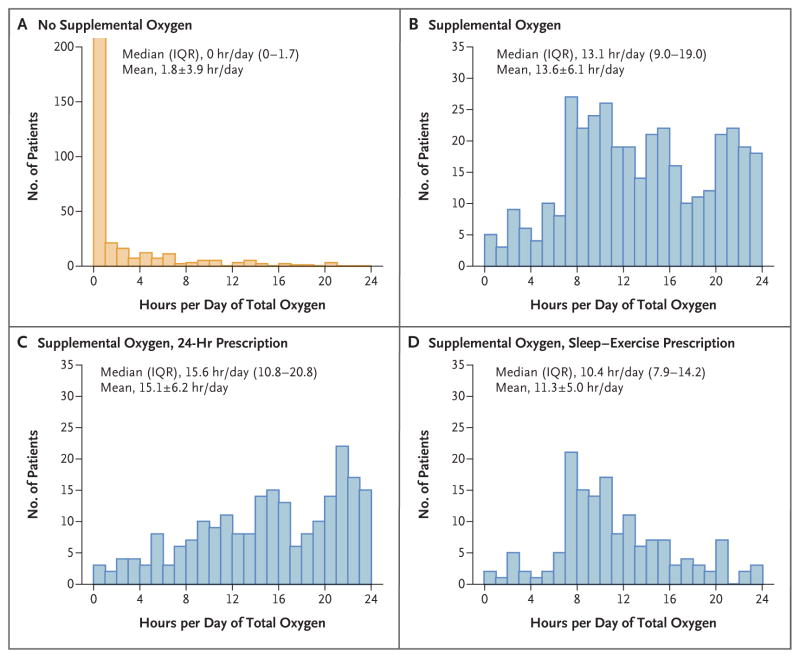
Histograms of self-reported use of supplemental oxygen as averaged over follow-up indicate much longer daily mean (±SD) use in the supplemental-oxygen group than in the no-supplemental-oxygen group (13.6±6.1 vs. 1.8±3.9 hours per day) (Fig. 2). There was a separation of patients in the supplemental-oxygen group according to prescription (15.1±6.2 hours per day in the 24-hour group vs. 11.3±5.0 hours per day in the sleep–exercise group), but there was considerable overlap. A comparison of self-reported stationary concentrator use with use that was calculated from meter readings in 100 patients in the supplemental-oxygen group who had available data showed a significant linear trend in bias (P<0.001), in which patients with less-than-average hours of daily use tended to overestimate their use and those with greater-than-average hours of daily use tended to underestimate their use (Fig. S2 in the Supplementary Appendix).
Figure 2. Self-Reported Use of Supplemental Oxygen during Follow-up.
Shown are histograms of total self-reported hours of supplemental-oxygen use per day (sum of stationary use and portable use) according to randomized assignment and prescription for supplemental oxygen (24-hour use or use during sleep and exercise). Plus–minus values are means ±SD. The value plotted for a patient is the mean of all the patient’s self-reports during follow-up. Self-reports were obtained 3 times yearly in the no-supplemental-oxygen group. In the supplemental-oxygen group, self-reports were more frequent during year 1 (12 times) and were obtained 3 times yearly thereafter. The median number of self-reports for a patient was 20 in the supplemental-oxygen group (range, 6 to 20) and 8 in the no-supplemental-oxygen group (range, 0 to 18). All the patients in the supplemental-oxygen group provided at least one assessment; 363 patients (98%) in the no-supplemental-oxygen group provided at least one assessment. IQR denotes interquartile range.
COMPARISON WITH DESIGN ASSUMPTIONS
Fewer enrollees than expected were hospitalized in the year before screening. However, more patients than expected were hospitalized during follow-up. Observed mortality rates compared well with the design assumptions (Table S8 in the Supplementary Appendix).
OTHER OUTCOMES
The two trial groups did not differ significantly with regard to the rates of all hospitalizations (rate ratio, 1.01; 95% CI, 0.91 to 1.13), COPD exacerbations (rate ratio, 1.08; 95% CI, 0.98 to 1.19), COPD-related hospitalizations (rate ratio, 0.99; 95% CI, 0.83 to 1.17), or non–COPD-related hospitalizations (rate ratio, 1.03; 95% CI, 0.90 to 1.18). (Fig. S3 and Table S9 in the Supplementary Appendix). We found no consistent differences between groups in the change from baseline in measures of quality of life, anxiety, depression, or in lung function, distance walked in 6 minutes, or other measures of functional status (Fig. S4 and Table S10 in the Supplementary Appendix).
ADVERSE EVENTS
A total of 51 adverse events were attributed to the use of supplemental oxygen (Table S11 in the Supplementary Appendix). There were 23 reports of tripping over equipment, with two patients requiring hospitalization. Five patients reported a total of six instances of fires or burns, with one patient requiring hospitalization.
DISCUSSION
We found that the prescription of supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to death, time to first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status. We found no effect on the primary outcome in subgroups of patients defined according to desaturation type, prescription type, or adherence to the regimen. The consistency of the null findings strengthens the overall conclusion that long-term supplemental oxygen in patients with stable COPD and resting or exercise-induced moderate desaturation has no benefit with regard to the multiple outcomes measured.
Our data support the conclusions of earlier studies that among patients with COPD who have a resting SpO2 of more than 88%, long-term treatment with supplemental oxygen does not result in longer survival than no long-term supplemental oxygen therapy, regardless of whether the patients have exercise-induced desaturation.5,6,24 Our findings contrast with the prolonged survival that was observed among patients with COPD and severe desaturation who were treated with supplemental oxygen.1,2 Possible reasons for this discrepancy are the nonlinear threshold effects of oxygen saturation on pulmonary vasoconstriction, mediator release, and ventilatory drive,25,26 which occur with an SpO2 of 88% or less and which may be more important in patients with chronic hypoxemia.
A systematic review and meta-analysis suggested that oxygen therapy may reduce dyspnea in patients with COPD and mild or no hypoxemia.27 We found no consistent benefit of long-term supplemental oxygen with regard to measures of quality of life, depression, anxiety, or functional status.
This trial has some limitations. First, some patients may not have enrolled in the trial because they or their providers believed that they were too ill or that they benefited from oxygen. Highly symptomatic patients who declined enrollment might have had a different response to oxygen than what we observed in the enrolled patients. Second, the lack of masking may have influenced some of the patient-reported outcomes; however, it is unlikely to have influenced the primary outcome. Third, we did not use uniform devices for oxygen delivery; it is possible that there was variability in the amount of oxygen delivered. Fourth, the immediate effects of oxygen on symptoms or exercise performance were not assessed. We did not measure nocturnal oxygen saturation; some patients with COPD and severe nocturnal desaturation might benefit from nocturnal oxygen supplementation.28,29 Fifth, patients’ self-reported adherence may have been an overestimate of their actual oxygen use. However, we found good agreement with the use as measured by means of serial meter readings on the concentrator. The estimated mean hours per day of use in the supplemental-oxygen group (15.1±6.2 hours per day in the 24-hour group and 11.3±5.0 hours per day in the sleep–exercise group) (Fig. 2) was similar to the use observed in the Nocturnal Oxygen Therapy Trial (17.7 hours per day in the continuous-oxygen group and 12.0 hours per day in the nocturnal-oxygen group).1 However, we cannot exclude the possibility that longer exposures to oxygen in the supplemental-oxygen group might have given different results. Finally, because hospitalization was recorded from self-report every 4 months, it is possible that we underestimated the number of hospitalizations; however, there did not appear to be systematic bias in follow-up between groups.
In conclusion, among patients with stable COPD and resting or exercise-induced moderate desaturation, we found that long-term supplemental oxygen did not provide any benefit with respect to the time to death or first hospitalization or any sustained benefit with respect to any other measured outcome.
Supplementary Material
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute, National Institutes of Health and Department of Health and Human Services (contract nos., HHSN268200736183C, HHSN-268200736184C, HHSN268200736185C, HHSN268200736186C, HHSN268200736187C, HHSN268200736188C, HHSN268200736189C, HHSN268200736190C, HHSN268200736191C, HHSN268200736192C, HHSN268200736193C, HHSN268200736194C, HHSN268200736195C, HHSN268200736196C, HHSN268200736197C, Y1-HR-7019-01, and Y1-HR-8076-01), in cooperation with the Centers for Medicare and Medicaid Services, Department of Health and Human Services.
APPENDIX
The affiliations of the members of the writing group are as follows: University of Colorado, Denver (R.K.A.); Veterans Affairs (VA) Puget Sound Health Care System and University of Washington, Seattle (D.H.A.); Johns Hopkins University School of Medicine (A.L.B., R.W.) and Johns Hopkins University Bloomberg School of Public Health (D.S., J.T., A.L.S.), Baltimore; Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center (R.C.) and Cedars–Sinai Medical Center (S.P.) — both in Los Angeles; Birmingham VA Medical Center (J.A.C.) and the University of Alabama (J.A.C., W.B.), Birmingham; Lewis Katz School of Medicine at Temple University, Philadelphia (G.J.C.), and University of Pittsburgh, Pittsburgh (F.S.) — both in Pennsylvania; Ohio State University, Columbus (P.D.), Cincinnati VA Medical Center and University of Cincinnati College of Medicine, Cincinnati (R.J.P.), and Cleveland Clinic, Cleveland (J.K.S.) — all in Ohio; Brigham and Women’s Hospital and Harvard Medical School, Boston (A.L.F.); University of Michigan, Ann Arbor (S.E.G.); University of Utah Health Sciences Center, Salt Lake City (R.E.K.); Duke University Medical Center, Durham, NC (N.M.); Weill Cornell Medical Center, New York (F.J.M.); Kaiser Permanente Center for Health Research, Portland, OR (T.S.); and Washington University School of Medicine, St. Louis (R.D.Y.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dr. Au reports serving on a data monitoring committee for Novartis; Dr. Casaburi, serving on advisory boards for Boehringer Ingelheim, AstraZeneca, and Novartis and receiving consulting fees from GlaxoSmithKline and Astellas Pharma, lecture fees from Boehringer Ingelheim and AstraZeneca, and grant support to his institution from Boehringer Ingelheim and Novartis; Dr. Cooper, receiving grant support from AstraZeneca; Dr. Fuhlbrigge, serving on an adjudication committee for ICON Medical Imaging, serving as an unpaid consultant for AstraZeneca, and receiving consulting fees from GlaxoSmithKline and travel support from AstraZeneca; Dr. MacIntyre, receiving consulting fees from Breathe Technologies and Ventec Life Systems; Dr. Martinez, serving on steering committees for Bayer, Boehringer Ingelheim, Centocor, Gilead Sciences, Takeda Pharmaceuticals (formerly Nycomed), Afferent Pharmaceuticals, Forest Laboratories, Janssen, GlaxoSmithKline, AstraZeneca, and Pearl Therapeutics, serving on advisory boards for Boehringer Ingelheim, Genentech, Ikaria, Kadmon, Takeda Pharmaceuticals (formerly Nycomed), Pfizer, Veracyte, Forest Laboratories, Janssen, GlaxoSmithKline, AstraZeneca, Bellerophon Therapeutics (formerly Ikaria), Novartis, Pearl Therapeutics, Roche, Sunovion Pharmaceuticals, Theravance Biopharma, and Concert Pharmaceuticals, serving on a data and safety monitoring board for Biogen (formerly Stromedix) and GlaxoSmithKline, and receiving fees for participating in continuing medical education activities from AcademicCME, MedEd Consulting, Continuing Education, Potomac Center for Medical Education, CME Incite, Annenberg Center for Health Sciences at Eisenhower, Integritas Communications, in Thought Research, Miller Medical Communications, Paradigm Medical Communications, PeerVoice, HayMarket Communications, Prime Healthcare, WebMD, and PeerView Academic Network, consulting fees from Axon Communications, Johnson & Johnson, Clarion Communications, Adept Field Solutions, Amgen, Proterixbio (formerly Bioscale), Unity Biotechnology, and Lucid Communique Medical Education, and lecture fees from AstraZeneca; Dr. Stoller, receiving consulting fees from Baxalta, CSL Behring, Grifols, and Arrowhead Pharmaceuticals; and Dr. Wise, receiving consulting fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, ContraFect, GlaxoSmithKline, Janssen, Mylan, Novartis, Pfizer, Pulmonx, Roche, Spiration, Sunovion Pharmaceuticals, Teva Pharmaceutical Industries, Theravance, Verona Pharma, and Vertex Pharmaceuticals and grant support from Boehringer Ingelheim, GlaxoSmithKline, Teva Pharmaceutical Industries, and Pearl Therapeutics. No other potential conflict of interest relevant to this article was reported.
References
- 1.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 2.Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 3.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 5.Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14:1002–8. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 6.Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52:674–9. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:892–8. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 8.Ringbaek T, Martinez G, Lange P. The long-term effect of ambulatory oxygen in normoxaemic COPD patients: a randomised study. Chron Respir Dis. 2013;10:77–84. doi: 10.1177/1479972312473135. [DOI] [PubMed] [Google Scholar]
- 9.Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168:1034–42. doi: 10.1164/rccm.200212-1525OC. [DOI] [PubMed] [Google Scholar]
- 10.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the Long-term Oxygen Treatment Trial. Chest. 2010;138:179–87. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2013 CMS statistics: CMS publication 03504. Washington, DC: Office of Information Products and Data Analytics; Aug, 2013. [Google Scholar]
- 12.Drummond MB, Blackford AL, Benditt JO, et al. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: retrospective analysis of the National Emphysema Treatment Trial. Chest. 2008;134:497–506. doi: 10.1378/chest.08-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37:85–95. doi: 10.1016/0021-9681(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD. 2005;2:91–7. doi: 10.1081/copd-200052090. [DOI] [PubMed] [Google Scholar]
- 15.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire Clin Ther. 2000;22:1121–45. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW. St. George’s Respiratory Questionnaire: MCID COPD. 2005;2:75–9. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–79. [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the Hospital Anxiety and Depression Scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc [B] 1972;34:187–220. [Google Scholar]
- 22.Hsu JC. Multiple comparisons: theory and methods. London: Chapman and Hall; 1996. [Google Scholar]
- 23.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 24.Ameer F, Carson KV, Usmani ZA, Smith BJ. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev. 2014;6:CD000238. doi: 10.1002/14651858.CD000238.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timms RM, Tisi GM. The effect of short-term oxygen supplementation on oxygen hemoglobin affinity in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;131:69–72. doi: 10.1164/arrd.1985.131.1.69. [DOI] [PubMed] [Google Scholar]
- 26.Williamson W, Fuld J, Westgate K, Sylvester K, Ekelund U, Brage S. Validity of reporting oxygen uptake efficiency slope from submaximal exercise using respiratory exchange ratio as secondary criterion. Pulm Med. 2012;2012:874020. doi: 10.1155/2012/874020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uronis HE, Ekström MP, Currow DC, McCrory DC, Samsa GP, Abernethy AP. Oxygen for relief of dyspnoea in people with chronic obstructive pulmonary disease who would not qualify for home oxygen: a systematic review and meta-analysis. Thorax. 2015;70:492–4. doi: 10.1136/thoraxjnl-2014-205720. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher EC, Luckett RA, Miller T, Fletcher JG. Exercise hemodynamics and gas exchange in patients with chronic obstruction pulmonary disease, sleep desaturation, and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1989;140:1237–45. doi: 10.1164/ajrccm/140.5.1237. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher EC, Miller J, Divine GW, Fletcher JG, Miller T. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mm Hg. Chest. 1987;92:604–8. doi: 10.1378/chest.92.4.604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.