Abstract
Varicella zoster virus (VZV) gives rise to two diseases, a primary infection, varicella, and a secondary infection, zoster. Morbidity and mortality from VZV in the United States has decreased by 80% to 90% due to the effective use of attenuated live viral vaccines. Because latent VZV continues to reactivate, however, serious VZV-induced disease persists. Newly developed molecular analyses have revealed that zoster is more common than previously realized; moreover, the establishment of VZV latency in neurons, such as those of the enteric nervous system, which do not project to the skin, leads to unexpected, serious, and clandestine manifestations of disease, including perforating gastrointestinal ulcers and intestinal pseudo-obstruction. The development of the first animal model of zoster, in guinea pigs, now enables the pathophysiology of latency and reactivation to be analyzed.
BACKGROUND
Varicella-zoster virus (VZV) is paradoxical in that it is highly infectious, but in vitro, it spreads poorly and slowly (1). Varicella (chickenpox) is transmitted wildly during epidemics, causing nearly universal infection of susceptibles; epidemics abate only when the susceptible population is depleted. The contagiousness of VZV approaches that of smallpox and measles. When cultured in vitro, however, VZV is highly cell-associated; infectious virions are not released into supernatant medium. Transmission requires direct cell-to-cell contact, enabling infected cells to fuse with their uninfected neighbors. Interestingly, cell-to-cell contact also appears to be the means by which VZV disseminates within a host. This is a slow process that has survival value because the virus does not overwhelm its host and gives the host time to mount an adaptive immune response to control the infection. It also provides VZV with time to establish latency in neurons, within which VZV can reside, safely ensconced for the lifetime of its host or until VZV reactivates months to years after the primary infection. Reactivation returns infectious virus to the population, presumably after the epidemic-depleted supply of susceptibles has been restocked. By this means, VZV has superbly become adapted to living with its only host and the virus is untroubled by its lack of an animal reservoir. VZV has become a nearly commensal organism (1).
Although VZV may be nearly commensal, nothing is perfect. Varicella is a serious, potentially lethal disease when it occurs in adults or in immunocompromised patients and, even in seemingly immunocompetent individuals, varicella can be lethal (1). The itching and scratching of the characteristic cutaneous vesicles of varicella can lead to dangerous streptococcal or staphylococcal superinfections. The discomfort and economic damage wrought by varicella epidemics, furthermore, is considerable. Zoster is often painful and can cause strokes, meningitis, and localized paralyses; moreover, in 15% of cases, zoster is followed by post-herpetic neuralgia, a pathological pain syndrome that is persistent, difficult to treat, and can lead to depression and suicide (1).
The natural history of VZV infections has seemed to be relatively straightforward. Although the virus spreads within a host from cell-to-cell, living human cells do not transmit VZV from one host to another. Virions do that. The portal of VZV entry is thought to be the upper respiratory tract, where inhaled infectious virions infect and proliferate within local lymphoid tissue, including tonsils (2). Here VZV infects T lymphocytes and can program them to home to the skin where the infection is transferred to the epidermis (3). VZV infects and proliferates within basal epidermal cells (4). Infectious nucleocapsids are assembled in the nuclei of infected cells and then bud through the inner nuclear membrane. VZV thus acquires a primary (temporary) envelope from the inner nuclear membrane during budding and is delivered to the perinuclear space, which is continuous with the rough endoplasmic reticulum (RER). The virus then buds again, fusing with the membrane of the RER, shedding its primary envelope as it does so and, in the process, is delivered to the cytosol. The glycoproteins of the final viral envelope are synthesized in the RER and are transferred by vesicular transport to the trans-Golgi network (TGN). Tegument proteins are synthesized on free polyribosomes in the cytosol but meet up with viral glycoproteins on the cytosolic faces of specialized wrapping structures that form from the TGN. The TGN membranes curve around nucleocapsids and tegument trapping them in the interiors of newly formed double-membrane enclosed organelles. The inner of the membranes is derived from the concave surface of the TGN wrapping structure and becomes the envelope of a newly produced virion, while the outer membrane, derived from the convex surface of the TGN wrapping structure, becomes a transport vesicle. These outer membranes contain mannose 6-phosphate receptors and the vesicles follow the itinerary of these receptors, which leads them to late endosomes. VZV is inactivated within the acidic interiors of late endosomes; therefore, subsequent exocytosis leads to the release of non-infectious viral particles. This process accounts for the paradoxical cell fusion-dependent cell-associated spread of VZV. Newly assembled virions, however, when they arise in cells that lack the mannose 6-phosphate-late endosome-lysosome pathway, avoid the acidic death that normally awaits them. That happens when VZV infects suprabasal cells of the epidermis, within which the mannose 6-phosphate-late endosome-lysosome pathway is inactivated as part of the process of maturation into squames, the dead waterproofing of the superficial epidermis. The fluid within the cutaneous vesicles of varicella is thus filled with highly infectious viral particles that slough off of the skin and waft through the air to be inhaled by the virus’ next unwitting host (4,5).
Sensory nerve fibers from dorsal root (DRG) and cranial nerve ganglia (CNG) innervate the epidermis. Pain- and temperature-sensitive fibers lose their Schwann cell sheaths as they enter the epidermis and ramify as naked nerve endings between the suprabasal epithelial cells (6). In this position, the nerve fibers become immersed in the free virions that subprabasal epithelial cells uniquely secrete (4). That immersion enables VZV to infect nerve endings and move by retrograde transport to the cell bodies in DRG and CNG where VZV establishes latency (7−9). Still, while this route accounts for the ability of VZV to gain access to ganglia, evolution would be remiss if it were to rely only on a single mechanism for anything as critical as gaining access to the neurons upon which the very survival of VZV as a species depends. VZV, of course, has already traveled in the blood, spreading in a T cell viremia to the skin (2,10). The blood supplies ganglia as well as the skin and experiments using the live attenuated vaccine as a probe have revealed that the viremic transit of VZV, in addition to retrograde transport in axons, delivers VZV to neurons where it establishes latency (11). Following vaccination, the vaccine virus (vOka), rather than wild-type (WT) VZV becomes latent in ganglia. In fact, vOka can even reactivate from latency to cause zoster, albeit at a frequency that is less than that of WT VZV (12). The vaccine is usually administered subcutaneously and vOka proliferates locally at the injection site (11). If retrograde transport were to have been the sole route by which VZV could reach ganglia, only those neurons that innervate the injection site would be infected. In fact, many neurons in multiple ganglia, including those at different axial levels and on the contralateral side, have been found (in autopsies of children who died suddenly from causes unrelated to VZV) to contain latent VZV. Viremia thus follows vaccination and disseminates VZV to a variety of neuronal residencies. There is another, perhaps more profound consequence of the viremic delivery of VZV to ganglia, than a wide distribution of latent VZV. Viremia delivers VZV to neurons that do not project to the skin (11,13,14). Given that zoster infects the skin as a result of the reactivated virus traveling by anterograde transport back down axons that innervate the epidermis, it is reasonable to expect that reactivation of VZV in neurons that lack cutaneous projections will result in a form of zoster that lacks a rash. Recent studies have now confirmed that such forms of zoster do occur, that they are menacing and dangerous, and are all the more so because complacent physicians rarely think of zoster when confronted with a patient who has no rash (14).
Among the ganglia that do not project to the skin but which have been found to harbor latent VZV are the nodose and autonomic ganglia, including the enteric nervous system (ENS) (14). The ENS is unique among autonomic ganglia in that the bulk of its neurons do not receive a direct innervation from the central nervous system (CNS) (15). It is thus neither sympathetic nor parasympathetic; moreover, it contains intrinsic primary afferent neurons that can initiate reflexes and interneurons arranged in microcircuits that allow the ENS to manifest integrative neuronal activity. The ENS contains more neurons than the remainder of the peripheral nervous system and can regulate the behavior of the bowel independently, even in the absence of input from the CNS. In a study of surgical specimens of gut removed from pediatric patients, transcripts encoding VZV gene products were detected in 12 of 13 VZV-exposed subjects (seven of seven with WT VZV; six of seven with vOka) (9). In contrast, no transcripts encoding VZV gene products were detected in controls (<1 year of age) that had not been exposed to VZV. Strikingly, in two VZV-exposed subjects with Hirschsprung’s disease (one natural varicella and one vaccinated), transcripts encoding VZV gene products were detected in the proximal ganglionated portion of bowel but not in the distal aganglionic gut (Figure 1). Latent VZV is thus a characteristic of the virome of the ENS of individuals who have been at some time in their lives, either through vaccination or natural varicella, exposed to VZV. Given the tendency of VZV to reactivate in DRG and CNG that project to the skin, there is no reason to believe that VZV would not similarly reactivate in neurons, such as those to the ENS that do not project to the skin. A rash usually characterizes cutaneous zoster because DRG and CNG project to the epidermis; painful cutaneous zoster without rash, “zoster sine herpete,” is relatively rare (10,14,16). Reactivation of zoster in the ENS, however, gives rise to enteric zoster, which is confined to the gut unless it occurs simultaneously with reactivation in a DRG or CNG (14). The detection of zoster when it occurs in a hidden internal site like the bowel, has, until recently been problematic. VZV has been discovered fortuitously after the fact in surgical or autopsy specimens done for intestinal pseudo-obstruction (Ogilivie’s syndrome) (17,18). This, however, is not an ideal diagnostic method. More recently, the appearance of DNA encoding VZV gene products in saliva has been found to reveal the presence of enteric zoster in patients suffering from abdominal pain for which no other cause was found after a thorough gastroenterological workup (14).
Fig. 1.
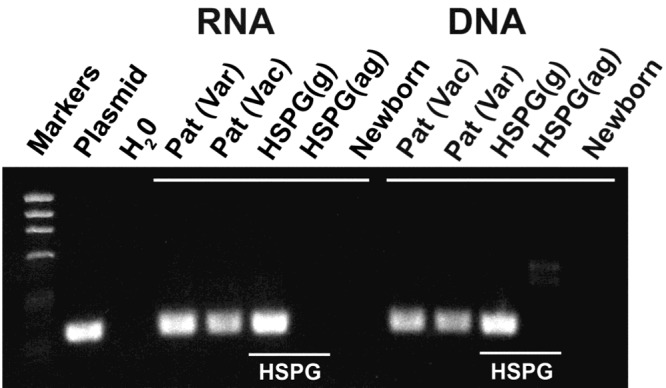
Transcripts encoding VZV gene products can only be found in segments of gut that contain ganglia. Transcripts and DNA encoding ORF63 were analyzed in surgically removed specimens of colon. ORF63, which is expressed during VZV latency, was analyzed as a marker for the presence of latently infected cells. Transcripts and DNA encoding ORF63 were each detected in a patient with a history of natural varicella (Var), a vaccinated patient (Vac) and in the proximal ganglionated region of bowel from a patient with Hirschsprung’s disease (HSPG). In contrast, neither transcripts nor DNA were detected in the distal aganglionic region of gut of the same patient with HSPG or in the intestine of a newborn infant, which was investigated as a control that neither experienced varicella, nor was vaccinated against it.
The VZV genome, with only 125,000 base pairs is the smallest of those of the eight human herpesviruses. VZV has 71 unique genes, divided into three categories: immediate early (IE), early (E), and late (L) (1). Gene synthesis is thought to occur in a cascade; regulatory IE genes are expressed before E genes, which are followed by L genes. Late genes encode structural gene products such as glycoproteins, which are important antigens involved in immune responses to VZV. When all 71 genes are expressed, infectious virus is produced, and the infection is “lytic.” Latency is thought to occur when only subsets of IE and E genes are expressed (19). Research involved in determining exactly which genes are expressed in latency is ongoing and results have been highly controversial (20). It is possible that VZV has more than one latency program, perhaps depending on the type of neurons in which it is latent. Latency of Epstein-Barr virus, a close relative of VZV, manifests three different latency patterns, associated respectively, with Burkett’s lymphoma, Hodgkin’s disease, and post-transplantation lymphoproliferative disease (21). There is evidence that the immunocytochemical detection of cytoplasmic expression of proteins encoded by VZV open reading frames (ORFs) 4, 21, 29, 63, and 66 reveal the presence in cells of latent VZV. In contrast, these proteins translocate to the nucleus when infection is lytic. The controversy is over the specificity of antibodies and, because much human research is carried out on cadavers, whether VZV reactivates after death (that is, during the time between death and the removal of ganglia during autopsy) (20). Most studies suggest that a low level of transcription of VZV genes occurs during latency and that the identification of transcripts encoding IE genes, especially those encoding ORF63, with no expression of L genes, helps to identify neurons with latent VZV.
WHY WERE VACCINES DEVELOPED AGAINST VZV?
Given that the vaccine virus, vOka, which becomes latent in ganglia of vaccinated hosts, can potentially cause zoster and that varicella is usually a benign childhood illness, it is fair to question why universal vaccination against varicella is useful. In fact, little attention was paid to VZV infections until immunosuppression became common. Patients who used to die uniformly began to survive cancer in response to chemotherapy, radiation, and allograft transplantation. The iatrogenic immunosuppression inherent to these medical advances drew attention to the lethal potential of VZV. The exaltation of cancer survival turned to ashes when treated patients succumbed to varicella (22). Fortunately, Thomas Weller, MD, achieved a breakthrough in 1953 by propagating VZV in cell culture for the first time (23). That success enabled Michiaki Takahashi, MD, to attenuate VZV in 1974 and prepare the live vOka varicella vaccine (24). Unfortunately, the gap between the preparation of the vaccine and its use to cope with the increasingly lethal issue of iatrogenic immunosuppression was large. Reasoned fears of vaccination included unknowns about the long-term effects of introducing modified DNA, the consequences of latency, and the passage of the virus in guinea pig cells during attenuation (25,26). There was also the unreasoned fear of vaccination that has persisted since Edward Jenner introduced vaccination against smallpox. It was impossible, moreover, to test the vaccine in an animal model. Probably the major fear of the varicella vaccine arose from the severity of varicella in adults. The anxiety was driven by the possibility that immunity introduced by the vaccine might wane and if it did, then vaccination could only postpone VZV infection to adulthood, thereby converting a mild illness of childhood to a severe illness of adults. Life-saving studies, however, were finally performed in children with leukemia who, if left without immunity, were threatened with death every time an epidemic of varicella occurred (27). The vOka vaccine was found to be safe and effective, even in this partially immunocompromised group. These children with leukemia were a proof-of-concept subset that made it morally possible subsequently to demonstrate the safety and efficacy of the varicella vaccine in healthy children and adults. The varicella vaccine was finally licensed for all healthy children in the United States in 1995 (19). Early studies suggested that the dreaded waning of immunity to varicella does not occur. The incidence of varicella in the United States is now 9 to 10 times lower than it was in the prevaccine era. The fatality rate has decreased by 88% (28,29). Millions of doses of vaccine have been distributed worldwide with a remarkable paucity of adverse events. In 2006, a second dose of varicella vaccine was added to the vaccine schedule to improve immunity and eliminate occasional epidemics and breakthrough infections (30). VZV is still the only human herpesvirus for which a vaccine exists. The success of the varicella vaccine eventually led to the development of a stronger live vaccine for adults to boost immunity and prevent zoster (31).
The critical question surrounding varicella vaccine today is not whether it is safe or effective but rather whether its universal use will, by decreasing circulation of WT VZV, cause epidemics of zoster. The hypothesis on which this idea is based is that repeated exposure of individuals is required to maintain specific cellular immunity to VZV and confine the virus to its latent state. This question was originally conceived and investigated using a mathematical model (32), which was expanded into a computer model; the data are therefore only indicative of reality if the assumptions on which they are based are correct (19). The fear of a raging zoster epidemic in elderly adults due to the vaccination of children has spawned an enormous number of publications, some of which have been hysterical. One report, for example, postulated the coming of an epidemic of zoster in young adults, with a 50% attack rate and 50 annual deaths in the United Kingdom (33). As a result, varicella vaccine is still not licensed for use in the United Kingdom, where children continue to experience considerable morbidity and mortality from varicella. Children, of course, lack political power and thus some of their elders think it perfectly reasonable to condemn the pediatric population to suffer through endless varicella epidemics with all of their consequences to maintain the immunity of their elders. Zoster and post-herpetic neuralgia are sufficiently insufferable that children’s welfare pales in comparison. Even more important than the questionable morality of the idea that children should be made to endure varicella to protect adults from zoster is the probable lack of validity of the concept.
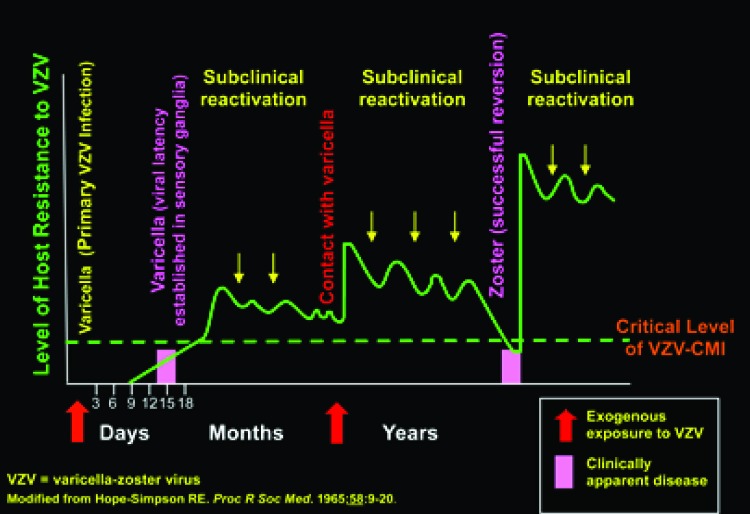
To understand the epidemiology of VZV, it is useful to reconsider the hypothesis of Edgar Hope-Simpson, an English general practitioner and member of the Royal Society of Medicine (Figure 2). He discovered, by observing his patients for many years, that zoster was rare in healthy children and young adults, became more common in middle age, and reached its peak in the elderly. He proposed that VZV might periodically, and sub-clinically reactivate internally from latency. He thought that these occurrences of occult disease were contained but each time they happened they would boost immunity to VZV. If this idea were to be correct, then the additional boosts to immunity derived from external exposures to VZV disseminated by patients with varicella or zoster would be beneficial but unnecessary. In old age, senescence of the immune system would occur regardless of boosting and susceptibility to zoster would increase. Hope-Simpson noted that zoster was 5 to 10 times more frequent in the aged than in the young, despite the absence, in his time, of varicella vaccination. Aging, cancer therapies, organ transplantation, increase in life span, and improvements in diagnosis have caused the incidence of zoster to increase the United States. This increase began approximately 60 years ago, predating the introduction of the varicella vaccine, and it is still ongoing at a rate that has remained remarkably constant. The postulated varicella vaccine-induced zoster epidemic has not yet occurred in the United States where the vaccine is nearly universally administered. The overall incidence rate of zoster in the United States is approximately 4.5 per 1,000 person-years (34), whereas that in the United Kingdom and indeed throughout Europe (35) is not significantly different. The independence of the incidence of zoster from the vaccination practices in place in various countries provides no support for the idea that vaccination against varicella provokes epidemic zoster.
Fig. 2.
The hypothesis of Hope-Simpson postulates that internal reactivation of VZV maintains immunity. A person is immunized during an initial attack of varicella but the virus becomes latent in neurons. Periodic subclinical reactivations (contained reversions) keep up immunity. This can be supplemented by external contacts with varicella. Immunity declines, however, as a function of age. This decline allows a periodic reactivation to cross the critical threshold of cell-mediated immunity (CMI) and zoster occurs (successful reversion). The zoster vaccine is designed to boost CMI and deter a periodic reactivation from crossing of the clinical threshold and causing active zoster.
Abundant epidemiologic data suggest that the increase in zoster in various populations is unrelated to use of varicella vaccine. There has been no increase the incidence of zoster in young or old people in the North America that can be identified as temporally related to the implementation of varicella vaccination programs (36−39). An especially enlightening retrospective study of the incidence of zoster was performed in France in sequestered religious groups who were not exposed to children; the sequestered nuns and monks were compared with the general public in the same locale, evaluating medical records during a 30-year period. The incidence of zoster during this period, approximately 15%, was the same in the sequestered and general populations (40,41). This study demonstrated that exposure to children who spread varicella is not required to maintain long-term immunity to zoster. Hope-Simpson was evidently prescient in his hypothesis that subclinical reactivation, as well as external exposure to VZV, could be adequate to maintain long-lasting immunity. Models that attempt to predict the future incidence of zoster on the assumption that maintenance of immunity requires active exposure to circulating virus derived from children with varicella omit the effect of internal boosting. Models that are incomplete and do not account for important contributing variables cannot be correct and accumulating evidence with respect to the incidence of zoster suggests that they are not.
Asymptomatic reactivation of VZV from latency has amply been demonstrated. For example, patients undergoing renal transplantation have been found to exhibit many asymptomatic increases in antibody titer to VZV in the absence exposure to external VZV (42). Asymptomatic immunocompromised patients experienced increases in cellular immunity and subclinical VZV viremia (43−45). One of the most dramatic findings is that one-third of astronauts experienced transient non-infectious VZV DNA in their saliva following space flight (46). Similarly 17% of children hospitalized in an intensive care unit had VZV DNA in their saliva transiently without specific symptoms (47). Zoster sine herpete is, of course, a symptomatic reactivation of VZV but, because no rash occurs, these occurrences are probably mostly undiagnosed and thus, like a tree that falls unwitnessed in the forest, are essentially undocumented events (14,16).
CAN LATENT VZV INFECTION OF THE ENS EXPLAIN ASYMPTOMATIC REACTIVATION OF VZV?
The discovery that VZV is latent in the ENS of virtually everyone who has either experienced varicella or been vaccinated with vOka (1,4,11) raises the question of whether gastrointestinal disease that is now thought to be idiopathic might actually be related to reactivation of VZV. A patient with a VZV-associated enteric illness would be unlikely to manifest a zosteriform rash, because the ENS does not innervate the skin. The demonstration of VZV DNA in saliva of patients with suspected enteric zoster is a promising method to use to screen patients if enteric zoster is suspected. VZV DNA in saliva is not, by itself, diagnostic of enteric zoster. It is present in patients during acute episodes of varicella or cutaneous zoster (14,48,49) as well as during episodes of presumed severe stress (46,47). Salivary VZV DNA, however, is not found in control subjects; recent studies revealed it to be present in 0 of 20 controls in one study (14) and in 0 of 80 controls in another (50). Salivary VZV DNA, moreover, is cleared from the saliva of patents with zoster upon recovery (14). Salivary VZV DNA, therefore, appears to be an indicator that active (lytic) VZV disease is present somewhere in the body. Where that disease is located has to be deduced from the full clinical picture. For example, if a patient with no rash has allodynia in a dermatomal distribution and salivary VZV DNA, the patient can be presumed to have zoster sine herpete; in fact, the salivary VZV DNA is usually the only laboratory evidence of that condition that one can obtain. On the other hand, when, as in a recent study, salivary VZV DNA was observed in 6 of 11 tested patients with unexplained abdominal pain and no rash, enteric zoster was suspected in the subset of patients with salivary VZV DNA (14). In the same study, salivary VZV DNA was found in 0 of 8 patients with known gastrointestinal disorders unrelated to VZV. Three of the six patients with unexplained abdominal pain in whom salivary VZV DNA was found were treated with valacyclovir; the pain remitted within 2 days of treatment and the VZV DNA disappeared from their saliva. Of the zoster patients, seven of seven patients tested no longer had VZV DNA detected in saliva (14). These observations are consistent with using salivary VZV DNA to test for enteric zoster. A therapeutic trial with valacyclovir is also suggestive, but direct confirmation, such as finding VZV DNA in an endoscopic specimen would provide definitive evidence of the diagnosis.
It was possible to make a tissue diagnosis of VZV infection in the gastrointestinal tract in one patient in whom salivary VZV DNA was found (14). This patient was a 16-year-old male who had received two doses of varicella vaccine at the appropriate age. The patient experienced the sudden onset of severe abdominal pain and weakness. A clinical examination revealed gastric bleeding and free air under the diaphragm was observed on an abdominal X-ray. A partial gastrectomy was performed to control perforated ulceration of the stomach. VZV DNA was detected in saliva, which prompted examination of the excised tissue. VZV DNA was observed in the resected portion of the stomach and immunocytochemical examination of the tissue revealed the presence of both late protein (gE) and immediate early protein (ORF63) in cells along the margins of the ulcers (Figure 3B, compare with Figure 3A). Despite extensive examination of serial sections and use of antibodies to pan-neuronal marker proteins, there was no evidence of surviving enteric neurons in the tissue. Genotyping of the VZV DNA revealed that the lesions were due to vOka and not WT VZV. After recovery, 1 month after the illness, salivary VZV DNA was no longer detected and a post-convalescent biopsy no longer contained immunocytochemically detectable VZV protein. This case was a clear demonstration of enteric zoster because the disease due to vOka which was administered to the patient years earlier. The absence of enteric neurons is consistent with the idea that VZV (vOka) reactivated in the ENS and killed the neurons in which it became lytic. It is likely that mucosal projections of these neurons delivered the virus to the gastric epithelium in which VZV proliferated and caused ulceration and, ultimately, perforation. Difficulty swallowing persisted but the patient’s mother refused further evaluation for the presence of an underlying immunological defect, such a deficient NK cell function that might have been responsible for the severe reactivation of vOka. No gross immunodeficiency in cellular or humoral immunity was detected.
Fig. 3.
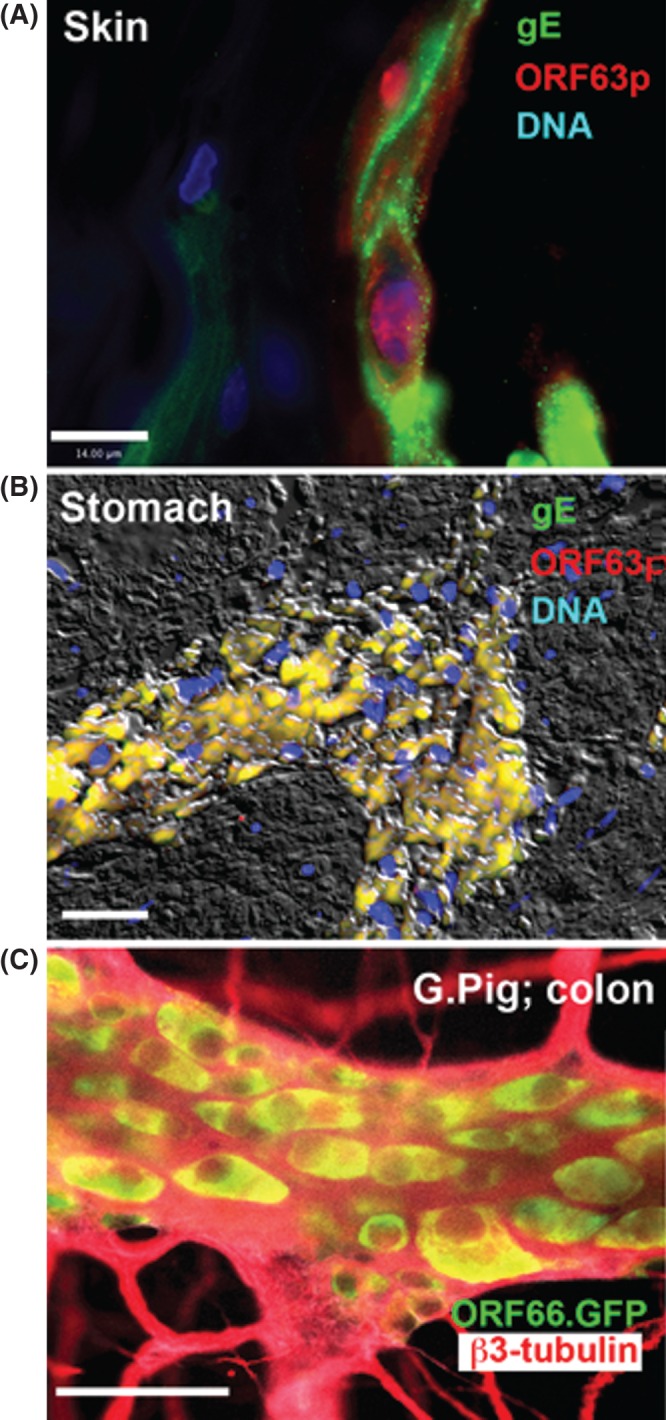
Immunoreactivity of VZV proteins in lesions. (A) Human skin. Persistent VZV in the epidermis of a man who died of recurrent episodes of disseminated zoster with granulomas (59). The virus had acquired resistance to acyclovir. The immunoreactivity of a late protein (gE; green) and an immediate early protein (ORF63p; red) can be seen. Note that the infection is lytic and ORF63p is predominantly intranuclear in location. The marker = 14 µm. (B) Human stomach. The fluorescence of VZV was detected in the excised stomach of a 16-year-old vaccinated male. The immunoreactivities of gE (green) and ORF63p (red) are co-localized; the image is merged so that the co-localized fluorescence is yellow. DNA fluorescence is blue. Interference microscopy with white light is superimposed to help with orientation. The marker = 50 µm. (C) Guinea pig colon from an animal in which with VZV-infected lymphocytes were injected intravenously. The VZV expresses green fluorescent protein (GFP) under the control of the ORF66 promoter (courtesy of Dr. Paul Kinchington). b3-tubulin immunoreactivity (red) is used as a neural marker and shows the limits of the ganglia. Only cell bodies have GFP fluorescence (yellow) in the merged image with red fluorescence of b3-tubulin.
Until recently, the absence of an animal model was a major impediment to understanding VZV latency and the transition from latency to reactivation that causes zoster. Multiple passages through guinea pig cells, however, were employed to attenuate the vOka strain of VZV to produce the varicella vaccine (19,24). In addition to yielding an attenuated virus, this process made clear that VZV can proliferate in guinea pig cells and encouraged us to determine whether VZV can infect guinea pig neurons in vitro. Although pure neurons, separated from fibroblasts and other non-neuronal cells cannot be obtained from DRG or CNG, they can be isolated from the ENS, which, similar to the CNS, lacks internal collagenous sheaths (51). We found that VZV infects enteric neurons isolated from guinea pig gut (52,53); moreover, when cell-free virions are used to infect isolated neurons, the type of infection is latent. The infected neurons survive for as long as the cultures can be maintained. In contrast, if cell-associated virus is employed, or if fibroblasts are present at the time cultures are inoculated with VZV, lytic infection occurs. Neurons begin to express glycoproteins and die within 48 to 72 hours. These observations are compatible with the idea that latency occurs when neurons are infected with cell-free VZV, as in sensory nerve endings ramifying within the suprabasal epidermis during varicella (4). Strikingly, after latency has been established in vitro, expression either of ORF61p of VZV or its herpes simplex virus orthologue, ICP0, in the infected guinea pig enteric neurons reactivates VZV (53). Reactivation is made evident by the expression of late proteins, cell death within 48 to 72 hours, electron microscopic visualization of viral particles, and the transmission of infection to co-cultured MeWo cells. Very recently, additional in vitro models of VZV infection, utilizing stem cell-derived human neurons have confirmed the tendency of cell-free VZV to induce latent infection, particularly when infection occurs through axon terminals (7, 54−56).
All models of human infection are imperfect. That is why they are models. In vitro models of VZV infection of animal or human neurons are all deficient in the sense that infection occurs in the absence of an immune system and the potential effects on the infected neurons of inflammation, hormones, and neighboring cells. The guinea pig enteric neurons are not human and human neurons derived from stem cells are heterogeneous and not identical to the normal targets of VZV. We therefore sought to develop an animal model in which VZV infection could be investigated in vivo (13,57,58). Hairless guinea pigs were employed to conveniently examine the skin. We found, essentially that three different routes of administration are capable of infecting the guinea pig ENS; 1) multiple intradermal injections in a belt around the animal at lumbar levels; 2) direct injection of the intestinal wall with VZV-infected cells; and 3) intravenous injection of guinea pigs with VZV-infected human or guinea pig lymphocytes (13). In the latter case, virtually the entire ENS as well as the DRG became infected and the infection was latent (Figure 3C; compare with Figure 3A). More recently, we found that we could reactivate VZV in the guinea pigs in which latent infection had been established as a result of intravenous administration of VZV-infected lymphocytes (57,58). This could be accomplished if the animals were subjected to a combination of immunosuppression with tacrolimus and administration of a stress hormone, corticotropic-releasing hormone. Thus far, the reactivation has been extremely severe, affecting the skin, intestine, liver, muscle, heart, and kidney. The reactivation mimics the effects of disseminated zoster in humans, which is also an extremely severe disease and also occurs in the setting of immunosuppression and stress (Figure 3A) (59). In the future, the animal model will have to be modified to scale back the degree of immunosuppression and stress to more closely mimic zoster. Still, the animal model now provides a valuable tool to look for future therapies for zoster and vaccines to prevent it. The model has already revealed the potency of VZV-infected lymphocytes as vehicles for the transfer of VZV. The lymphocytes, moreover, do not secrete infectious cell-free viral particles. As a result, it is apparent that, in contrast to VZV-infected fibroblasts, VZV-infected lymphocytes are remarkably able to transfer latent VZV infection to neurons. How lymphocytes are able to do this remains to be determined.
Enteric zoster is a problem of unknown extent. The availability of salivary VZV DNA and its use in combination with endoscopy should, in the future, provide evidence of its incidence and prevalence. The guinea pig model may help to clarify how VZV the condition can be eliminated. Most important, the possibility that enteric zoster contributes to the derivation of gastrointestinal disorders that are now considered to be iatrogenic can be definitively evaluated.
Footnotes
Potential Conflict of Interest: A.A.G.: Service contracts (molecular VZV diagnosis for vaccine safety): Merck; Ad hoc consulting and chair DSMB: GSK (on VZV). Supported by NIH R01 DK093094.
REFERENCES
- 1.Gershon A, Breuer J, Cohen JI, et al. Varicella zoster virus infection. Nat Rev Primers. 2015;1:15016–34. doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Topics Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen N, Mukherjee G, Sen A, et al. Single-cell mass cytometry analysis of human tonsil T cell remodeling by varicella zoster virus. Cell Reports. 2014;8:633–45. doi: 10.1016/j.celrep.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JJ, Zhu Z, Gershon AA, Gershon MD. Mannose 6-phosphate receptor dependence of varicella zoster virus infection in vitro and in the epidermis during varicella and zoster. Cell. 2004;119:915–26. doi: 10.1016/j.cell.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Gershon MD, Gershon AA. VZV infection of keratinocytes: production of cell-free infectious virions in vivo. Curr topics Microbiol Immunol. 2010;342:173–88. doi: 10.1007/82_2010_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newbold P, Brain SD. An investigation into the mechanism of capsaicin-induced oedema in rabbit skin. Br J Pharmacol. 1995;114:570–7. doi: 10.1111/j.1476-5381.1995.tb17177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grigoryan S, Yee MB, Glick Y, et al. Direct transfer of viral and cellular proteins from varicella-zoster virus-infected non-neuronal cells to human axons. PloS One. 2015;10:e0126081. doi: 10.1371/journal.pone.0126081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markus A, Grigoryan S, Sloutskin A, et al. Varicella-zoster virus (VZV) infection of neurons derived from human embryonic stem cells: direct demonstration of axonal infection, transport of VZV, and productive neuronal infection. J Virol. 2011;85:6220–33. doi: 10.1128/JVI.02396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gershon AA, Chen J, Davis L, et al. Latency of varicella zoster virus in dorsal root, cranial, and enteric ganglia in vaccinated children. Trans Am Clin Climatol Assoc. 2012;123:17–33. discussion 33-5. [PMC free article] [PubMed] [Google Scholar]
- 10.Levin MJ. Varicella-zoster virus and virus DNA in the blood and oropharynx of people with latent or active varicella-zoster virus infections. J Clin Virol. 2014;61:487–95. doi: 10.1016/j.jcv.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Gershon AA, Li Z, Cowles RA, Gershon MD. Varicella zoster virus (VZV) infects and establishes latency in enteric neurons. J Neurovirol. 2011;17:578–89. doi: 10.1007/s13365-011-0070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinmann S, Chun C, Schmid DS, et al. Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009. J Infect Dis. 2013;208:1859–68. doi: 10.1093/infdis/jit405. [DOI] [PubMed] [Google Scholar]
- 13.Gan L, Wang M, Chen JJ, Gershon MD, Gershon AA. Infected peripheral blood mononuclear cells transmit latent varicella zoster virus infection to the guinea pig enteric nervous system. J Neurovirol. 2014;20:442–56. doi: 10.1007/s13365-014-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershon AA, Chen J, Gershon MD. Use of saliva to identify varicella-zoster virus (VZV) infection of the gut. Clin Infect Dis. 2015;61:536–44. doi: 10.1093/cid/civ320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 16.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Topics Microbiol Immunol. 2010;342:243–53. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelman DA, Antaki F, Basson MD, Salwen WA, Gruber SA, Losanoff JE. Ogilvie syndrome and herpes zoster: case report and review of the literature. J Emerg Med. 2009;39:696–700. doi: 10.1016/j.jemermed.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Pui JC, Furth EE, Minda J, Montone KT. Demonstration of varicella-zoster virus infection in the muscularis propria and myenteric plexi of the colon in an HIV-positive patient with herpes zoster and small bowel pseudo-obstruction (Ogilvie’s syndrome) Am J Gastroenterol. 2001;96:1627–30. doi: 10.1111/j.1572-0241.2001.03808.x. [DOI] [PubMed] [Google Scholar]
- 19.Gershon A, Takahashi M, Seward JF. Live attenuated varicella vaccine. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. Philadelphia: WB Saunders; 2013. pp. 837–69. [Google Scholar]
- 20.Kennedy PG, Rovnak J, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96:1581–602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leach C, Syumaya C, Harrison G. Epstein-Barr Virus. In: Cherry J, Harrison G, Kaplan S, Steinbach W, Hotez P, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 7th ed. Philadelphia: Elsevier; 2014. pp. 1992–2015. [Google Scholar]
- 22.Feldman S, Hughes W, Daniel C. Varicella in children with cancer: 77 cases. Pediatrics. 1975;80:388–97. [PubMed] [Google Scholar]
- 23.Weller TH. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc Soc Exp Biol Med. 1953;83:340–6. doi: 10.3181/00379727-83-20354. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi M, Otsuka T, Okuno Y, Asano Y, Yazaki T, Isomura S. Live vaccine used to prevent the spread of varicella in children in hospital. Lancet. 1974;2:1288–90. doi: 10.1016/s0140-6736(74)90144-5. [DOI] [PubMed] [Google Scholar]
- 25.Brunell PA. Live varicella vaccine. Lancet. 1975;i:98. doi: 10.1016/s0140-6736(82)90006-x. [DOI] [PubMed] [Google Scholar]
- 26.Brunell PA. Varicella vaccine: the crossroads is where we are not! Pediatrics. 1978;62:858–9. [PubMed] [Google Scholar]
- 27.Gershon AA, Steinberg S, Gelb L. NIAID-Collaborative-Varicella-Vaccine-Study-Group. Live attenuated varicella vaccine: efficacy for children with leukemia in remission. JAMA. 1984;252:355–62. doi: 10.1001/jama.252.3.355. [DOI] [PubMed] [Google Scholar]
- 28.Baxter R, Ray P, Tran TN, et al. Long-term effectiveness of varicella vaccine: a 14-year, prospective cohort study. Pediatrics. 2013;131:e1389–96. doi: 10.1542/peds.2012-3303. [DOI] [PubMed] [Google Scholar]
- 29.Marin M, Zhang JX, Seward JF. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics. 2011;128:214–20. doi: 10.1542/peds.2010-3385. [DOI] [PubMed] [Google Scholar]
- 30.Anon. Prevention of varicella: recommendations for use of varicella vaccines in children, including a recommendation for a routine 2-dose varicella immunization schedule. Pediatrics. 2007;120:221–31. doi: 10.1542/peds.2007-1089. [DOI] [PubMed] [Google Scholar]
- 31.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 32.Garnett GP, Grenfell BT. The epidemiology of varicella-zoster virus infections: the influence of varicella on the prevalence of herpes zoster. Epidemiol Infect. 1992;108:513–28. doi: 10.1017/s0950268800050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisson M, Gay N, Edmonds WJ, Andrews NJ. Exposure to varicella boosts immunity to herpes-zoster: implications for mass vaccination against chickenpox. Vaccine. 2002;20:2500–7. doi: 10.1016/s0264-410x(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 34.Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502. doi: 10.1186/s12879-015-1262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinchinat S, Cebrian-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis. 2013;13:170. doi: 10.1186/1471-2334-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159:739–45. doi: 10.7326/0003-4819-159-11-201312030-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 38.Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993–2006 : evaluation of impact of varicella vaccination. Clin Infect Dis. 2011;52:332–40. doi: 10.1093/cid/ciq077. [DOI] [PubMed] [Google Scholar]
- 39.Russell ML, Dover DC, Simmonds KA, Svenson LW. Shingles in Alberta: before and after publicly funded varicella vaccination. Vaccine. 2014;32:6319–24. doi: 10.1016/j.vaccine.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Crumpacker C. Absence of exposure to varicella does not increase the risk of zoster. Clin Infect Dis. 2011;53:412–3. doi: 10.1093/cid/cir439. [DOI] [PubMed] [Google Scholar]
- 41.Gaillat J, Gajdos V, Launay O, et al. Does monastic life predispose to the risk of Saint Anthony’s Fire (herpes zoster)? Clin Infect Dis. 2011;53:405. doi: 10.1093/cid/cir436. [DOI] [PubMed] [Google Scholar]
- 42.Luby J, Ramirez-Ronda C, Rinner S, Hull A, Vergne-Marini P. A longitudinal study of varicella zoster virus infections in renal transplant recipients. J Infect Dis. 1977;135:659–63. doi: 10.1093/infdis/135.4.659. [DOI] [PubMed] [Google Scholar]
- 43.Ljungman P, Wilczek H, Gahrton G, et al. Long-term acyclovir prophylaxis in bone marrow transplant recipients and lymphocyte proliferation responses to herpes virus antigens in vitro. Bone Marrow Transpl. 1986;1:185–92. [PubMed] [Google Scholar]
- 44.Schunemann S, Mainka C, Wolff MH. Subclinical reactivation of varicella-zoster virus in immunocompromised and immunocompetent individuals. Intervirology. 1998;41:98–102. doi: 10.1159/000024920. [DOI] [PubMed] [Google Scholar]
- 45.Wilson A, Sharp M, Koropchak CM, Ting SF, Arvin AM. Subclinical varicella-zoster virus viremia, herpes zoster, and T lymphocyte immunity to varicella-zoster viral antigens after bone marrow transplantation. J Infect Dis. 1992;165:119–26. doi: 10.1093/infdis/165.1.119. [DOI] [PubMed] [Google Scholar]
- 46.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 47.Papaevangelou V, Quinlivan M, Lockwood J, et al. Subclinical VZV reactivation in immunocompetent children hospitalized in the ICU associated with prolonged fever duration. Clin Microbiol Infect. 2013;19:E245–51. doi: 10.1111/1469-0691.12131. [DOI] [PubMed] [Google Scholar]
- 48.Mehta SK, Tyring SK, Cohrs RJ, et al. Rapid and sensitive detection of varicella zoster virus in saliva of patients with herpes zoster. J Virol Methods. 2013;193:128–30. doi: 10.1016/j.jviromet.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta SK, Tyring SK, Gilden DH, et al. Varicella-zoster zirus in the saliva of patients with herpes zoster. J Infect Dis 2008. 2008:654–7. doi: 10.1086/527420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Birlea M, Cohrs RJ, Bos N, Mehta SK, Pierson DL, Gilden D. Search for varicella zoster virus DNA in saliva of healthy individuals aged 20-59 years. J Med Virol. 2008;197:360–2. doi: 10.1002/jmv.23834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2014;86:268–80. discussion 80. [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Gershon A, Silverstein SJ, Li ZS, Lungu O, Gershon MD. Latent and lytic infection of isolated guinea pig enteric and dorsal root ganglia by varicella zoster virus. J Med Virol. 2003;70:S71–8. doi: 10.1002/jmv.10325. [DOI] [PubMed] [Google Scholar]
- 53.Gershon AA, Chen J, Gershon MD. A model of lytic, latent, and reactivating varicella-zoster virus infections in isolated enteric neurons. J Infect Dis. 2008;197(suppl 2):S61–5. doi: 10.1086/522149. [DOI] [PubMed] [Google Scholar]
- 54.Sloutskin A, Kinchington PR, Goldstein RS. Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose. Virology. 2013;443:285–93. doi: 10.1016/j.virol.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grigoryan S, Kinchington PR, Yang IH, et al. Retrograde axonal transport of VZV: kinetic studies in hESC-derived neurons. J Neurovirol. 2012;18:462–70. doi: 10.1007/s13365-012-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Markus A, Lebenthal-Loinger I, Yang IH, Kinchington PR, Goldstein RS. An in vitro model of latency and reactivation of varicella zoster virus in human stem cell-derived neurons. PLoS Pathogens. 2015;11:e1004885. doi: 10.1371/journal.ppat.1004885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohrs RJ, Gilden D. 2014 Colorado alphaherpesvirus latency symposium. J Neurovirol. 2014;20:644–56. doi: 10.1007/s13365-014-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohrs RJ, Gilden D. 2015 Colorado Alphaherpesvirus Latency Society Symposium. J Neurovirol. 2015;21:706–16. doi: 10.1007/s13365-015-0396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhalla P, Forrest GN, Gershon M, et al. Disseminated, persistent, and fatal infection due to the vaccine strain of varicella-zoster virus in an adult following stem cell transplantation. Clin Infect Dis. 2015;60:1068–74. doi: 10.1093/cid/ciu970. [DOI] [PMC free article] [PubMed] [Google Scholar]