Abstract
Lipid bodies are cytoplasmic inclusions that develop within leukocytes, including eosinophils and neutrophils, associated with inflammation. Our investigations of the formation and function of lipid bodies have revealed that they are distinct, inducible endoplasmic reticulum−derived, membrane- and ribosome-containing organelles with diverse functional roles in inflammatory responses of leukocytes. Leukocyte lipid bodies contain all enzymes required for synthesizing cyclo-oxygenase- and lipoxygenase-derived eicosanoids. Lipid body formation, rapidly inducible in vitro and in vivo by specific intracellular signaling pathways, enhances leukocyte formation of cyclo-oxygenase– and lipoxygenase-derived eicosanoids. Lipid bodies are discrete sites of eicosanoid synthesis, as documented for immunolocalized leukotriene C4, leukotriene B4, and prostaglandin E2. Lipid body−derived eicosanoids function as both paracrine and intracrine mediators of inflammation. Based on combined proteomic and ultrastructural studies, leukocyte lipid bodies are complex organelles with internal membranes and ribosomes. Structurally and functionally leukocyte lipid bodes are distinct from lipid droplets in adipocytes.
INTRODUCTION
Lipid bodies (LBs) are lipid-rich cytoplasmic “inclusions” that form in diverse cell types ranging from yeast and Drosophila to mammalian cells. In mammalian cells, LBs (also called lipid droplets) are characteristic of adipocytes and steroidogenic cells (1). Although little had been known about the origins, composition, or functions of LBs (2), in the last decade or so there has been a renaissance of interest in LBs and lipid droplets (3) that led to the recognition that these structures are multifunctional organelles (1,3−6). This broader recognition of LBs as organelles was bolstered by proteomic analyses of LBs from several cell types (7−12). Unexpected results from LB proteomic analyses included a common recognition that LBs contained many Rab guanosine triphosphate hydrolase (GTPase) proteins potentially involved in vesicular trafficking. In addition, LB-associated proteins of the PAT/PLIN family, perilipins, adipophilin, tail-interacting protein of 47 kDa (TIP47) and S3-12, initially associated only with adipocyte LBs, were uniformly found with LBs in other cells (12,13). Thus, although there was an evolving recognition of likely commonalities in the biogenesis and structure of LBs in varied mammalian cell types, the origins and range of functions of LB organelles, especially those in leukocytes and not adipocytes, remained ill understood.
Leukocyte LBs are often not recognized because LBs dissolve in common alcohol-based stains, such as Wright’s and Giemsa hematological stains. In limited numbers, LBs are normally present within mammalian cells, including neutrophils (polymorphonuclear leukocytes [PMNs]), eosinophils, mast cells, macrophages, endothelial cells, and fibroblasts. LB numbers uniformly increase in cells participating in inflammatory processes (5,14−19). Our interests in LBs were occasioned both by the prominence of LB organelles within leukocytes associated with inflammation in vivo and by the roles that these lipid-rich organelles might play in arachidonic acid (AA) metabolism by leukocytes and other cells involved in inflammation.
RESULTS
LB Formation In Vitro
As we have shown, leukocyte LB formation is a rapid, highly regulated process involving specific agonist-elicited signal transduction pathways (5,20−27). Thus, LBs are inducible “early response” organelles that may participate in inflammation-associated responses of leukocytes.
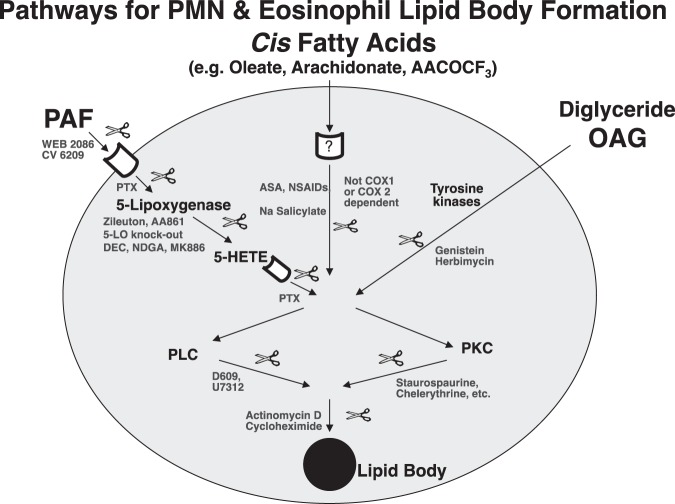
Signaling pathways mediating leukocyte LB formation. Our studies delineating mechanisms of leukocyte LB induction identified several agonists and intracellular signaling pathways (20−22,25,28). For PMNs and eosinophils (Figure 1), one pathway is initiated by receptor-mediated platelet activating factor (PAF) signaling via 5-lipoxygenase (5-LO) activation to generate 5-hydroxyeicosatetraenoic acid (5-HETE), which then signals via a pertussis toxin (PTX) −inhibitable receptor-dependent mechanism. The second pathway initiated by cis-fatty acids is aspirin (ASA), salicylate and non-steroidal anti-inflammatory drug (NSAID) inhibitable, but cyclo-oxygenase 1 (COX1) and cyclooxygenase 2 (COX2) independent. Cell-permeant diglyceride constitutes a third initiating signal. For all three pathways, common downstream signaling requires in part protein kinase C and phospholipase C activation and new mRNA and protein synthesis. In addition for eosinophils, eotaxin/RANTES acting via CCR3 activate downstream phosphatidylinositol 3 kinase (PI3K) and ERK 1/2 and p38 MAP kinases to elicit LB formation. Eosinophils from normal subjects after exposure to granulocyte-macrophage colony stimulating factor behave like eosinophils from hypereosinophilic syndrome donors in which PAF-induced LB formation is tyrosine kinase-dependent (20). Interleukin 5 (IL-5) and immunoglobulin G stimulate eosinophil LB formation and leukotriene C4 (LTC4) production via endogenous PAF formation (29). IL-16 by stimulating eotaxin/RANTES release stimulates LB formation and LTC4 production (30). Thus, leukocyte LB formation is a highly regulated, rapid cellular response mediated by several distinct activating and signaling mechanisms.
Fig. 1.
Pathways of lipid body formation in neutrophils (polymorphonuclear leukocytes) and eosinophils. Stimuli include platelet activating factor, cis fatty acids and diglycerides (OAG). Abbreviations: PAF, platelet activating factor; WEB, ; CV, ; PTX, pertussis toxin; 5-LO, 5-lipoxygenase; DEC, diethylcarbamazine; NDGA, ; MK866, ; ASA, aspirin; NSAIDs, non-steroidal anti-inflammatory drugs; COX, cyclo-oxygenase; OAG, oleoyl-acetyl glycerol; PLC, phospholipase C; PKC, protein kinase C; 5-HETE, 5-hydroxyeicosatetraenoic acid; D609, ; U7312, .
LB Formation In Vivo
While normal blood PMNs and eosinophils contain ~1 and ~5 LBs/cell, respectively, blood PMNs from patients with infections and eosinophils from eosinophilic patients contain many more LBs/cell (14,15,31). Recruited PMNs and eosinophils in airways, tissues, blood, and exudative effusions of humans and experimental animals with infectious and inflammatory reactions typically contain abundant LBs (15−17,21,32,33). LB formation in leukocytes in vivo is a common correlate of their participation in inflammation.
LBs as “Eicosasomes” — Sites of Arachidonate Localization and Eicosanoid Formation
We hypothesized that LBs have roles in the regulated formation of AA-derived eicosanoids in leukocytes. COX pathways, mediated by COX1 or COX2, form prostaglandins (PGs) and thromboxanes. The 5-LO pathway in PMNs, eosinophils, monocytes, mast cells, and macrophages, forms 5-HETE and leukotrienes (LT). The formation of eicosanoids that are not stored pre-formed within cells is tightly regulated; and enhanced formation of eicosanoids, necessary for their intracrine, autocrine, and paracrine activities, occurs at cell membranes in response to activating stimuli. Recognized intracellular sites of eicosanoid formation have included the membranes of the nuclear envelope and cytosolic LBs (34−36).
LBs are sites of substrate arachidonate and eicosanoid-forming enzyme localization. We have shown that eicosanoid precursor AA localizes within LBs in many leukocyte types (14,15,25,37,38) and that AA was esterified in phospholipids of eosinophil LBs (38). Esterified AA localization within PMN LBs has been confirmed using Raman microscopy (39). A key enzyme needed to release AA, cytosolic phospholipase A2, activating kinases (MAP and PI3 kinases), and all relevant eicosanoid-forming enzymes (COXs, 5- and 15-LO, 5-LO−activating protein, and LTC4 synthase) have been localized to LBs (20−22,24,31,32,40−49). These findings support the capacity of LBs to serve as distinct sites for eicosanoid formation.
Lipid bodies correlate with enhanced (“primed”) leukocyte eicosanoid formation and release. In studies of PMNs, there had been a paradox. In response to the Ca++ ionophore, A23187, PMNs generated large quantities of leukotriene B4 (LTB4) and 5-HETE; whereas more natural, receptor-mediated stimulation, for example, N- formylmethionyl-leucyl-phenylalanine (fMLP), elicited little eicosanoid formation (50,51), unless PMNs are first “primed.” Priming consists of pre-incubating PMNs with oleoyl-acetyl-glycerol (OAG), PAF, AA, phorbol myristate acetate or lipopolysaccharide (50−53). Priming agents also augment eicosanoid formation by A23187-stimulated PMNs (21,24,54,55). Our studies established that increased leukocyte LB formation consistently correlated with primed responses for enhanced eicosanoid formation. With each class of LB inducer (e.g., eotaxin/RANTES, PAF, OAG, cis-fatty acids, lipopolysaccharide), numbers of induced LBs correlated with enhanced magnitudes of released COX- and 5-LO−derived eicosanoids (21,22,24,31,47,56,57). Conversely, inhibition of LB induction correlated with suppression of enhanced eicosanoid release (21,22,24,27,56). For instance, ASA and NS-398 inhibition of oleate-induced LB formation suppressed generation of 5-LO (not itself ASA or NS-398 inhibitable)-derived leukotrienes (24,27). Thus, induction of LB formation uniformly correlated with enhanced primed generation of eicosanoids, and inhibition of LB formation correlated with diminished capacity to form both COX- and LO-derived eicosanoids.
To further ascertain a role for LBs in enhanced eicosanoid formation independent of nuclei, we established that: 1) anucleate eosinophil cytoplasts form LBs in response to PAF stimulation, 2) LB numbers in cytoplasts correlated with levels of primed LTC4 and prostaglandin E2 (PGE2) released by cytoplasts following A23187 challenge, and 3) LBs in anucleate eosinophil cytoplasts were sites of immunolocalized 5-LO, COX, and LTC4 synthase proteins (58). Thus, in the absence of nuclei and nuclear envelopes, LBs were sites of enhanced eicosanoid formation.
Direct localization of eicosanoid synthesis at LBs. Previously, intracellular sites of eicosanoid formation had never been directly demonstrated in any cell, but were only inferred based in immunolocalization of eicosanoid-synthesizing enzymes. Because immunolocalization of eicosanoid-forming proteins might not reflect their state of enzymatic activity, we developed a novel method to immunolocalize newly synthesized LTC4 at its sites of formation within eosinophils. We localized newly synthesized LTC4 at LBs in eosinophils stimulated in vitro with eotaxin-1, RANTES, or anti-CD9 monoclonal antibody and at LBs in eosinophils elicited in vivo by allergic challenges (22,30,32,33,59). In contrast, in eosinophils activated by A23187 alone, newly formed LTC4 was localized at nuclear membranes (22,59), concordant with the perinuclear immunolocalization of 5-LO protein in A23187-activated eosinophils (60). Our colleagues and collaborators utilized our same eicosanoid immunolocalization methods to likewise localize newly synthesized PGE2 and LTB4 directly at LBs in macrophages (56,57,61). Likewise, PGD2 formation has been localized to LBs in eosinophils (62). These findings provided the first direct “smoking gun” evidence that LBs are sites of synthesis of both 5-LO- and COX-derived eicosanoids.
Functions of Leukocyte LB-Derived Arachidonate and Eicosanoids
One function of induced LBs is to serve as distinct sites for enhanced synthesis of COX- and/or LO-derived eicosanoids that are paracrine mediators of inflammation (63). As noted above, our studies correlated elicited formation of LBs with enhanced extracellular release of COX- and 5-LO−pathway-formed eicosanoids by PMNs, eosinophils, and macrophages. Utilization of depots of LB-derived AA for generating paracrine-acting mediators, rather than AA derived from perinuclear membranes, would obviate stoichiometric structural perturbation of nuclear membranes.
A second functional role for leukocyte LBs is to provide AA and eicosanoids that act as intracrine signal-transducing mediators. The first evidence for this intracellular mediator role for LTC4 arose from our studies. Our LTC4 immunolocalization technique with its heightened sensitivity detected low level LTC4 formation at LBs in eotaxin-1- stimulated eosinophils when no extracellular LTC4 release was detectable (22,30,64). Eotaxin-1 stimulates the release of preformed IL-4 from eosinophil granules (30,65). We established that eotaxin-1−elicited release of IL-4 is: 1) dependent on intracellular activation of 5-LO to form LTC4 at LBs, and 2) intracellular (not extracellular) LTC4 functions as an obligate signal-transducing mediator (64). Moreover, we have documented that eosinophil granule membranes express functional cysteinyl leukotriene (CysLT) receptors that mediate secretion from within eosinophil granules (66). Thus, for the first time, we demonstrated an intracrine, signal-transducing role for a CysLT and established that the regulated formation of LTC4 at LBs is critically involved in controlling a response of eosinophils, including their capacity to secrete the cytokine IL-4.
Another intracrine signaling role for LBs was revealed by Raman microscopic studies that showed that AA-rich LBs in PMNs rapidly move to and associate with phagosomes, suggesting that LB-derived AA functions to locally activate nicotinamide adenine dinucleared phosphate, reduced (NADPH) oxidase at phagosomes (39). Thus, LBs are functional as sites of regulated formation of eicosanoids for their paracrine mediator functions and are intimately involved in intracellular signal transduction fully pertinent to leukocyte functioning in inflammation.
Consonant with a central role for leukocyte LBs as principal sites of eicosanoid formation, increasingly findings in other cell types, including colon cancer cells (67), Pseudomonas ExoU toxin-stimulated airway epithelial cells (68), and Mycobacteria Bacillus Calmette Guerin (BCG) vaccine challenged macrophages in vivo (61), are documenting that LBs in varied cell types have central and primary functions in the synthesis of eicosanoids (69−71). These findings fully substantiate the functional importance of LBs in leukocytes, in common with other cells, in eicosanoid synthesis pertinent to host inflammatory and immune responses, and exemplify how our focused studies of LBs in leukocytes likely have broader implications for other cells.
DISCUSSION
LBs in leukocytes and other cells have engendered considerable recent interest (4,67,72,73). For leukocyte LBs involved in eicosanoid formation, we have significantly advanced our understanding of these inducible organelles. We established that LTC4, LTB4, PGD2, and PGE2 are formed at LBs. We have provided evidence that LBs have functional roles pertinent to allergic inflammation as a source of eicosanoids with both extracellular-paracrine and intracellular-intracrine roles as mediators of leukocyte responses pertinent to allergic inflammation. Our proteomic/electron microscopy studies revealed endoplasmic reticulum (ER)−like membranes and ribosomes in LBs. Recognition of ER membranes within leukocyte LBs resolves a conundrum about how membrane-associated eicosanoid-forming enzymes could function at LBs. In accord with their content of ribosomal and mRNAs, we demonstrated that LBs are sites of de novo 5-LO protein synthesis. In addition to their roles in generating eicosanoid lipid mediators, active intra- and extracellularly, leukocyte LBs likely have roles in cytokine-mediated responses. Moreover, the leukocyte LB proteome in concert with proteomes of LBs from other cells supports the capacities of leukocyte LBs to be multifunctional organelles. Whereas lipid droplets in adipocytes and other cells are recognized to contain neutral lipids surrounded by a monolayer of phospholipid membranes (74), our studies of leukocyte LBs differentiate them from most lipid droplets. With our insights that leukocyte LBs are complex ER-derived organelles, leukocyte LBs differ in form and function from lipid droplets found in adipocytes.
ACKNOWLEDGMENTS
I am grateful for and fully acknowledge the collaborative assistance of Anne M. Dvorak, MD, Patricia T. Bozza, MD, PhD, Christiane Bandeira-Melo, PhD, Wengui Yu, MD, PhD, Josiane Neves, PhD, and Rossana Melo, PhD, as well as others, in our studies of leukocyte lipid bodies. The studies have been supported by NIH grants to the author (NIAID R01AI022571, R01/R37AI020241).
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Moore, New York City: Thank you Peter, it’s wonderful to see some real hematology presented at the meeting. Are there any congenital abnormalities of lipid bodies to explain any problems handling infections or inflammation that have been described?
Weller, Boston: Probably not. Not that we know of in terms of the lipid bodies that form within leukocytes, there aren’t. There are though other congenital inherited abnormalities that probably affect lipid droplet formation. These are due to alterations in some of the associated proteins that form within lipid droplets.
Schreiner, Los Altos: Thank you for a very interesting presentation. I have two questions, is there any evidence or role for these lipid bodies as extruded elements?
Weller, Boston: Excellent question and the evidence for that would largely rest with EM studies in order to detect them. In general, from what I am aware of, there really is no recognized role for these being extruded. There may be intracellular associations within cells. For instance within mast cells, lipid bodies have been shown to associate with degranulating phagosomes; and there is some data in neutrophils that actually shows that lipid bodies are quite motile within cells and move to potentially deliver activating arachidonic acid to help facilitate the respiratory burst.
Schreiner, Los Altos: Thank you, and my second question is that I am struck by the number of initiating factors that are either lipids or glycolipids particularly with respect to bacterial glycolipids. Is there any evidence that some of the initiating elements particularly bacterial glycolipids are actually part of the lipid body or incorporated as they come into the cell and play a continuing role so to speak in activity within the lipid body?
Weller, Boston: There is no indication that bacterial-derived lipids are actually incorporated into the lipid body. Curiously, part of the signaling to initiate leukocyte lipid body formation that occurs by the cis-fatty acids is not a reflection of the incorporation the exogenous cis-fatty acid into the lipid bodies. Hence, if one uses a cis-fatty acid that has a blocked carboxyl group, it still is acting as a signaling agent even though it cannot become esterified within neutrophil or eosinophil lipid bodies. Bacterial-derived products, including lipopolysaccharide, can signal to initiate lipid body formation.
Boxer, Ann Arbor: Excellent talk Peter. What is the natural history of the lipid bodies in malignant eosinophilia and do they play in the role in the pathogenesis of malignancy? And then you know when on peripheral blood smears we see often activated eosinophils that apparently degranulate, do lipid bodies re-accumulate in these activated eosinophils?
Weller, Boston: Good questions Larry, I think lipid bodies probably do re-accumulate within eosinophils that have degranulated so that it is a dynamic process. In terms of relationships with neoplasms, lipid bodies are not unique to leukocytes; and there is actually a literature with epithelial cells and adenocarcinomas in the GI tract that would identify lipid bodies as sites of eicosanoid production within some of the neoplasms. These lipid bodies might be one of the sites where taking aspirin or NSAID can have a therapeutic benefit by inhibiting the formation or the function of these lipid bodies.
Boxer, Ann Arbor: What about the malignant eosinophil itself? Is there any unusual morphology under EM that you could recognize?
Weller, Boston: No, with malignant eosinophils there is no alternate morphology that would distinguish a malignant eosinophil enough from an activated eosinophil that might have more lipid bodies.
Dale, Seattle: Very interesting talk….thank you Peter… neutrophil is a very short-lived cell, are these cells in the death pathway or do the lipid bodies actually promote the cell survival?
Weller, Boston: We have no data that would link the formation of these with a death pathway. We think that it’s very much a functional capacity of an intact viable cell and not necessarily a cell that is heading toward death.
Schiffman, Providence: Beautiful talk Peter. Any down-the-road implications for either enhancement or blocking of the formation of lipid bodies in order to interfere with either inflammatory or infectious pathways?
Weller, Boston: Thank you. I think that is a good question. Curiously, when we began to look at the capacities of cis fatty acids stimulate lipid body formation, we found that aspirin blocked lipid body formation as did sodium salicylate. Cis fatty acid−elicited lipid body formation was also blocked in cells deficient in cyclo-oxygenase 1 and cyclo-oxygenase 2. This blockade of lipid body formation impaired the formation of both prostaglandins and leukotrienes, so there is the potential for a therapeutic intervention in the formation of lipid bodies that would then limit the quantities of eicosanoids being produced.
Michael Gershon, New York City: The question I had, since neutrophils are pretty much loaded guns, that is they finish the protein synthesis by the time they are out there in tissue….
Weller, Boston: I would question that, I think that is the old dogma and at the same time I would likewise question the limited capacities of eosinophils for further protein synthesis.
Michael Gershon, New York City: Alright, the question I was asking whether the lipid bodies get made with precursors already loaded into the cell and as a progenitor of the lipid body before it’s a neutrophil that while it is still in the forming stage?
Weller, Boston: Lipid body formation in leukocytes requires in part de novo protein synthesis. Lipid bodies arise from the endoplasmic reticulum which folds back on itself. Local synthesis of lipid develops and overlies the folded endoplasmic reticulum and accounts for the staining with lipophilic stains or with osmium. We do think that mature neutrophils and eosinophils retain the capacity for ongoing protein synthesis and for de novo lipid body formation. Thank you.
REFERENCES
- 1.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–8. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/s0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 3.Melo RC, Weller PF. Unraveling the complexity of lipid body organelles in human eosinophils. J Leukoc Biol. 2014;96:703–12. doi: 10.1189/jlb.3RU0214-110R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckman M. Cell biology. Great balls of fat. Science. 2006;311:1232–4. doi: 10.1126/science.311.5765.1232. [DOI] [PubMed] [Google Scholar]
- 5.Bozza PT, Melo RC, Bandeira-Melo C. Leukocyte lipid bodies regulation and function: contribution to allergy and host defense. Pharmacol Ther 2007. 2007;113:30–49. doi: 10.1016/j.pharmthera.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Fujimoto T, Ohsaki Y. Cytoplasmic lipid droplets: rediscovery of an old structure as a unique platform. Ann N Y Acad Sci. 2006;1086:104–15. doi: 10.1196/annals.1377.010. [DOI] [PubMed] [Google Scholar]
- 7.Liu P, Ying Y, Zhao Y, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–92. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 8.Umlauf E, Csaszar E, Moertelmaier M, et al. Association of stomatin with lipid bodies. J Biol Chem. 2004;279:23699–709. doi: 10.1074/jbc.M310546200. [DOI] [PubMed] [Google Scholar]
- 9.Fujimoto Y, Itabe H, Sakai J, et al. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta. 2004;1644:47–59. doi: 10.1016/j.bbamcr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Sato S, Fukasawa M, Yamakawa Y, et al. Proteomic profiling of lipid droplet proteins in hepatoma cell lines expressing hepatitis C virus core protein. J Biochem (Tokyo) 2006;139:921–30. doi: 10.1093/jb/mvj104. [DOI] [PubMed] [Google Scholar]
- 11.Brasaemle DL, Dolios G, Shapiro L, et al. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–42. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 12.Wan HC, Melo RC, Jin Z, et al. Roles and origins of leukocyte lipid bodies: proteomic and ultrastructural studies. FASEB J. 2007;21:167–78. doi: 10.1096/fj.06-6711com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Londos C, Sztalryd C, Tansey JT, et al. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–9. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Weller PF, Dvorak AM. Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood. 1985;65:1269–74. [PubMed] [Google Scholar]
- 15.Weller PF, Ackerman SJ, Nicholson-Weller A, et al. Cytoplasmic lipid bodies of human neutrophilic leukocytes. Am J Pathol. 1989;135:947–59. [PMC free article] [PubMed] [Google Scholar]
- 16.Coimbra A, Lopes-Vaz A. The presence of lipid droplets and the absence of stable sudanophilia in osmium-fixed human leukocytes. J Histochem Cytochem. 1971;19:551–7. doi: 10.1177/19.9.551. [DOI] [PubMed] [Google Scholar]
- 17.Robinson JM, Karnovsky ML, Karnovsky MJ. Glycogen accumulation in polymorphonuclear leukocytes, and other intracellular alterations that occur during inflammation. J Cell Biol. 1982;95:933–42. doi: 10.1083/jcb.95.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triggiani M, Oriente A, Seeds MC, et al. Migration of human inflammatory cells into the lung results in the remodeling of arachidonic acid into a triglyceride pool. J Exp Med. 1995;182:1181–90. doi: 10.1084/jem.182.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melo RC, Fabrino DL, Dias FF, et al. Lipid bodies: structural markers of inflammatory macrophages in innate immunity. Inflamm Res. 2006;55:342–8. doi: 10.1007/s00011-006-5205-0. [DOI] [PubMed] [Google Scholar]
- 20.Bozza PT, Yu W, Cassara J, et al. Pathways for eosinophil lipid body induction: differing signal transduction in cells from normal and hypereosinophilic subjects. J Leukocyte Biol. 1998;64:563–9. doi: 10.1002/jlb.64.4.563. [DOI] [PubMed] [Google Scholar]
- 21.Bozza PT, Payne JL, Goulet JL, et al. Mechanisms of PAF-induced lipid body formation: a central role for 5-lipoxygenase in the compartmentalization of leukocyte lipids. J Exp Med. 1996;183:1515–25. doi: 10.1084/jem.183.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandeira-Melo C, Phoofolo M, Weller PF. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem. 2001;276:22779–87. doi: 10.1074/jbc.M101436200. [DOI] [PubMed] [Google Scholar]
- 23.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins. Contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol. 2001;24:653–7. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- 24.Bozza PT, Payne JL, Morham SG, et al. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci U S A. 1996;93:11091–6. doi: 10.1073/pnas.93.20.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller PF, Ryeom SW, Picard ST, et al. Cytoplasmic lipid bodies of neutrophils: formation induced by cis-unsaturated fatty acids and mediated by protein kinase C. J Cell Biol. 1991;113:137–46. doi: 10.1083/jcb.113.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bozza PT, Weller PF. Arachidonyl trifluoromethyl ketone induces lipid body formation in leukocytes. Prostaglandins, Leuko Essentl Fatty Acids. 2001;64:227–30. doi: 10.1054/plef.2001.0264. [DOI] [PubMed] [Google Scholar]
- 27.Bozza PT, Pacheco P, Yu W, et al. NS-398: Cyclooxygenase-2 independent inhibition of leukocyte priming for lipid body formation and enhanced leukotriene generation. Prostag Leukotr Ess. 2002;67:237–44. doi: 10.1054/plef.2002.0425. [DOI] [PubMed] [Google Scholar]
- 28.Scarfo LM, Weller PF, Farber HW. Induction of endothelial cell cytoplasmic lipid bodies during hypoxia. Am J Physiol Heart Circ Physiol. 2001;280:H294–301. doi: 10.1152/ajpheart.2001.280.1.H294. [DOI] [PubMed] [Google Scholar]
- 29.Bartemes KR, McKinney S, Gleich GJ, et al. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 1999;162:2982–9. [PubMed] [Google Scholar]
- 30.Bandeira-Melo C, Sugiyama K, Woods L, et al. Interleukin-16 promotes leukotriene C4 and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–63. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 31.Pacheco P, Bozza FA, Gomes RN, et al. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular loci involved in eicosanoid metabolism. J Immunol. 2002;169:6498–506. doi: 10.4049/jimmunol.169.11.6498. [DOI] [PubMed] [Google Scholar]
- 32.Vieira-de-Abreu A, Assis EF, Gomes GS, et al. Allergic challenge-elicited lipid bodies compartmentalize in vivo leukotriene C4 synthesis within eosinophils. Am J Respir Cell Mol Biol. 2005;33:254–61. doi: 10.1165/rcmb.2005-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesquita-Santos FP, Vieirade-Abreu A, Calheiros AS, et al. Cutting edge: prostaglandin D2 enhances leukotriene C4 synthesis by eosinophils during allergic inflammation: synergistic in vivo role of endogenous eotaxin. J Immunol. 2006;176:1326–30. doi: 10.4049/jimmunol.176.3.1326. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene biosynthesis. Am J Respir Crit Care Med. 2000;161:S36–40. doi: 10.1164/ajrccm.161.supplement_1.ltta-8. [DOI] [PubMed] [Google Scholar]
- 35.Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostag Leukotr Ess. 2003;69:135–43. doi: 10.1016/s0952-3278(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 36.Bandeira-Melo C, Bozza PT, Weller PF. The cellular biology of eosinophil eicosanoid formation and function. J Allergy Clin Immunol. 2002;109:393–400. doi: 10.1067/mai.2002.121529. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak AM, Dvorak HF, Peters SP, et al. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J Immunol. 1983;131:2965–76. [PubMed] [Google Scholar]
- 38.Weller PF, Monahan-Earley RA, Dvorak HF, et al. Cytoplasmic lipid bodies of human eosinophils: subcellular isolation and analysis of arachidonate incorporation. Am J Pathol. 1991;138:141–8. [PMC free article] [PubMed] [Google Scholar]
- 39.van Manen HJ, Kraan YM, Roos D, et al. Single-cell Raman and fluorescence microscopy reveal the association of lipid bodies with phagosomes in leukocytes. Proc Natl Acad Sci U S A. 2005;102:10159–64. doi: 10.1073/pnas.0502746102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weller PF, Dvorak AM. Lipid bodies: intracellular sites for eicosanoid formation. J Allergy Clin Immunol. 1994;94:1151–6. doi: 10.1016/0091-6749(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 41.Dvorak AM, Morgan E, Tzizik DM, et al. Prostaglandin endoperoxide synthase (cyclooxygenase): ultrastructural localization to nonmembrane-bound cytoplasmic lipid bodies in human eosinophils and murine 3T3 fibroblasts. Int Arch Allergy Immunol. 1994;105:245–50. doi: 10.1159/000236764. [DOI] [PubMed] [Google Scholar]
- 42.Dvorak AM, Weller PF, Harvey VS, et al. Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to isolated, purified fractions of guinea pig peritoneal macrophages and line 10 hepatocarcinoma cells. Int Arch Allergy Immunol. 1993;101:136–42. doi: 10.1159/000236511. [DOI] [PubMed] [Google Scholar]
- 43.Dvorak AM, Schleimer RP, Dvorak AM, et al. Human lung mast cell and alveolar macrophage cytoplasmic lipid bodies contain arachidonic acid and prostaglandin endoperoxide synthase (cyclooxygenase), the substrate and enzyme necessary for prostaglandin production. Int Arch Allergy Immunol. 1992;99:208–17. doi: 10.1159/000236250. [DOI] [PubMed] [Google Scholar]
- 44.Dvorak AM, Morgan E, Schleimer RP, et al. Ultrastructural immunogold localization of prostaglandin endoperoxide synthase (cyclooxygenase) to nonmembrane-bound cytoplasmic lipid bodies in human lung mast cells, alveolar macrophages, type II pneumocytes and neutrophils. J Histochem Cytochem. 1992;40:759–69. doi: 10.1177/40.6.1316915. [DOI] [PubMed] [Google Scholar]
- 45.Yu W, Bozza PT, Tzizik DM, et al. Co-compartmentalization of MAP kinases and cytosolic PLA2 at cytoplasmic lipid bodies of U937 cells. Am J Pathol. 1998;152:759–69. [PMC free article] [PubMed] [Google Scholar]
- 46.Yu W, Cassara J, Weller PF. Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid bodies in human polymorphonuclear leukocytes and other myeloid-derived cells. Blood. 2000;95:1078–85. [PubMed] [Google Scholar]
- 47.Bozza PT, Yu W, Weller PF. Mechanisms of formation and function of eosinophil lipid bodies: inducible intracellular sites involved in arachidonic acid metabolism. Mem Inst Oswaldo Cruz. 1997;92(Suppl II):135–40. doi: 10.1590/s0074-02761997000800018. [DOI] [PubMed] [Google Scholar]
- 48.Dvorak AM. Cyclooxygenase, a key enzyme family for production of prostaglandins, is present in human mast cell lipid bodies. Chem Immunol Allergy. 2005;85:68–71. doi: 10.1159/000086506. [DOI] [PubMed] [Google Scholar]
- 49.Wooten RE, Willingham MC, Daniel LW, et al. Novel translocation responses of cytosolic phospholipase A(2)alpha fluorescent proteins. Biochim Biophys Acta. 2008;1783:1544–50. doi: 10.1016/j.bbamcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bauldry SA, Wykle RL, Bass DA. Phospholipase A2 activation in human neutrophils. Differential actions of diacylglycerols and alkylacylglycerols in priming cells for stimulation by N-formyl-met-leu-phe. J Biol Chem. 1988;263:16787–95. [PubMed] [Google Scholar]
- 51.Haines KA, Giedd KN, Rich AM, et al. The leukotriene B4 paradox: neutrophils can, but will not, respond to ligand-receptor interactions by forming leukotriene B4 or its omega-metabolites. Biochem J. 1987;241:55–62. doi: 10.1042/bj2410055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raulf M, König W. Modulation of leukotriene release from human polymorphonuclear leucocytes by PMA and arachidonic acid. Immunology. 1988;64:51–9. [PMC free article] [PubMed] [Google Scholar]
- 53.Doerfler ME, Weiss J, Clark JD, et al. Bacterial lipopolysaccharide primes human neutrophils for enhanced release of arachidonic acid and causes phosphorylation of an 85-kD cytosolic phospholipase A2. J Clin Invest. 1994;93:1583–91. doi: 10.1172/JCI117138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee TH, Mencia-Huerta J-M, Shih C, et al. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J Clin Invest. 1984;74:1922–33. doi: 10.1172/JCI111612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liles WC, Meier KE, Henderson WR. Phorbol myristate acetate and the calcium ionophore A23187 synergistically induce release of LTB4 by human neutrophils: involvement of protein kinase C activation in regulation of the 5-lipoxygenase pathway. J Immunol. 1987;138:3396–402. [PubMed] [Google Scholar]
- 56.Maya-Monteiro CM, Almeida PE, D’Avila H, et al. Leptin induces macrophage lipid body formation by a phosphatidylinositol 3-kinase- and mammalian target of rapamycin-dependent mechanism. J Biol Chem. 2008;283:2203–10. doi: 10.1074/jbc.M706706200. [DOI] [PubMed] [Google Scholar]
- 57.Pacheco P, Vieirade-Abreu A, Gomes RN, et al. Monocyte chemoattractant protein-1/CC chemokine ligand 2 controls microtubule-driven biogenesis and leukotriene B4-synthesizing function of macrophage lipid bodies elicited by innate immune response. J Immunol. 2007;179:8500–8. doi: 10.4049/jimmunol.179.12.8500. [DOI] [PubMed] [Google Scholar]
- 58.Bozza PT, Yu W, Penrose JF, et al. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med. 1997;186:909–20. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tedla N, Bandeira-Melo C, Tassinari P, et al. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:1174–9. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brock TG, Anderson JA, Fries FP, et al. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol. 1999;162:1669–76. [PubMed] [Google Scholar]
- 61.D’Avila H, Melo RCN, Parreira GG, et al. Mycobacterium bovis Bacillus Calmette-Guerin induces TLR2-mediated formation of lipid bodies: intracellular domains for eicosanoid synthesis in vivo. J Immunol. 2006;176:3087–97. doi: 10.4049/jimmunol.176.5.3087. [DOI] [PubMed] [Google Scholar]
- 62.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol. 2011;187:6518–26. doi: 10.4049/jimmunol.1101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christie PE, Henderson WR., Jr Lipid inflammatory mediators: leukotrienes, prostaglandins, platelet-activating factor. Clin Allergy Immunol. 2002;16:233–54. [PubMed] [Google Scholar]
- 64.Bandeira-Melo C, Woods LJ, Phoofolo M, et al. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med. 2002;196:841–50. doi: 10.1084/jem.20020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bandeira-Melo C, Sugiyama K, Woods LJ, et al. Cutting edge: eotaxin elicits rapid, vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–7. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 66.Neves JS, Radke AL, Weller PF. Cysteinyl leukotrienes acting via granule membrane-expressed receptors elicit secretion from within cell-free human eosinophil granules. J Allergy Clin Immunol. 2010;125:477–82. doi: 10.1016/j.jaci.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Accioly MT, Pacheco P, Maya-Monteiro CM, et al. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 2008;68:1732–40. doi: 10.1158/0008-5472.CAN-07-1999. [DOI] [PubMed] [Google Scholar]
- 68.Plotkowski MC, Brandao BA, de Assis MC, et al. Lipid body mobilization in the ExoU-induced release of inflammatory mediators by airway epithelial cells. Microb Pathog. 2008;45:30–7. doi: 10.1016/j.micpath.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Fazolini NP, Cruz AL, Werneck MB, et al. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle. 2015;14:2667–76. doi: 10.1080/15384101.2015.1041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Araujo-Santos T, Rodriguez NE, Moura-Pontes S, et al. Role of prostaglandin F2alpha production in lipid bodies from Leishmania infantum chagasi: insights on virulence. J Infect Dis. 2014;210:1951–61. doi: 10.1093/infdis/jiu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bozza PT, Bakker-Abreu I, Navarro-Xavier RA, et al. Lipid body function in eicosanoid synthesis: an update. Prostag Leukotr Ess. 2011;85:205–13. doi: 10.1016/j.plefa.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 72.Fujimoto T, Parton RG. Not just fat: the structure and function of the lipid droplet. Cold Spring Harb Perspect Biol. 2011;3(3):a004838. doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robenek H, Buers I, Hofnagel O, et al. Compartmentalization of proteins in lipid droplet biogenesis. Biochim Biophys Acta. 2009;1791:408–18. doi: 10.1016/j.bbalip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Wilfling F, Haas JT, Walther TC, et al. Lipid droplet biogenesis. Curr Opin Cell Biol. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]