Abstract
Dystrophin is a large sub-sarcolemmal protein. Its absence leads to Duchenne muscular dystrophy (DMD). Binding to the sarcolemma is essential for dystrophin to protect muscle from contraction-induced injury. It has long been thought that membrane binding of dystrophin depends on its cysteine-rich (CR) domain. Here, we provide in vivo evidence suggesting that dystrophin contains three additional membrane-binding domains including spectrin-like repeats (R)1-3, R10-12 and C-terminus (CT). To systematically study dystrophin membrane binding, we split full-length dystrophin into ten fragments and examined subcellular localizations of each fragment by adeno-associated virus-mediated gene transfer. In skeletal muscle, R1-3, CR domain and CT were exclusively localized at the sarcolemma. R10-12 showed both cytosolic and sarcolemmal localization. Importantly, the CR-independent membrane binding was conserved in murine and canine muscles. A critical function of the CR-mediated membrane interaction is the assembly of the dystrophin-associated glycoprotein complex (DGC). While R1-3 and R10-12 did not restore the DGC, surprisingly, CT alone was sufficient to establish the DGC at the sarcolemma. Additional studies suggest that R1-3 and CT also bind to the sarcolemma in the heart, though relatively weak. Taken together, our study provides the first conclusive in vivo evidence that dystrophin contains multiple independent membrane-binding domains. These structurally and functionally distinctive membrane-binding domains provide a molecular framework for dystrophin to function as a shock absorber and signaling hub. Our results not only shed critical light on dystrophin biology and DMD pathogenesis, but also provide a foundation for rationally engineering minimized dystrophins for DMD gene therapy.
Introduction
Dystrophin is an essential cytoskeletal protein in the muscle. It constitutes a primary linkage between the extracellular matrix (ECM) and the actin cytoskeleton (1,2). In muscle cells, dystrophin plays an important role in maintaining membrane integrity and preventing membrane rupture. Loss of dystrophin, as seen in Duchenne muscular dystrophy (DMD) (3), leads to sarcolemmal leakage, myofiber degeneration and necrosis. Full-length dystrophin is a large rod-shaped protein. It contains four functional domains including N-terminus (NT), the mid-rod domain, the cysteine-rich (CR) domain and C-terminus (CT). The mid-rod domain consists of 24 spectrin-like repeats (R). Four hinges (H) are interspersed in the mid-rod domain (4). Dystrophin NT and spectrin-like repeats R11-17 bind to cytoskeletal filamentous actin (5,6). The CR domain anchors dystrophin to the muscle membrane via interaction with the transmembrane protein β-dystroglycan (7–9). β-dystroglycan further connects with basal lamina proteins to complete the axis from the ECM to the cytoskeleton (10). This mechanical linkage protects the muscle membrane from contraction-induced damages. In this well-established model, the dystrophin CR domain is solely responsible for dystrophin membrane binding (Supplementary Material, Fig. S1).
Despite compelling evidence suggesting that the CR domain mediates dystrophin-sarcolemma interaction, case reports from some rare-occurring patients suggest that dystrophin may bind to the sarcolemma through CR domain-independent mechanisms. In these patients, biochemical and genetic analyses confirmed a complete deletion of the CR domain. Yet, immunostaining showed clear sarcolemmal localization of the truncated dystrophin protein (Supplementary Material, Fig. S2B) (11–13).
To better understand how dystrophin interacts with the sarcolemma in the absence of the CR domain, we performed a comprehensive in vivo screening for alternative membrane binding domains (MBDs) in dystrophin. We identified R1-3, R10-12 and CT as new dystrophin MBDs in mouse muscle. We further confirmed that these MBDs are conserved in dog muscle. To determine whether these MBDs are functionally equivalent, we evaluated their ability to establish the dystrophin-associated glycoprotein complex (DGC) at the sarcolemma. Our results showed that only the CR domain and CT are capable of restoring the DGC. We also evaluated these newly discovered MBDs in the heart. We found that R1-3 and CT interact with the sarcolemma in cardiac muscle. Taken together, our studies suggest that dystrophin-sarcolemma interaction is much more complex than it has been perceived. Our findings reveal a new model of dystrophin membrane binding. This model may better explain the dynamic participation of dystrophin in maintaining the integrity of the muscle cell membrane.
Results
Identification of dystrophin R1-3, R10-12 and CT as new dystrophin MBDs
To thoroughly understand how dystrophin interacts with the sarcolemma, we performed a comprehensive screening in mouse muscle. According to the fact that dystrophin has four functional domains and its mid-rod domain can be further divided into sub-regions (14), we split the full-length human dystrophin protein into ten subdomains, including NT-H1, R1-3, R4-6, R7-9, R10-12, R13-15, R16-19, R20-24, H4-CR and CT. We fused each subdomain with a green fluorescent protein (GFP) tag and individually expressed them in the tibialis anterior (TA) muscle of dystrophin-null mdx mice by adeno-associated virus (AAV)-mediated gene transfer (Supplementary Material, Fig. S3).
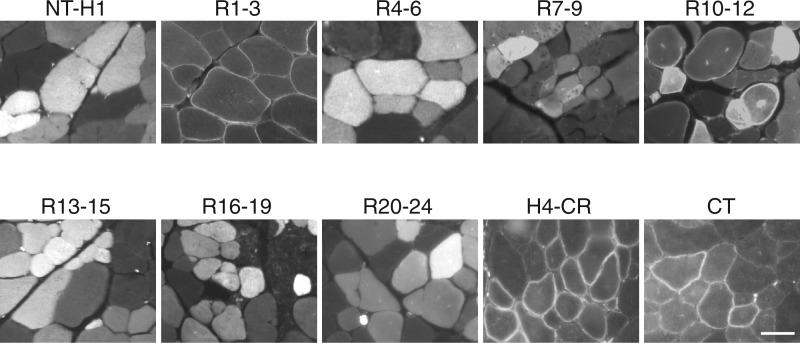
To determine subcellular localization of each dystrophin subdomain, we visualized the GFP signal under a fluorescence microscope (Fig. 1) . In line with the literature, we observed the sarcolemmal localization of the H4-CR subdomain. Unexpectedly, we found that subdomains R1-3 and CT were exclusively restricted at the muscle cell membrane. Subdomains NT-H1, R4-6, R7-9, R13-15, R16-19, and R20-24 were only detected in the cytosol. Interestingly, the R10-12 subdomain was found both at the sarcolemma and in the cytoplasm (Fig. 1).
Figure 1.
Dystrophin R1-3, R10-12, CR and CT are independent membrane-binding domains. Full-length human dystrophin was split into ten subdomains and each subdomain fused with a GFP tag. The fusion proteins were individually expressed in mdx muscle by AAV gene transfer. Representative GFP photomicrographs of each indicated dystrophin subdomain are shown. Dystrophin R1-3, H4-CR and CT were exclusively localized at the sarcolemma. R10-12 was found at the sarcolemma and in the cytosol. NT-H1, R4-6, R7-9, R13-15, R16-19 and R20-24 were exclusively localized in the cytosol. Scale bar: 50 μm.
To confirm these intriguing observations, we performed immunoblot with whole muscle lysates and microsomal preparations (Fig. 2). In whole muscle lysates, we found an efficient expression of all ten dystrophin subdomains (Fig. 2A). However, only subdomains R1-3, R10-12, H4-CR and CT were detected in the membrane-enriched microsomal preparations (Fig. 2B). These data are in agreement with immunostaining results suggesting that these subdomains are indeed dystrophin MBDs.
Figure 2.
Microsomal western blot suggests the association of R1-3, R10-12, CR and CT with the sarcolemma. (A) Whole muscle lysate western blots revealing AAV-mediated expression of GFP-fused dystrophin subdomains in mdx muscle. (B) Detection of dystrophin R1-3, R10-12, H4-CR and CT in the membrane fraction by microsomal western blots. GAPDH marks the cytosolic fraction. (C) cytosolic fraction; M, membrane fraction.
Preservation of the membrane-binding property of R1-3, R10-12, CR and CT in canine muscle
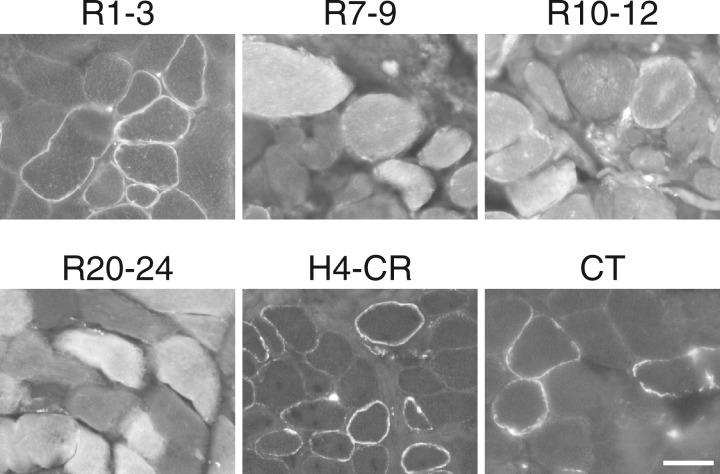
To examine whether the membrane-binding property of R1-3, R10-12, H4-CR and CT is conserved in different species, next we delivered the corresponding AAV vectors to dystrophic dog muscle by local injection. As controls, we also injected R7-9 and R20-24 AAV vectors. Two months later, we examined GFP expression under a fluorescence microscope. Similar to what we saw in mdx muscle, R1-3, H4-CR and CT subdomains were exclusively localized in the muscle membrane, while the R10-12 subdomain was found both at the sarcolemma and in the cytoplasm. Subdomains R7-9 and R20-24, which localized exclusively in the cytosol in mdx muscle, were only detected in the cytosol of dystrophic dog muscle (Fig. 3)
Figure 3.
Dystrophin R1-3, R10-12, H4-CR and CT bind to the sarcolemma in canine muscle. Indicated GFP fusion dystrophin subdomains were expressed in dystrophic dog muscle by AAV gene transfer. Representative GFP photomicrographs show the membrane binding of R1-3, R10-12, H4-CR and CT and cytosolic localization of R7-9 and R20-24. R10-12 is also seen in the cytosol. Scale bar: 50 μm.
Independent restoration of the DGC by the CR domain and CT
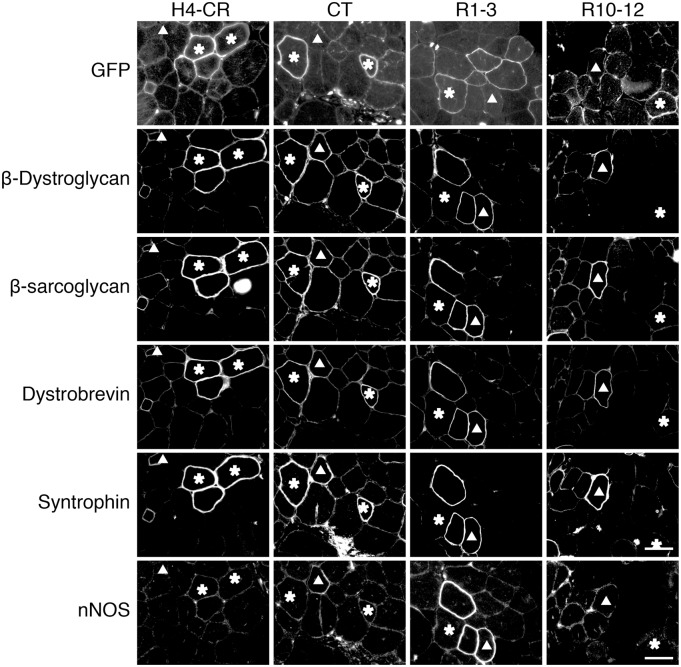
In the canonical model (Supplementary Materials, Figs S1 and S4), the CR domain is solely responsible for nucleating dystroglycan, sarcoglycans, dystrobrevin and syntrophin into the DGC at the sarcolemma (15–18). To determine whether the newly identified MBDs had similar functions, we evaluated DGC components on serial muscle sections by immunostaining (Fig. 4). As expected, the H4-CR subdomain successfully restored β-dystroglycan, β-sarcoglycan, dystrobrevin and syntrophin to the sarcolemma. Surprisingly, myofibers that were transduced with the CT subdomain AAV vector also resulted in sarcolemmal localization of these DGC components. In muscles infected with R1-3 and R10-12 AAV vectors, DGC components were detected in GFP-negative revertant fibers but not in transduced GFP-positive myofibers (Fig. 4).
Figure 4.
Dystrophin CT restores the DGC at the sarcolemma. Representative serial section photomicrographs of GFP and immunostaining for β-dystroglycan, β-sarcoglycan, dystrobrevin and syntrophin in mdx muscle expressing the indicated GFP-dystrophin subdomain fusion proteins. Asterisk, the GFP-positive myofiber in serial sections; Triangle, the GFP-negative revertant fiber in serial sections. GFP signals co-localize with DGC components in myofibers transduced by the H4-CR and CT but not R1-3 and R10-12 subdomain AAV vectors. Scale bar: 50 μm.
Conservation of the membrane-binding property of R1-3, CR and CT in cardiac muscle
To determine whether our findings in skeletal muscle can be extended to cardiac muscle, we delivered GFP-fusion subdomain AAV vectors via the tail vein (Fig. 5). Compared with un-injected BL10 and mdx controls, systemic AAV injection resulted in robust GFP signals in the myocardium. Several different patterns were observed. The H4-CR subdomain was restricted at the sarcolemma while subdomains NT-H1, R4-6, R10-12, R13-15, R16-19 showed exclusive cytosolic expression. The R1-3 subdomain was found in the cytosol and the intercalated disk. In the mice infected with the CT-GFP AAV vector, we only detected a few GFP positive cardiomyocytes. Interestingly, GFP signals in these cells were found predominantly at the sarcolemma (Fig. 5).
Figure 5.
Dystrophin R1-3, CR and CT bind to the sarcolemma in the heart. Indicated GFP fusion dystrophin subdomains were delivered to the mdx heart by systemic AAV injection. Uninjected BL10 and mdx hearts were used as negative controls. Subdomain H4-CR and CT showed membrane localization. Subdomain R1-3 was found in the intercalated disk and cytosol. Remaining subdomains were only seen in the cytosol. Scale bar: 50 μm.
Discussion
In this study, we performed the first comprehensive in vivo evaluation of the subcellular localizations of dystrophin subdomains. We demonstrated that in addition to the well-known CR domain, dystrophin contains several highly conserved MBDs that can independently interact with the sarcolemma. These newly identified MBDs are R1-3, R10-12 and CT (Fig. 6). The CT subdomain bound to the sarcolemma in both skeletal muscle and cardiac muscle. Further, it restored the DGC. Subdomain R1-3 showed exclusive membrane binding in skeletal muscle (Fig. 6A) but a preference for the intercalated disk in the heart (Fig. 6B). Subdomain R10-12 only demonstrated partial membrane localization in skeletal muscle (Fig. 6A).
Figure 6.
A new model of dystrophin-sarcolemma interaction. (A) In muscle, dystrophin binds to the sarcolemma through four independent membrane-binding subdomains. (B) In the heart, dystrophin binds to the sarcolemma through three independent membrane-binding domains. These subdomains are marked by thick red lines.
Interaction with the sarcolemma is central to how dystrophin protects the muscle. A wealth of molecular, biochemical and structural studies has provided unequivocal proof that the CR domain anchors dystrophin to the sarcolemma via the formation of the DGC (7–9). Hence, it has been quite puzzling why dystrophins that lack the CR domain still appear to bind to the sarcolemma in some atypical patients (11–13). Studies performed in mdx mice suggest that these puzzling patient observations may well be true. Of notice, forced expression of fragmented dystrophins that lack the CR domain has been repeatedly detected at the sarcolemma in mdx mice (Supplementary Material, Fig. S2C) (19–24). Collectively, it is reasonable to hypothesize that dystrophin may carry additional membrane localization domain(s).
To better understand dystrophin-sarcolemma interaction, investigators have turned to the artificial in vitro systems. These studies identified a number of potential regions capable of membrane binding such as R2, R1-3, R4-19, R11-15, R16-21 (Supplementary Material, Fig. S2D) (14,25–30). Essentially, 21 out of 24 spectrin-like repeats in the rod domain were found to carry the membrane binding property in these in vitro studies. Such a broad range makes it almost impossible to pinpoint the identity of true dystrophin MBDs. Considering the fact that in vivo performance of dystrophin spectrin-like repeats cannot be accurately predicted by in vitro analysis (31), it becomes even more challenging to characterize the CR domain-independent dystrophin-sarcolemma interaction in test tubes. Here, we took a systematic and unbiased approach with an emphasis on the in vivo interaction in rodents and large mammals. We found four structurally defined regions in dystrophin that are capable of interacting with the sarcolemma. These include the well-studied CR domain and three new MBDs (two in the rod domain and one in CT). While R1-3 and R10-12 have been implicated in some in vitro studies, direct binding of CT to the sarcolemma has never been reported. Intriguingly, CT also restores the DGC (Fig. 4). It is intriguing that we observed striking differences in the membrane binding behavior of the newly identified rod domain MBDs. Specifically, R1-3 is not restricted to the sarcolemma in the heart and R10-12 has no membrane binding activity in the heart (Fig. 5). This is reminiscent of different nNOS-binding properties of dystrophin in skeletal muscle and the heart (32,33). Collectively, these data suggest that dystrophin may have different functional roles in skeletal muscle and the heart.
The mechanism(s) by which these newly identified MBDs bind to the sarcolemma await future investigations. It is possible that electrostatic and/or hydrophobic interactions may play a role. However, considering what is known about other spectrin family proteins, we suspect that such interactions may likely involve specified membrane domains (such as lipid rafts) and palmitoylation (34).
Restoration of the DGC by CT is another unexpected finding in this study. We speculate that CT may utilize its syntrophin/dystrobrevin binding motifs to recruit syntrophin and dystrobrevin first. Subsequently, these two proteins scaffold sarcoglycans and dystroglycan to the complex (Supplementary Material, Fig. S4) (35–38).
Another important area that requires further analysis is the kinetic mode of interaction between different MBDs and the sarcolemma. A recent study in the zebrafish suggests that dystrophin can associate with the sarcolemma either via stable tight interaction or via reversible dynamic shuttling between the sarcolemma and the cytosol (39). While additional studies are needed, the results of our microsomal preparation western blot seem to hint that the CR domain is responsible for stable membrane binding (GFP signals were barely detected in the cytosolic fraction) and three newly discovered MBDs may contribute to dynamic membrane binding (abundant GFP signals also presented in the cytosol) (Fig. 2B).
There are a few limitations in our study. First, we have not included hinges 2 and 3 in our constructs. Due to the structural properties of hinges (proline-rich, neither α-helix nor β-sheet), we suspect that these hinge regions may play a nominal role in membrane binding. Nevertheless, future studies are needed to confirm this. Second, we have used an over-expression system in our studies and also the fragmented dystrophin domains are not in their natural protein environment. It remains to be determined whether the membrane binding properties of the newly discovered MBDs are preserved under physiological concentration of dystrophin in wild type animals.
Taken together, we have discovered a new model for dystrophin membrane binding (Fig. 6). Our results offer critical insights into dystrophin function, DMD pathogenesis and gene therapy.
Materials and Methods
Animals
All animal experiments were approved by the Animal Care and Use Committee of the University of Missouri, and the animal use and handling were strictly in accordance with the National Institutes of Health guidelines. Dystrophin-null mdx mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Dystrophin-deficient dogs were generated in house by artificial insemination.
AAV production and delivery
The GFP gene was fused in-frame to the C-terminal ends of the human dystrophin subdomains (Supplementary Material, Fig. S3). The fusion constructs were cloned into the cis AAV packaging constructs by PCR and confirmed by sequencing. Expression was driven by the cytomegalovirus promoter and the SV40 poly-adenylation signal. Y731F AAV-9 vectors were generated by transient transfection and purified through two rounds of CsCl gradient ultracentrifugation (40,41). The viral titer was determined by quantitative PCR.
AAV vectors were delivered by intramuscular injection to limb muscles to adult mdx mice (4-7x1011 vg particles/muscle) and adult dystrophic dogs (0.8–4 × 1014 vg particles/muscle). In dog studies, we applied 5-week transient immune suppression with cyclosporine and mycophenolate mofetil according to our published protocol (42).
Muscle harvesting, microscopic examination and western blot
Eight weeks after injection, the animals were euthanized and muscles were harvested according to Liadaki et al through serial sucrose gradient to preserve the GFP signal (43). GFP was visualized directly under the fluorescein isothiocyanate channel using a fluorescence microscope. Immunostianing was performed as we published before (31,44). Whole muscle lysates were generated as we published before (31,44). The cytosolic and microsomal preparations were obtained with the Plasma Membrane Protein Extraction kit (ab65400, Abcam). Muscle lysates were resolved in a 6% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. Antibodies used in immunostaining and western blot are listed in Supplementary Material, Table S1.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. D.D. is a member of the scientific advisory board for and an equity holder of Solid GT, a subsidiary of Solid Biosciences.
Funding
This work was supported by the grants from Duchenne Parent Project, the Netherlands [to Y.L.]; the National Institutes of Health [NS90634 to D.D., AR67985 to D.D. and Y.L.]; Department of Defense [MD130014 to D.D.]; and Jesse’s Journey-The Foundation for Gene and Cell Therapy [to D.D.].
Supplementary Material
References
- 1.Straub V., Rafael J.A., Chamberlain J.S., Campbell K.P. (1997) Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol., 139, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H.L. (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. U S A, 90, 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman E.P., Brown R.H.J., Kunkel L.M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell, 51, 919–928. [DOI] [PubMed] [Google Scholar]
- 4.Koenig M., Kunkel L.M. (1990) Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J. Biol. Chem., 265, 4560–4566. [PubMed] [Google Scholar]
- 5.Amann K.J., Renley B.A., Ervasti J.M. (1998) A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J Biol. Chem., 273, 28419–28423. [DOI] [PubMed] [Google Scholar]
- 6.Rybakova I.N., Amann K.J., Ervasti J.M. (1996) A new model for the interaction of dystrophin with F-actin. J. Cell Biol., 135, 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell K.P., Kahl S.D. (1989) Association of dystrophin and an integral membrane glycoprotein. Nature, 338, 259–262. [DOI] [PubMed] [Google Scholar]
- 8.Jung D., Yang B., Meyer J., Chamberlain J.S., Campbell K.P. (1995) Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J. Biol. Chem., 270, 27305–27310. [DOI] [PubMed] [Google Scholar]
- 9.Huang X., Poy F., Zhang R., Joachimiak A., Sudol M., Eck M.J. (2000) Structure of a WW domain containing fragment of dystrophin in complex with beta-dystroglycan. Nat. Struct. Biol., 7, 634–638. [DOI] [PubMed] [Google Scholar]
- 10.Ervasti J.M., Campbell K.P. (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell, 66, 1121–1131. [DOI] [PubMed] [Google Scholar]
- 11.Helliwell T.R., Ellis J.M., Mountford R.C., Appleton R.E., Morris G.E. (1992) A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am. J. Hum. Genet., 50, 508–514. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman E.P., Garcia C.A., Chamberlain J.S., Angelini C., Lupski J.R., Fenwick R. (1991) Is the carboxyl-terminus of dystrophin required for membrane association? A novel, severe case of Duchenne muscular dystrophy. Ann. Neurol., 30, 605–610. [DOI] [PubMed] [Google Scholar]
- 13.Recan D., Chafey P., Leturcq F., Hugnot J.P., Vincent N., Tome F., Collin H., Simon D., Czernichow P., Nicholson L.V.et al. (1992) Are cysteine-rich and COOH-terminal domains of dystrophin critical for sarcolemmal localization? J. Clin. Invest., 89, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Rumeur E., Winder S.J., Hubert J.F. (2010) Dystrophin: more than just the sum of its parts. Biochim. Biophys. Acta, 1804, 1713–1722. [DOI] [PubMed] [Google Scholar]
- 15.Cox G.A., Sunada Y., Campbell K.P., Chamberlain J.S. (1994) Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nat. Genet., 8, 333–339. [DOI] [PubMed] [Google Scholar]
- 16.Crawford G.E., Faulkner J.A., Crosbie R.H., Campbell K.P., Froehner S.C., Chamberlain J.S. (2000) Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J. Cell Biol., 150, 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapaport D., Greenberg D.S., Tal M., Yaffe D., Nudel U. (1993) Dp71, the nonmuscle product of the Duchenne muscular dystrophy gene is associated with the cell membrane. FEBS Lett., 328, 197–202. [DOI] [PubMed] [Google Scholar]
- 18.Judge L.M., Haraguchiln M., Chamberlain J.S. (2006) Dissecting the signaling and mechanical functions of the dystrophin-glycoprotein complex. J. Cell Sci., 119, 1537–1546. [DOI] [PubMed] [Google Scholar]
- 19.Rafael J.A., Cox G.A., Corrado K., Jung D., Campbell K.P., Chamberlain J.S. (1996) Forced expression of dystrophin deletion constructs reveals structure-function correlations. J. Cell Biol., 134, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritz J.D., Danko I., Roberds S.L., Campbell K.P., Latendresse J.S., Wolff J.A. (1995) Expression of deletion-containing dystrophins in mdx muscle: implications for gene therapy and dystrophin function. Pediatr. Res., 37, 693–700. [DOI] [PubMed] [Google Scholar]
- 21.Maconochie M.K., Simpkins A.H., Damien E., Coulton G., Greenfield A.J., Brown S.D. (1996) The cysteine-rich and C-terminal domains of dystrophin are not required for normal costameric localization in the mouse. Transgenic Res., 5, 123–130. [DOI] [PubMed] [Google Scholar]
- 22.Gardner K.L., Kearney J.A., Edwards J.D., Rafael-Fortney J.A. (2006) Restoration of all dystrophin protein interactions by functional domains in trans does not rescue dystrophy. Gene Ther., 13, 744–751. [DOI] [PubMed] [Google Scholar]
- 23.Barnabei M.S., Sjaastad F.V., Townsend D., Bedada F.B., Metzger J.M. (2015) Severe dystrophic cardiomyopathy caused by the enteroviral protease 2A-mediated C-terminal dystrophin cleavage fragment. Sci. Transl. Med., 7, 294ra106.. [DOI] [PubMed] [Google Scholar]
- 24.Dunckley M.G., Wells K.E., Piper T.A., Wells D.J., Dickson G. (1994) Independent localization of dystrophin N- and C-terminal regions to the sarcolemma of mdx mouse myofibres in vivo. J. Cell Sci., 107, 1469–1475. [DOI] [PubMed] [Google Scholar]
- 25.Hir S.A., Raguenes-Nicol C., Paboeuf G., Nicolas A., L Rumeur E., Vie V., (2014) Cholesterol favors the anchorage of human dystrophin repeats 16 to 21 in membrane at physiological surface pressure. Biochim. Biophys. Acta, 1838, 1266–1273. [DOI] [PubMed] [Google Scholar]
- 26.DeWolf C., McCauley P., Sikorski A.F., Winlove C.P., Bailey A.I., Kahana E., Pinder J.C., Gratzer W.B. (1997) Interaction of dystrophin fragments with model membranes. Biophys. J., 72, 2599–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Rumeur E., Fichou Y., Pottier S., Gaboriau F., Rondeau-Mouro C., Vincent M., Gallay J., Bondon A. (2003) Interaction of dystrophin rod domain with membrane phospholipids. Evidence of a close proximity between tryptophan residues and lipids. J. Biol. Chem., 278, 5993–6001. [DOI] [PubMed] [Google Scholar]
- 28.Le Rumeur E., Pottier S., Da Costa G., Metzinger L., Mouret L., Rocher C., Fourage M., Rondeau-Mouro C., Bondon A. (2007) Binding of the dystrophin second repeat to membrane di-oleyl phospholipids is dependent upon lipid packing. Biochim. Biophys. Acta, 1768, 648–654. [DOI] [PubMed] [Google Scholar]
- 29.Legardinier S., Hubert J.F., Le Bihan O., Tascon C., Rocher C., Raguenes-Nicol C., Bondon A., Hardy S., Le Rumeur E. (2008) Sub-domains of the dystrophin rod domain display contrasting lipid-binding and stability properties. Biochim. Biophys. Acta, 1784, 672–682. [DOI] [PubMed] [Google Scholar]
- 30.Legardinier S., Raguenes-Nicol C., Tascon C., Rocher C., Hardy S., Hubert J.F., Le Rumeur E. (2009) Mapping of the lipid-binding and stability properties of the central rod domain of human dystrophin. J. Mol. Biol., 389, 546–558. [DOI] [PubMed] [Google Scholar]
- 31.Lai Y., Zhao J., Yue Y., Duan D. (2013) alpha2 and alpha3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc. Natl. Acad. Sci. U S A, 110, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson E.K., Zhang L., Adams M.E., Phillips A., Freitas M.A., Froehner S.C., Green-Church K.B., Montanaro F. (2012) Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS One, 7, e43515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai Y., Thomas G.D., Yue Y., Yang H.T., Li D., Long C., Judge L., Bostick B., Chamberlain J.S., Terjung R.L., Duan D. (2009) Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest., 119, 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennett V., Lorenzo D.N. (2016) An Adaptable Spectrin/Ankyrin-Based Mechanism for Long-Range Organization of Plasma Membranes in Vertebrate Tissues. Curr Top Membr, 77, 143–184. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida M., Hama H., Ishikawa-Sakurai M., Imamura M., Mizuno Y., Araishi K., Wakabayashi-Takai E., Noguchi S., Sasaoka T., Ozawa E. (2000) Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum. Mol. Genet., 9, 1033–1040. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki A., Yoshida M., Ozawa E. (1995) Mammalian alpha 1- and beta 1-syntrophin bind to the alternative splice-prone region of the dystrophin COOH terminus. J. Cell Biol., 128, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang B., Jung D., Rafael J.A., Chamberlain J.S., Campbell K.P. (1995) Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J. Biol. Chem., 270, 4975–4978. [DOI] [PubMed] [Google Scholar]
- 38.Bunnell T.M., Jaeger M.A., Fitzsimons D.P., Prins K.W., Ervasti J.M. (2008) Destabilization of the dystrophin-glycoprotein complex without functional deficits in alpha-dystrobrevin null muscle. PLoS One, 3, e2604.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bajanca F., Gonzalez-Perez V., Gillespie S.J., Beley C., Garcia L., Theveneau E., Sear R.P., Hughes S.M. (2015) In vivo dynamics of skeletal muscle Dystrophin in zebrafish embryos revealed by improved FRAP analysis. Elife, 4, e06541.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shin J.H., Yue Y., Duan D. (2012) Recombinant adeno-associated viral vector production and purification. Methods Mol. Biol., 798, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhong L., Li B., Mah C.S., Govindasamy L., Agbandje-McKenna M., Cooper M., Herzog R.W., Zolotukhin I., Warrington K.H.J., Weigel-Van Aken K.A., et al. (2008) Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U S A, 105, 7827–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J.H., Yue Y., Srivastava A., Smith B., Lai Y., Duan D. (2012) A Simplified Immune Suppression Scheme Leads to Persistent Micro-dystrophin Expression in Duchenne Muscular Dystrophy Dogs. Hum. Gene Ther., 23, 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liadaki K., Luth E.S., Kunkell L.M. (2007) Co-detection of GFP and dystrophin in skeletal muscle tissue sections. BioTechniques, 42, 699–700. [DOI] [PubMed] [Google Scholar]
- 44.Lai Y., Zhao J., Yue Y., Wasala N.B., Duan D. (2014) Partial restoration of cardiac function with ΔPDZ nNOS in aged mdx model of Duchenne cardiomyopathy. Hum. Mol. Genet., 23, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.