Abstract
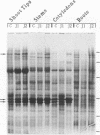
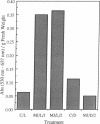
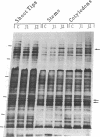
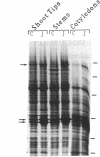
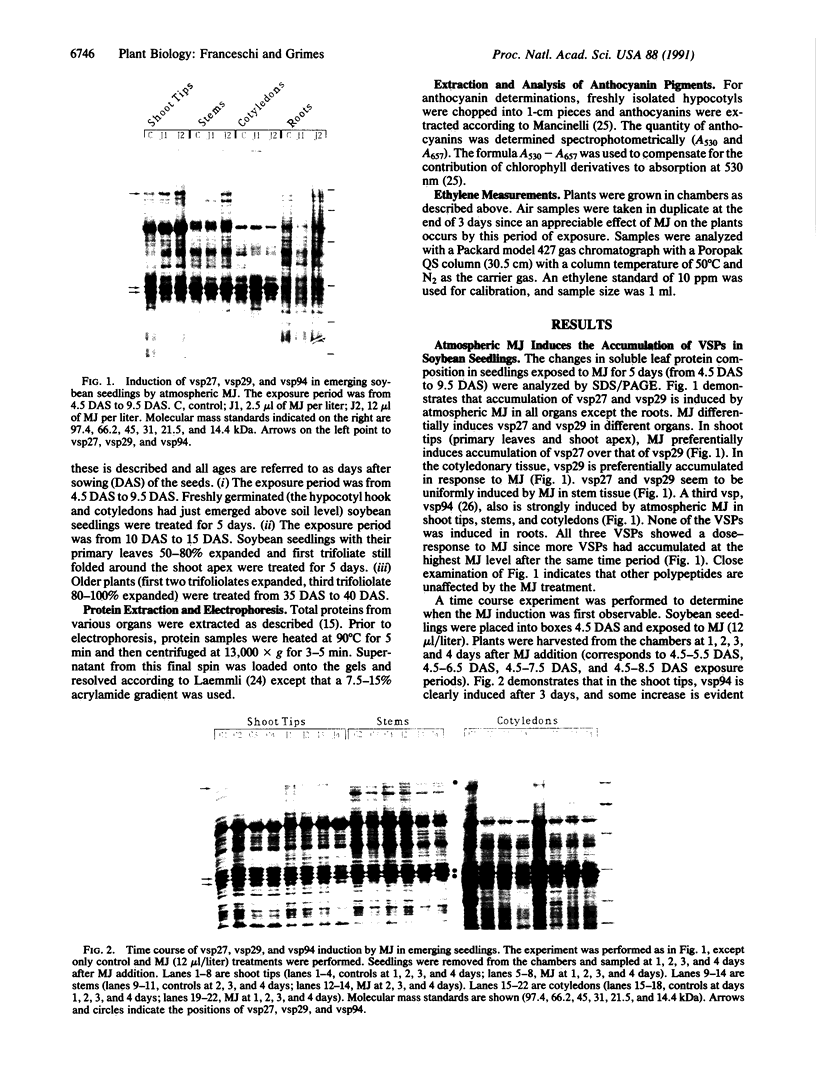
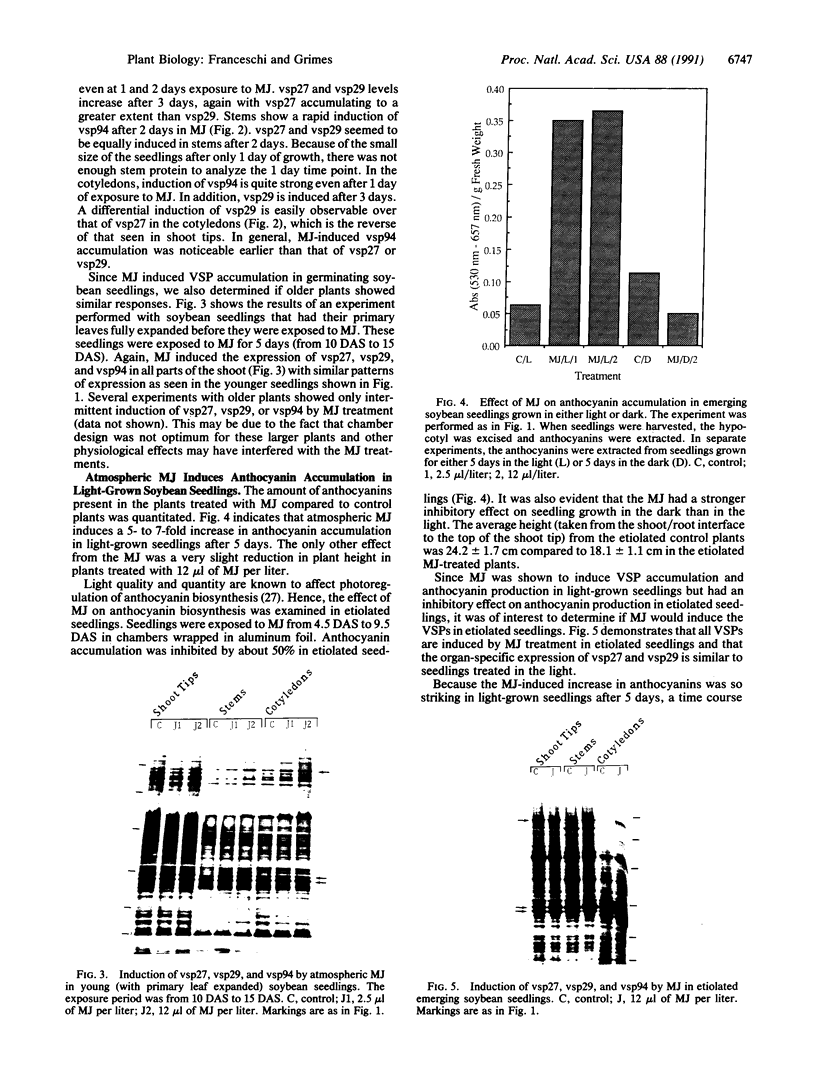
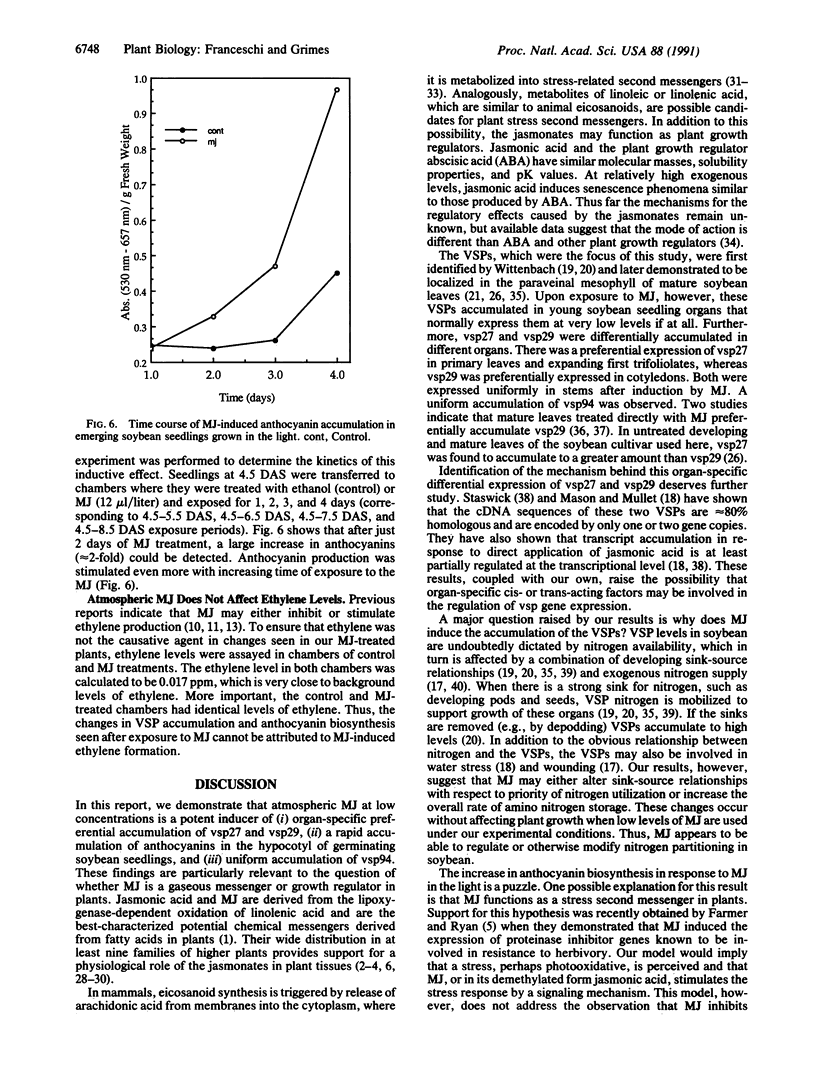
Soybean seedlings were exposed to atmospheric methyl jasmonate (MJ) to determine if low levels of this compound could regulate the expression and accumulation of the vegetative storage proteins (VSPs) in soybeans. Low levels of atmospheric MJ induced the accumulation of three VSPs with molecular masses of 27 kDa, 29 kDa, and 94 kDa (vsp27, vsp29, and vsp94, respectively). Atmospheric MJ caused vsp94 to be accumulated in all above-ground organs of the seedling uniformly after just 3 days of exposure. vsp27 preferentially accumulated in shoot tips and primary leaves, whereas vsp29 preferentially accumulated in the cotyledons. In addition to these effects, MJ also induced the biosynthesis of anthocyanins in light-grown seedlings but inhibited anthocyanin biosynthesis in etiolated seedlings. It is concluded that low levels of atmospheric MJ regulate anthocyanin biosynthesis and the organspecific accumulation of VSPs in developing soybean seedlings. The organ-specific differential accumulation may reflect changes in the pattern of nitrogen partitioning between various compounds and/or organs. These results lend substance to the hypothesis that volatile MJ may act as a gaseous messenger or growth regulator in plants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Wittenbach V. A., Giaquinta R. T. Paraveinal Mesophyll of Soybean Leaves in Relation to Assimilate Transfer and Compartmentation : III. Immunohistochemical Localization of Specific Glycopeptides in the Vacuole after Depodding. Plant Physiol. 1983 Jun;72(2):586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mancinelli A. L. Interaction between Light Quality and Light Quantity in the Photoregulation of Anthocyanin Production. Plant Physiol. 1990 Apr;92(4):1191–1195. doi: 10.1104/pp.92.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli A. L. Photoregulation of Anthocyanin Synthesis : VIII. Effect of Light Pretreatments. Plant Physiol. 1984 Jun;75(2):447–453. doi: 10.1104/pp.75.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason H. S., Mullet J. E. Expression of two soybean vegetative storage protein genes during development and in response to water deficit, wounding, and jasmonic acid. Plant Cell. 1990 Jun;2(6):569–579. doi: 10.1105/tpc.2.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Soybean vegetative storage protein structure and gene expression. Plant Physiol. 1988 May;87(1):250–254. doi: 10.1104/pp.87.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 1983 Sep;73(1):121–124. doi: 10.1104/pp.73.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veau E. J., Robinson J. M., Warmbrodt R. D., van Berkum P. Photosynthesis and photosynthate partitioning in n(2)-fixing soybeans. Plant Physiol. 1990 Sep;94(1):259–267. doi: 10.1104/pp.94.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]