Abstract
The avian influenza subtype H9N2 is considered a low pathogenic virus which is endemic in domestic poultry of a majority of Asian countries. Many reports of seropositivity in occupationally poultry-exposed workers and a number of confirmed human infections with an H9N2 subtype of avian influenza have been documented up to now. Recently, the human infections with both H7N9 and H10N8 viruses highlighted that H9N2 has a great potential for taking a part in the emergence of new human-infecting viruses. This review aimed at discussing the great potential of H9N2 virus which is circulating at avian-human interface, for cross-species transmission, contribution in the production of new reassortants and emergence of new pandemic subtypes. An intensified surveillance is needed for controlling the future risks which would be created by H9N2 circulation at avian-human interfaces.
Keywords: Avian influenza, future pandemics, H9N2, human infection, human interface, reassortment potential
INTRODUCTION
Respiratory viral infections in adults cause significant morbidity and mortality, especially in high-risk patients.[1,2] Influenza is considered an important respiratory infection. The influenza syndrome has typically sudden onset and is characterized by fever, headache, sore throat, cough, nasal congestion, myalgia, vomiting, and loss of appetite.[3] Influenza viruses are recognized as single-stranded, enveloped negative-sense RNA particles, and they belong to the viral family of orthomyxoviridae. Three types of them exist, including influenza A, B, and C. All types can infect humans, but both types A and B are considered human pathogens. Only viruses of the Group A genus have been isolated from birds and termed avian influenza viruses (AIVs).[4] Influenza type A viruses are divided into subtypes based on genetic and antigenic differences in the two surface spike proteins, hemagglutinin (HA) and neuraminidase (NA).[5] Most influenza infections are spread by virus-laden respiratory droplets several microns in diameter that are expelled during coughing and sneezing.[6] Fomites represent another mode of transmission. Influenza has the potential to transmit into human by birds or pigs.[6] The negative-sense RNA genome of influenza A virus is composed of eight segments; these segments are responsible for encoding 12 proteins. In the last stage of viral assembly, these genomic virion RNAs are incorporated into the virion as it buds from the apical plasma membrane of the cell.[7] Genome segmentation confers evolutionary advantages for the virus, however also causes a limitation during the virion assembling because at least one copy of each of the eight segments is required to form a fully infectious virus particle.[7]
The potential of genetic reassortment of influenza A viruses, due to segmented genome of them, from different animal species is thought to be a mechanism for the development of influenza viruses with pandemic potential.[8]
Avian and animal influenza viruses can sporadically enter to humans, causing outbreaks with different levels of severity. In some cases, the human-to-human virus transmission does not take place. In other cases, the human-to-human transmission can occur, resulting in worldwide influenza pandemics.[9]
Previous reports[10,11] showed that the avian to human transmission of two avian viruses including H5N1 and H9N2 is possible. This review focused on H9N2, its infections in human, pandemic potential, surveillance, and prevention of its spread.
AVIAN INFLUENZA H9N2
The type A of influenza viruses is naturally circulating viruses in waterfowls, which does not cause any clear symptoms in them. However, for domestic fowls including chickens, geese, ducks, and turkeys the transmission of influenza A viruses can promote some difficulties. The basis for classification of influenza A viruses is the two glycoproteins of them. HA and NA glycoproteins are expressed on the surface of influenza viruses. Up to now, 15 (or 16)[12] HA and 9 NA types have been reported in birds worldwide.
The H9N2 subtype was isolated for the first time from turkeys in the Northern state of United States in 1966.[13] Afterward, this subtype was isolated from wild birds of that area, then detected from domestic poultry of Europe, Africa, Asia, and the Middle East.[4] The subtype H9N2 is just limited to the wild birds in North America.[14] However, H9N2 is endemic in domestic poultry and frequent outbreaks in Asian countries including Iran, Saudi Arabia, Pakistan, and Iraq[4] have been reported previously. In Iran, an H9N2 outbreak in poultry farms took place in 1998[15] and now circulates endemically, and vaccination of poultry is a routine program.[16] Hence, infections with this subtype are often asymptomatic or with mild illness. From deadliness point of view, there are two groups of AIVs high pathogenicity (HP) and low pathogenicity (LP) AIVs. H5 or H7 subtypes are considered HP but H9N2 is an LP one. LP viruses usually cause mild or asymptomatic disease in poultry whereas HPs are associated with severe symptoms and high mortality. H9N2 circulation in domestic birds has both economic and health consequences. Despite the LP nature of H9N2, co-infection of poultry with noninfluenza respiratory pathogens can enhance the severity of the clinical syndrome in poultry, reduction in the yield of products derived from chicken and higher rates of morbidity. On the other hand, lower range of pathogenicity and to some extent mild illness of poultry through infection with this subtype in domestic poultry reinforces the likely hood of being a carrier host for this subtype; consequently, result in raising concerns on crossing the species barrier, contribution in the emergence of novel viruses and causing new pandemics.
AVIAN INFLUENZA H9 AT AVIAN-HUMAN INTERFACE
The illness of domestic poultry either in asymptomatic or mortal form is public health threat because infected poultry excretes high amounts of flu viruses in nasal fluid, saliva, and other body fluids also via feces. According to Food and Agricultural Organization of the United Nations, one gram of viral contaminated feces has sufficient viruses that potentially can infect 1 million other fowls.[17] The potential of AIV for transmission into domestic poultry raises great concerns regarding both occupational and public health.
Health and safety executive states that occupational exposure to avian influenza may occur in those who are in close contact with infected birds or humans; work with materials or products from infected birds; or are in contact with waste products from infected birds.[7] Previous reports[8,9] showed that the avian to human transmission of two avian viruses including H5N1 and H9N2 is possible. Several reports of seropositivity in occupationally or nonoccupationally exposed people have been documented. Table 1 summarizes the evidence of seropositivity in poultry workers, slaughterhouse workers, and other handlers of poultry.
Table 1.
Seropositivity for H9N2 in occupationally poultry-exposed groups
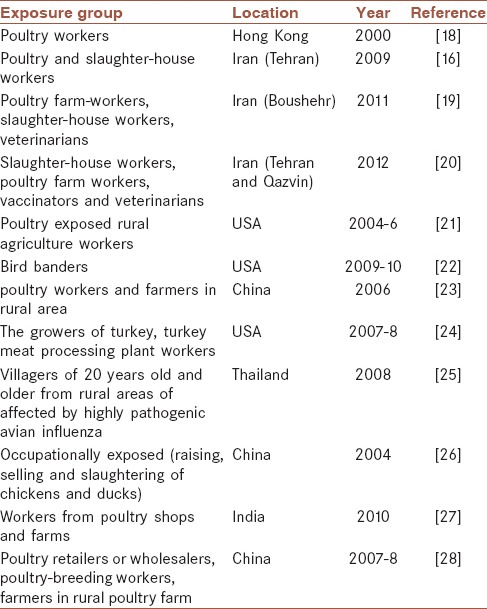
Table 1 shows that transmission of H9N2 from poultry to occupationally exposed groups can occur; these workers are more likely to be seropositive for H9N2 than nonexposed persons.
Although the vaccination history of seasonal influenza was reported by exposed workers in a majority of studies. None of them examined the effectiveness of human vaccination on genetic reassortment of AIVs. In a number of studies, a cross-reactivity between avian and human influenza viruses was detected[16,20,29] which may cause a type of immunity to prevent generation of reassortant novel pandemic viruses. This hypothesis has not been confirmed; however, immunization is highly recommended for poultry exposed workers because of their usual contacts with infected birds. By the way, several studies documented that slaughter-house workers showed a higher percentage of seropositivity than other groups of workers. Because butchers in slaughter-houses are in close contact with visceral parts of chickens, therefore, it will result in more than usual exposure to viral infected material and surfaces.
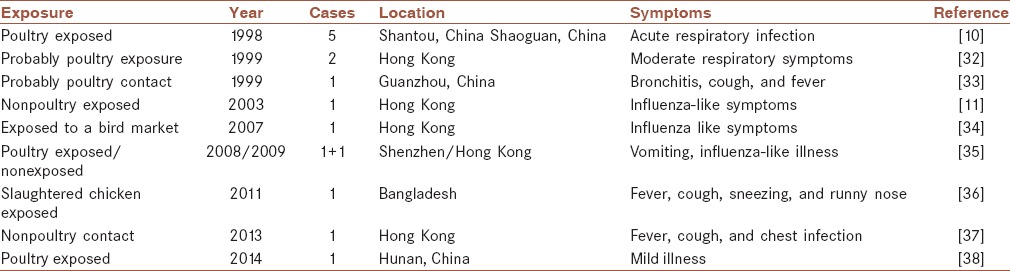
In 1999, isolation of H9N2 viruses from nasopharyngeal aspirate specimens of two children was confirmed for the first time in Hong Kong.[29] In fact, the H9N2 viruses had obtained the receptors very close to those of human influenza viruses that had transmitted directly to humans.[30] The transmission of H9N2 can cause mild respiratory symptoms in human. Freidl et al. reported that there have been 15 confirmed human infections with AIV A (H9N2), up to now.[31] Table 2 summarizes the cases of human infection with H9N2. According to Table 2, only two of 15 cases had not been exposed to birds. The transmissions of H9N2 to human that grow or work with the birds are documented in the literature, but some studying limitations restricted the estimation of real frequency of the cross-species transmission of H9N2. Among AIVs H5N1 has been caused the highest death cases (~370) since its first emergence in Hong Kong in 1997. In comparison, infections of human with H9N2 were not fatal. Matrosovich et al. reported that Asian poultry H9N2 viruses show human virus receptors like specificity.[39] Therefore, H9N2 has the potential to become human-to-human transmissible, but there is not any report of transmission of it between humans.
Table 2.
Reported human infections with H9N2
The genetically reassortment of AIVs from different species is considered to be a mechanism for new influenza virus development having pandemic potential. Each influenza viruses from different animals such as human, avian and swine are capable to circulate particularly within their original species. However, influenza viruses have the ability to transmit to nonnative hosts.
The previous literature emphasized that ideal mixing vessel for the generation of novel AIVs is swine. Peiris et al. reported co-circulation of H9N2 and human H3N2 subtypes in pigs that increase the possibility of being an intermediate host for the emergence of new reassortants pandemic subtypes.[40]
However, recent research archive shows that both swine and human have the potential to act as AIVs re-assortment mixing vessels. A comparison between the receptors of human, swine, and birds clearly reveals that the reverse human to avian transmission of human influenza viruses is less probable. However, mammals including human and swine have closer receptors.[41] Therefore, human in particular people with occupational close contacts with infected birds may act as mixing host for the emergence of novel reassortants [Figure 1]. This reassortment between avian and human viruses is an antigenic shift. In 1999 first human-avian reassortant of H9N2 was isolated from humans in Europe.[42]
Figure 1.
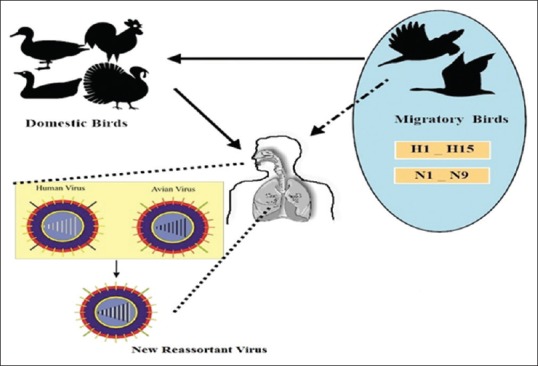
Antigenic shift of influenza viruses. Occupationally exposed workers may act as mixing host for emergence of novel influenza reassortants
Recently, antigenic and phylogenetic studies of H9N2 viruses isolated from markets in Hong Kong showed that in the H9N2 genome, six segments were originated from Chinese H9N2 virus, but the PB1 and PB2 genes are very near to both the H5N1 viruses and an H9N2 isolated from Hong Kong quail.[5] These reports highlight the antigenic shifts of H9N2 because of endemic status of this subtype in Asian countries. On the other hand, Liu et al.,[51] showed that the real incubators for human-avian adapted influenza viruses are poultry with H9N2 infection. The emergence of novel reassorted virus emphasizes the potential high risk of, transmission of subtype H9N2 viruses to humans.[43] Furthermore, according to the WHO report, the genotype of H7N9 viruses which isolated from Chinese infected humans in 2013 possibly have originated by reassortment between H9N2 viruses of poultry and H7 and N9 genes career ducks in China.[44]
Due to the lack of immunologic memory for newly emerged influenza viruses that earned the capacities of both transmissions to and among humans, will be spread quickly in human populations, consequently will be resulted in pandemic. There have been a period of 10–50 years between the emergence of pandemics since the 16th century.[45] Therefore, it is not predictable when a new pandemic influenza strain will emerge. There are no reports of human-to-human transmission of H9N2, previously.[29] Furthermore, there has not observed any cases up to now.
H9N2 VIRUS SURVEILLANCE
Avian influenza is a global threat for public health but sustained, coordinated, and comprehensive global programs to monitor the genetic diversity of AIVs circulating in avian human interfaces are very few.[46] AIVs surveillance can be directed in three main groups including wild birds, domestic poultry, and also in occupationally exposed populations. The surveillance programs in those groups are critical for awareness of the persistence, intraspecies and interspecies transmission and evolution of AIVs.
In recent literature, H9N2 surveillance was reported from different countries.[46,47,48,49] However, wild birds which are the main reservoir for all AIV species and key players of evolution and spread, surveillance in wild birds for AIVs is lacking, limited to the last outbreak virus and geographically biased.[46] In the West Bengal State from India, active AIV surveillance in wild, resident, migratory birds, and domestic poultry was performed from 2009 to 2011.[50] During aforementioned period, the presence of low pathogenic H9N2 and H4N6 viruses was confirmed in chickens, and domestic ducks by researchers which necessitate implementation of controlling investigations to prevent the spread of AIVs.[50] Two surveillance program in Egypt performed from 2010 to 2013 and from 2012 to 2013 among various regions and poultry production sectors that showed co-circulation of subtype H9N2 viruses with subtype H5N1 viruses, as well as frequent co-infection of the same avian host. The co-circulation of these two subtypes poses a concern for probable reassortment.[47,48]
A serosurveillance in occupationally poultry-exposed workers was conducted in Shanghai, China, from 2008 to 2010. Evidence for the presence of anti-H9 antibodies in a high percentage of workers was detected. Furthermore, more than 200 H9N2 AIVs were isolated from cloacal and tracheal swabs collected from the poultry in live poultry, and more than 700 influenza viruses (H3N2, H1N1, B) were isolated from nasal/throat of patients having influenza symptoms.[49] This co-circulation strengthens the probability of reassortment between avian and human influenza viruses. Therefore, long-term surveillance of AIVs in occupationally exposed workers has great importance.
CONCLUSION
The novel avian-human influenza virus that could cause a pandemic is a great concern; H9N2 AIV that has the potential to grow in human is prone to generate it. Liu et al. suggested eradication of poultry carrying AIV H9N2 as the incubators for wild AIV by slaughtering them that prevent human infection, elimination of live poultry markets, and disinfection of these places.[51]
Obtaining enough knowledge on the influenza A H9N2 and other viruses circulating in avian-human interfaces and vaccination against seasonal influenza for occupational groups in direct contact particularly in slaughter-houses and development of effective antiviral agents is recommended. International surveillance programs for managing the future risks of H9N2 circulation at avian-human interface seem necessary.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHORS’ CONTRIBUTION
ShRR and AA contributed to the conception and design of the work; SMH and EA (guarantor) participated in the acquisition, interpretation of data for the work and final approval; and AA drafted the work and revised/proofed it.
Acknowledgments
The authors would like to thank Dr. Jahangir for reviewing the manuscript.
REFERENCES
- 1.Rahimi-Rad MH, Alizadeh E, Samarei R. Aquatic leech as a rare cause of respiratory distress and hemoptysis. Pneumologia. 2011;60:85–6. [PubMed] [Google Scholar]
- 2.Rad MH, Alizadeh E, Ilkhanizadeh B. Recurrent laryngeal papillomatosis with bronchopulmonary spread in a 70-year-old man. Tuberk Toraks. 2007;55:299–302. [PubMed] [Google Scholar]
- 3.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 4.Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–44. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 5.Balish AL, Katz JM, Klimov AI. Influenza: Propagation, quantification, and storage. Curr Protoc Microbiol. 2013;(Chapter 15) doi: 10.1002/9780471729259.mc15g01s29. Unit 15G.1. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–45. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson EC, von Kirchbach JC, Gog JR, Digard P. Genome packaging in influenza A virus. J Gen Virol. 2010;91(Pt 2):313–28. doi: 10.1099/vir.0.017608-0. [DOI] [PubMed] [Google Scholar]
- 8.Warshawsky B, Gemmill NCI, Henry B, Kumar D, Mcneil S, Quach-thanh C, et al. Vol. 39. Warrington: 2013. Canada Communicable Disease Report CCDR; pp. 1–46. [Google Scholar]
- 9.Coelingh KL, Luke CJ, Jin H, Talaat KR. Development of live attenuated influenza vaccines against pandemic influenza strains. Expert Rev Vaccines. 2014;13:855–71. doi: 10.1586/14760584.2014.922417. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Li J, Cheng X. Discovery of men infected by avian influenza A (H9N2) virus. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 1999;13:105–8. [PubMed] [Google Scholar]
- 11.Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol. 2005;43:5760–7. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79:2814–22. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homme PJ, Easterday BC. Avian influenza virus infections. I. Characteristics of influenza A-turkey-Wisconsin-1966 virus. Avian Dis. 1970;14:66–74. [PubMed] [Google Scholar]
- 14.Alexander PE, De P, Rave S. Is H9N2 avian influenza virus a pandemic potential? Can J Infect Dis Med Microbiol. 2009;20:e35–6. doi: 10.1155/2009/578179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nili H, Asasi K. Avian influenza (H9N2) outbreak in Iran. Avian Dis. 2003;47(3 Suppl):828–31. doi: 10.1637/0005-2086-47.s3.828. [DOI] [PubMed] [Google Scholar]
- 16.Alizadeh E, Masoud HS, Tavassoti KM, Vahideh M, Roozbeh B, Mansoureh T. Avian influenza (H9N2) among poultry workers in Iran. Iran J Microbiol. 2009;1:3–6. [Google Scholar]
- 17.Smith E, Coghlan B, Leder K. Avian influenza: Clinical and epidemiological information for paramedics and emergency healthcare workers. Australas J Paramed. 2006;4:1–8. [Google Scholar]
- 18.Eick A, Hu-Primmer J, Rowe T, Masseoud F, Fukuda K, Lim W, et al., editors. Seroprevalence of Antibody to H9N2 Viruses in Poultry Workers of Hong Kong. Atlanta, GA: International Conference on Emerging Infectious Diseases; 2000. [Google Scholar]
- 19.Hadipour MM. Seroprevalence of H9N2 avian influenza virus in human population in boushehr province, Iran. Asian J Anim Vet Adv. 2011;6:196–200. [Google Scholar]
- 20.Anvar E, Hosseini SM, Kheiri MT, Mazaheri V, Fazaei K, Shabani M, et al. Serological survey of avian influenza (H9N2) among different occupational groups in Tehran and Qazvin provinces in IR Iran. Jundishapur J Microbiol. 2013;6:1–4. [Google Scholar]
- 21.Gray GC, McCarthy T, Capuano AW, Setterquist SF, Alavanja MC, Lynch CF. Evidence for avian influenza A infections among Iowa's agricultural workers. Influenza Other Respir Viruses. 2008;2:61–9. doi: 10.1111/j.1750-2659.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray GC, Ferguson DD, Lowther PE, Heil GL, Friary JA. A national study of US bird banders for evidence of avian influenza virus infections. J Clin Virol. 2011;51:132–5. doi: 10.1016/j.jcv.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia N, de Vlas SJ, Liu YX, Zhang JS, Zhan L, Dang RL, et al. Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J Clin Virol. 2009;44:225–9. doi: 10.1016/j.jcv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Kayali G, Ortiz EJ, Chorazy ML, Gray GC. Evidence of previous avian influenza infection among US Turkey workers. Zoonoses Public Health. 2010;57:265–72. doi: 10.1111/j.1863-2378.2009.01231.x. [DOI] [PubMed] [Google Scholar]
- 25.Khuntirat BP, Yoon IK, Blair PJ, Krueger WS, Chittaganpitch M, Putnam SD, et al. Evidence for subclinical avian influenza virus infections among rural Thai Villagers. Clin Infect Dis. 2011;53:e107–16. doi: 10.1093/cid/cir525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu CY, Lu JH, Chen WQ, Jiang LF, Tan BY, Ling WH, et al. Potential infections of H5N1 and H9N2 avian influenza do exist in Guangdong populations of China. Chin Med J (Engl) 2008;121:2050–3. [PubMed] [Google Scholar]
- 27.Pawar SD, Tandale BV, Raut CG, Parkhi SS, Barde TD, Gurav YK, et al. Avian influenza H9N2 seroprevalence among poultry workers in Pune, India, 2010. PLoS One. 2012;7:e36374. doi: 10.1371/journal.pone.0036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang M, Fu CX, Zheng BJ. Antibodies against H5 and H9 avian influenza among poultry workers in China. N Engl J Med. 2009;360:2583–4. doi: 10.1056/NEJMc0900358. [DOI] [PubMed] [Google Scholar]
- 29.Uyeki TM, Chong YH, Katz JM, Lim W, Ho YY, Wang SS, et al. Lack of evidence for human-to-human transmission of avian influenza A (H9N2) viruses in Hong Kong, China 1999. Emerg Infect Dis. 2002;8:154–9. doi: 10.3201/eid0802.010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: Relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci U S A. 2000;97:9654–8. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freidl GS, Meijer A, de Bruin E, de Nardi M, Munoz O, Capua I, et al. Influenza at the animal-human interface: A review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A (H5N1) Euro Surveill. 2014;19:20793. doi: 10.2807/1560-7917.es2014.19.18.20793. [DOI] [PubMed] [Google Scholar]
- 32.Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 33.Gou Y, Xie J, Wang M. A strain of influenza A H9N2 virus repeatedly isolated from human population in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2000;14:209–12. [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Avian Influenza A Infection of Humans; 2008. Available from: http://www.cdc.gov/flu/avian/gen-info/avian-flu-humans.htm .
- 35.Cheng VC, Chan JF, Wen X, Wu WL, Que TL, Chen H, et al. Infection of immunocompromised patients by avian H9N2 influenza A virus. J Infect. 2011;62:394–9. doi: 10.1016/j.jinf.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 36.International Centre for Diarrhoeal Disease Research. Outbreak of mild respiratory disease caused by H5N1 and H9N2 infections among young children in Dhaka. Health Sci Bull. 2011;9:5–12. [Google Scholar]
- 37.Mail P. Avian Influenza Human (164): China (HK) H9N2. Dec 2013. Archive Number 20131230.2143709. 2013 Dec 30; [Google Scholar]
- 38.Mail PM. Avian Influenza, Human (02): China (HK) H9N2 Ex (HN). 2014. Archive Number 20140102.2148327. 2014 Jan 2; [Google Scholar]
- 39.Matrosovich MN, Krauss S, Webster RG. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology. 2001;281:156–62. doi: 10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- 40.Peiris JS, Guan Y, Markwell D, Ghose P, Webster RG, Shortridge KF. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment? J Virol. 2001;75:9679–86. doi: 10.1128/JVI.75.20.9679-9686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warshawsky B, Crowcroft N, Gemmill I, Henry B, Kumar D, Mcneil S, et al. Evidence review on occupational exposure of swine and poultry workers. Can Commun Dis Rep. 2013;39:1–46. doi: 10.14745/ccdr.v39i00a04a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams JR, Chen PY, Cho CT, Chin TD. Influenza: prospect for prevention and control. Kaohsiung J Med Sci. 2002;18:421–34. [PubMed] [Google Scholar]
- 43.Shanmuganatham K, Feeroz MM, Jones-Engel L, Smith GJ, Fourment M, Walker D, et al. Antigenic and molecular characterization of avian influenza A(H9N2) viruses, Bangladesh. Emerg Infect Dis. 2013;19:1393–1402. doi: 10.3201/eid1909.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. Overview of the emergence and characteristics of the avian influenza A (H7N9) virus. Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 45.WHO. Avian Influenza: Assessing the Pandemic Threat. Geneva, Switzerland: WHO; 2005. [Google Scholar]
- 46.Machalaba CC, Elwood SE, Forcella S, Smith KM, Hamilton K, Jebara KB, et al. Global avian influenza surveillance in wild birds: A strategy to capture viral diversity. Emerg Infect Dis. 2015;21:e1–7. doi: 10.3201/eid2104.141415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kayali G, Kandeil A, El-Shesheny R, Kayed AS, Gomaa MM, Maatouq AM, et al. Active surveillance for avian influenza virus, Egypt, 2010-2012. Emerg Infect Dis. 2014;20:542–51. doi: 10.3201/eid2004.131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakal MA, Youssef YI, El-Zeedy SA, Ibrahim SM, Al Baroudi BM. Surveillance on avian influenza H5N1 and H9N2 subtypes in egypt 2012-2013. Poult Fish Wildl Sci. 2013;2:111. [Google Scholar]
- 49.Wang Q, Ju L, Liu P, Zhou J, Lv X, Li L, et al. Serological and virological surveillance of avian influenza A virus H9N2 subtype in humans and poultry in Shanghai, China, between 2008 and 2010. Zoonoses Public Health. 2015;62:131–40. doi: 10.1111/zph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pawar SD, Kale SD, Rawankar AS, Koratkar SS, Raut CG, Pande SA, et al. Avian influenza surveillance reveals presence of low pathogenic avian influenza viruses in poultry during 2009-2011 in the West Bengal State, India. Virol J. 2012;9:151. doi: 10.1186/1743-422X-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D, Shi W, Gao GF. Poultry carrying H9N2 act as incubators for novel human avian influenza viruses. Lancet. 2014;383:869. doi: 10.1016/S0140-6736(14)60386-X. [DOI] [PubMed] [Google Scholar]


